Abstract
In the workplace, the arsenic is used in the semiconductor production and the manufacturing of pigments, glass, pesticides and fungicides. Therefore, workers may be exposed to airborne arsenic during its use in manufacturing. The purpose of this study was to evaluate the potential toxicity of particulate matters (PMs) doped with arsenic (PMs-Arsenic) using a rodent model and to compare the genotoxicity in various concentrations and to examine the role of PMs-Arsenic in the induction of signaling pathway in the lung. Mice were exposed to PMs 124.4 ± 24.5 μg/m3 (low concentration) , 220.2 ± 34.5 μg/m3 (middle concentration) , 426.4 ± 40.3 μg/m3 (high concentration) doped with arsenic 1.4 μg/m3 (Low concentration) ,2.5 μg/m3 (middle concentration) , 5.7 μg/m3 (high concentration) for 4 wks (6 h/d, 5 d/wk) , respectively in the whole-body inhalation exposure chambers. To determine the level of genotoxicity, Chromosomal aberration (CA) assay in splenic lymphocytes and Supravital micronucleus (SMN) assay were performed. Then, signal pathway in the lung was analyzed. In the genotoxicity experiments, the increases of aberrant cells were concentration-dependent. Also, PMs-arsenic caused peripheral blood micronucleus frequency at high concentration. The inhalation of PMs-Arsenic increased an expression of phosphorylated Akt (p-Akt: protein kinase B) and phpsphorylated mammalian target of rapamycin (p-mTOR) at high concentration group. Taken together, inhaled PMs-Arsenic caused genotoxicity and altered Akt signaling pathway in the lung. Therefore, the inhalation of PMs-Arsenic needs for a careful risk assessment in the workplace.
Keywords: Akt (protein kinase B) , Arsenic, Genotoxicity, Inhalation, Particulate Matters (PMs)
INTRODUCTION
Particulate matters (PMs) are major pollutants in atmospheres and they are broadly classified by size into two groups: PMs 10 (particulate matter < 10 ㎛ in diameter) and PMs 2.5 (< 2.5 ㎛) . These PMs come from a variety of sources such as diesel engine combustion on roads, construction site, and manufacturing of metal casting. Previous studies have shown the relation between the exposure to PMs and pulmonary diseases such as asthma, chronic obstructive pulmonary disease (COPD) and lung cancer (Dockery and Pope, 1994; Pope, 2000; Pope et al., 2002).
Also, epidemiological studies have shown that it was associations of inhalation exposures to airborne PMs with risk for adverse effects on morbidity and mortality (Küzli et al., 2000).
PMs 2.5 or smaller particle reaches easily and deposits alveolar in the region of respiratory tract (Oberdorster et al., 2005). Reduced particle size shows the greater toxicity in the respiratory and cardiovascular system (Donaldson and Mac-Nee, 2001). Furthermore, PMs 2.5 contain diverse chemical elements than PMs 10 as a reason for anthropogenic emissions and these compositions of PMs affected their toxicity (Schins et al., 2004). Also, PMs can induce DNA damage, apoptosis through mitochondria-regulated death pathway and oxidative stress in vitro (de Kok et al., 2005; Noda et al., 2002). However, the exact mechanism is unknown.
Heavy metals such as lead, cadmium, copper and arsenic continue to increase in the environment by industrialization. They are stable and easy to accumulate with causing injury of the body. Arsenic is a kind of toxic metals found in the ground water, soil and airborne particles (Tchounwou et al., 1999). Arsenic induced genotoxicity by damage DNA and can alter cell signaling (Dong, 2002; Partridge et al., 2007). The major arsenicals formed in a natural environment are arsenite [As (III) ], arsenate [As (V) ], dimethylarsinic acid (DMA) and monomethylarsonate (MMA) . Inorganic arsenic is methylated into DMA and MMA in the body. In general, inorganic arsenic is known to more toxic than organic arsenic. However, some recent studies have reported that methylated arsenic metabolites are more toxic than inorganic arsenic (Petrick et al., 2000; Vega et al., 2001).
DMA has been used in herbicide, various pharmaceutical and medical products. The lung cancer can result from arsenic exposure either via drinking water (Hopenhayn-Rich et al., 1998) or inhalation (Enterline et al., 1995). DMA in drinking water acts as a complete carcinogen in A/J mice known to be susceptible to developing pulmonary tumors (Hayashi et al., 1998). In workplaces, arsenic is used in the semiconductor production and the manufacturing of pigments, glass, pesticides and fungicides. Furthermore, these works processing often generates PMs in the work-place. Therefore, workers may be exposed to airborne PMs doped with arsenic (PMs-Arsenic) during its use in manufacturing (Hathaway et al., 1991).
Recently, a few in vivo studies have reported that the inhalation of arsenic caused developmental toxicity and systemic uptake (Beck et al., 2002; Holson et al., 1999). However, the potential toxic effects of PMs-Arsenic in genotoxicity and signal transduction studies have not been explored completely. Therefore, the objective of this study is to evaluate the potential toxicity of PMs-Arsenic using a rodent model and to compare the genotoxicity in various concentrations and to examine the role of PMs-Arsenic in the induction of Akt signaling pathway in the lung.
MATERIALS AND METHODS
Particulate matters (PMs) doped with arsenic generating system.
PMs generating system and concentration analysis adopted from the method of Park et al. (2005). Briefly, the configuration used was that of a classical arrangement for the burner nozzle surrounded by a co-flowing stream of air. Mass flow meters and controller (MR-3000, Clark, MA, USA) were used to regulate the flows of argon gas and fuels. Argon gas, effective for the prevention of iron oxide deposition on the nozzle tip, was used for annular flow. Primary fuel ethylene (C2H4) was mixed with strong sooting propense acetylene to maintain a constant soot formation in the exhaust stream. Dimethylarsinic acid (DMA, C2H7AsO2) solution (Sigma-Aldrich, St. Louis, MO, USA) was added to the fuel mixture as a vapor, which was generated by passing ethylene through the DMA liquid in a 100-mm glass vessel. All lines from the vessel to the burner were heated using a heating tape to prevent the condensation of DMA solution. The concentration of PMs and As in the chamber was determined using a high-volume sampler equipped with a cascade impactor and inductively coupled plasma mass spectrometry (ICP-MS) , respectively.
Animals and experiment design.
The NF-κB luciferase transgenic male mice (4~5 weeks old) were used for experiments and acclimatized for at least 1 wk prior to the beginning of the study. We randomized animals into 4 groups, ten mice each per group, with one group as the unexposed control. Mice were exposed to PMs 124.4 ± 24.5 μg/m3 (low concentration) , 220.2 ± 34.5 μg/m3 (middledle concentration) , 426.4 ± 40.3 μg/m3 (high concentration) doped with arsenic 1.4 μg/m3 (low concentration) , 2.5 μg/m3 (middle concentration) , 5.7 μg/m3 (high concentration) , respectively for 6 h/day, 5 days per week for 4 weeks in the whole-body exposure chambers (WBEC) . Animals were given sterilized commercial pellet diet and tap water ad libitum. Environment condition in the WBEC was maintained constant at a temperature of 21~23℃ and a humidity of 35~70% with 12~15 air changes per hour. All experiments were performed according to the guideline for care and use of Seoul National University.
Chromosomal aberration (CA) assay in splenic lymphocytes.
For determination of genotoxcity, we adapted methods from an approach described in our previous study (Kim et al., 2002). Spleens were dissected, and gored through a sterilized nylon filter, which was then washed in phosphate buffer solution (PBS) to collect the splenocytes. The cell suspension was then gently layered on an equal volume of Histopaque 1077 (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged at 400 ×g for 30 min. The interface was carefully collected and washed by suspending in equal volume of PBS and centrifuging at 200 ×g for 15 min. The cell pellet was then resuspended in PBS, centrifuged at 200 ×g for 10 min, and finally suspended in 1 ml of PBS. The cell concentration was determined using a haemocytometer. Splenocyte cultures were initiated at a concentration of 1 × 106 cells/㎖ in a complete medium. Growth medium consisted of RPMI 1640 (Gibco, UK) supplemented with 15% fetal bovine serum (Life Technology, Sweden) , 2 mM L-glutamine,and antibiotics. Concanavalin A (Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 5 μg/ml was used as a mitogen. At least two separate cultures were prepared from each animal for each experiment. Cultures were allowed to grow at 37℃ in a 5% CO2 atmosphere with 95% humidity for 72 hr. Mitotic cells were blocked with colcemid and harvested through centrifugation at 400 ×g for 10 min. The supernatant was removed, and the cell pellet was suspended in 10 ㎖ prewarmed hypotonic KCl solution (0.075 M) for 20 min at 37℃. The supernatant was removed after the tubes were centrifuged for 8 min at 400 ×g. The cell pellet was suspended in 10 ml fixative (acetic acid : methanol, 1 : 3, v/v) , which was changed four times, and the cells were resuspended in 0.3~0.5 ㎖ fixative prior to the slide preparation. Samples of the cell suspension were added to precleaned slides and air-dried. The chromosomes were stained with Giemsa and evaluated by a single observer.
Supravital micronucleus (SMN) assay.
We used a modified method of Hayashi et al. (2000). Briefly, whole blood samples were taken via small cut in the lateral tail vein from mice after the termination of exposure. Five micro liters of blood was dropped on to the center of acridine orange (AO, 1 mg/ml, Sigma-Aldrich, St. Louis, MO, USA) coated slides and clean cover slips were placed carefully over the drops. Mouse peripheral blood erythrocytes were stained with acridine orange (AO, 1 mg/ml, Sigma-Aldrich, St. Louis, MO, USA) and carefully mounted on glass cover slip. The slides were subsequently incubated at 4℃ for 2 hr and examined under oil immersion optics using a fluorescence microscope (HBO100W/Z, Carl Zeiss, Germany) fitted with blue excitation and yellow barrier filters.The only types I, II, and III erythrocytes were observed. At least 2,000 erythrocytes per animals were examined for micronuclei. Frequencies of the micronucleated reticulocytes provided indices of the induced genetic damages.
Western blot analysis.
The lungs were dissected, the protein concentration of the homogenized lysates using a Bradford kit (Bio-Rad, Hercules, CA, USA) equal amounts of protein sample were separated on SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked for 1 hr in TBST (Tris-buffered saline + Tween 20) containing 5% skim milk; immunoblotting was performed by incubating the membranes overnight with their corresponding primary antibodies at 4℃. All antibodies for western blot were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) . After washing in TBST, the membranes were incubated with a horseradish peroxidase (HRP) -labeled secondary antibody (Zymed, CA, USA) and the bands-of-interest were detected using a luminescent image analyzer, LAS-3000 (Fujifilm, Japan) .
Statistical analysis.
All results are expressed as means ±standard error (SE). A multiple variance of analysis (ANOVA) test and Student’s t-test (Graphpad Software, San Diego, CA, USA) were used to compare exposure groups with those obtained from unexposed control group. Statistical significance of the differences in micronucleus occurrences among various treated groups was checked through the method of Kim et al., 2000.
RESULTS AND DISSCUSION
Characterization of generated PMs-Arsenic in wholebody exposure chamber.
Size distribution of PMs in the WBEC from our generating system was sufficiently narrow range of PMs with approximately 95% of PM2.5 and most of PM 2.5 was 0.1 ㎛ and 0.2 ㎛ which occupied 86% to 94%. The actual concentrations of fine PMs with As were 124.4 ± 24.5 (low) , 220.2 ± 34.5 (middle) , and 426.5 ± 40.3 (high) μg/m3 in the WBEC, respectively.
Genotoxicity.
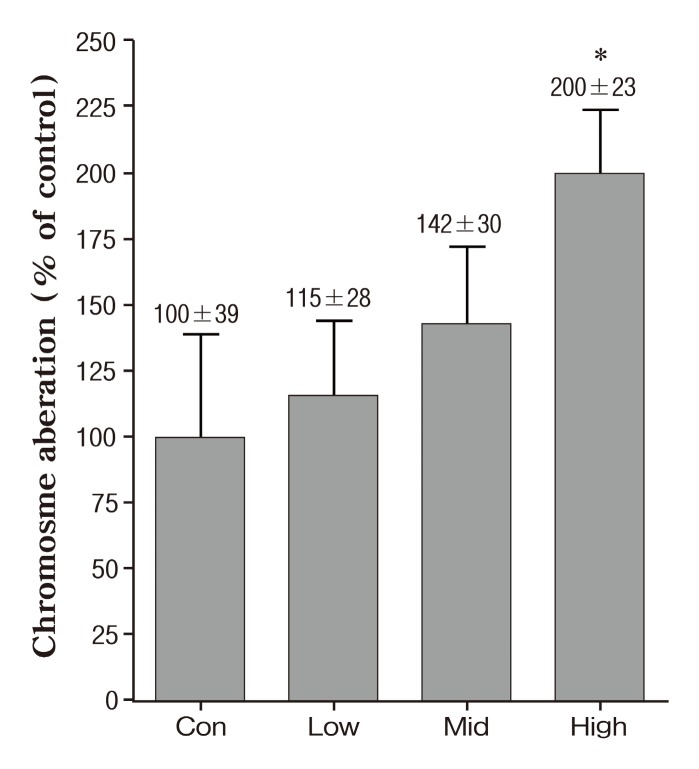
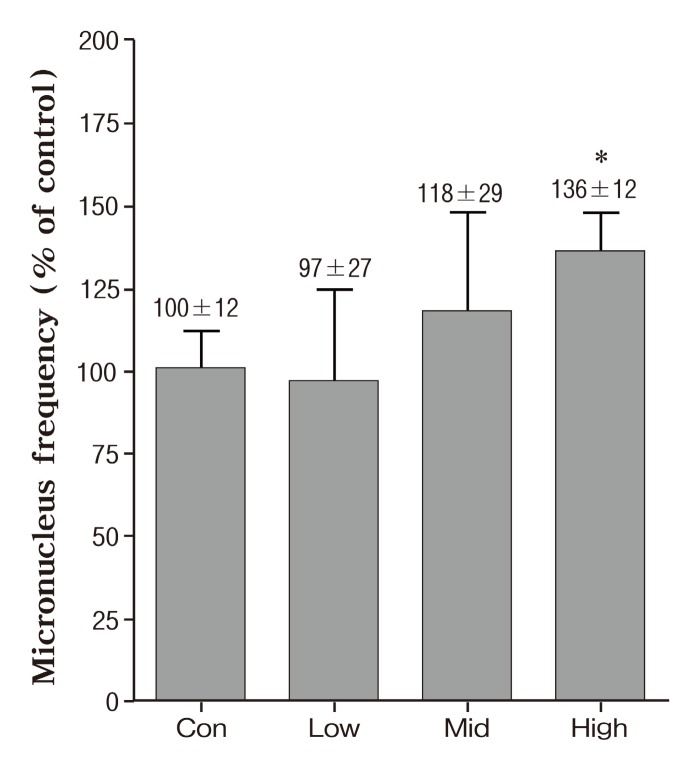
We performed chromatid/chromosome (CA) assay and SMN assay for genotoxicity. The frequencies of cells with CA breaks and exchanges in mice exposed various concentration of PMs-As are shown in Fig. 1. All exposed groups were increased dose-dependent in the frequencies of cells with chromosomal aberration compared with the unexposed group, with only significantly at the 426.4 ± 40.3 μg/m3 (high concentration) , whereas the critical changes have not been found in other groups. As shown in Fig. 2, there was a 96% increase in the CA as compared to the control at the 426.4 ± 40.3 μg/m3 (high concentration) .The effects of exposure to PMs-As on peripheral blood micronucleus frequencies of mice are showed in Fig.3. In the result of SMN, a high concentration group was a 36% significantly increase in peripheral blood micronucleus frequencies compared with control (Fig. 2) . Also,these results are in close agreement with other studies which showed genotoxicity of PMs (de Kok et al., 2005; Roubicek et al., 2007) and arsenic (Noda et al., 2002; Rudel et al., 1996).
Fig. 1. Frequency of chromosomal aberrations (%) in mice of inhaled PMs-Arsenic. Data represents mean ± SE. Significant difference with control group (*p < 0.05) .
Fig. 2. Peripheral blood micronucleus frequency (%) in mice after PMs-Arsenic exposure. Data represents mean ± SE. Significant difference with control group (*p < 0.05) .
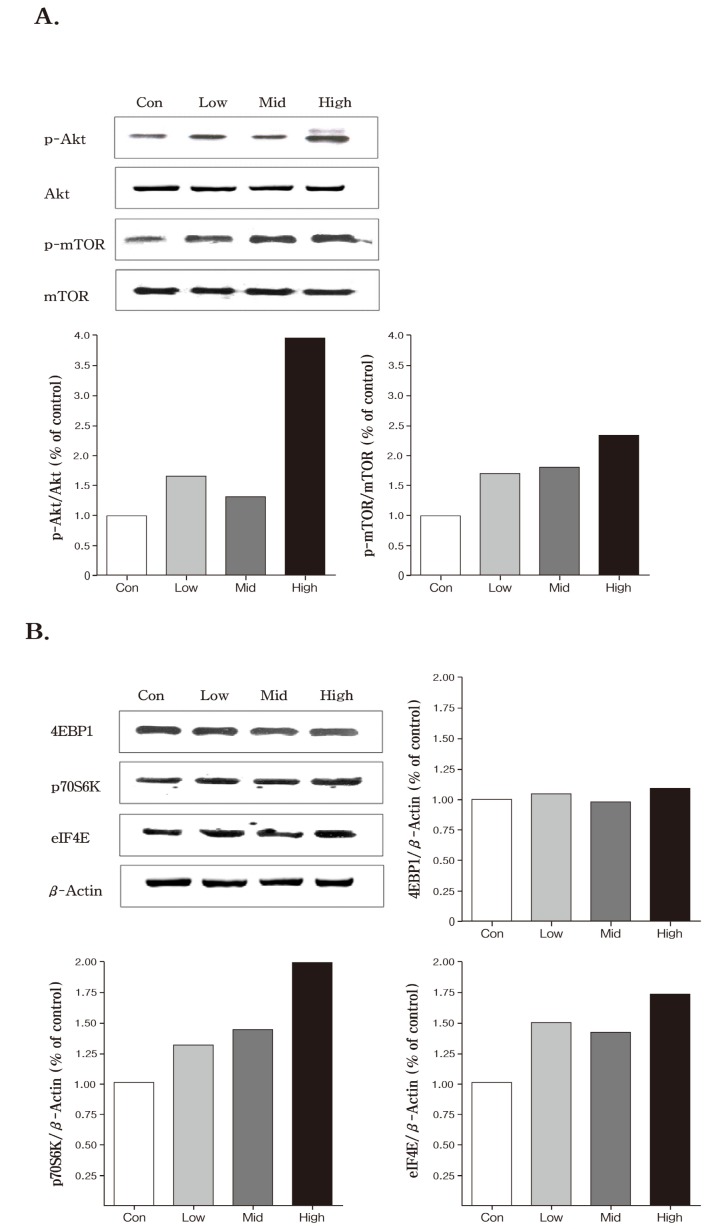
Fig. 3. Western blot analysis of proteins on inhaled PMs-Arsenic in the lung. Mice were exposed to PMs 124.4 ± 24.5 μg/m3 (Low) 220.2 ± 34.5 μg/m3 (Middle) and 426.4 ± 40.3 μg/m3 (High) doped with Arsenic 1.4 μg/m3 (Low) 2.5 μg/m3 (Middle) and 5.7 μg/m3 (High) for 4 wks (6 h/d 5 d/wk) respectively in the whole-body exposure chambers.
In our previous study, inhalation of PMs (low: 117.89 ± 13.2, middle: 204.79 ± 15.4 and high: 414.29 ± 29.8 μg/m3, respectively) and PMs doped with iron (PMs-Iron, low: 105.59 ± 19.5, middle: 205.79 ± 15.8 and high: 428.89 ± 38.7 μg/m3, respectively) caused a significant concentration-dependent increase of genotoxicity (Park et al., 2005). Also, high concentration of PMs-Iron caused a significantly higher frequency of chromosome aberration than PMs only exposed. Taken together, PMs genotoxicity affect by chemical composition and concentration.
Western blot analysis of Akt/mTOR cascade in the lung.
In the Akt (protein kinase B) signaling pathways, the expression of phosphorylated Akt (p-Akt) was increased in all PMs-Arsenic exposed groups compared with control. Greatest expression of p-Akt was observed in high dose group compared to other groups (Fig. 3) . Similar pattern was found in phpsphorylated mammalian target of rapamycin (p-mTOR) . The expression of the p-mTOR was increased in a dosedependent manner, however, the crucial change was not found in the un-phosphorylated form of the two molecules, Akt and mTOR (Fig. 3) . Akt/mTOR cascade is an important mediator in signaling pathways, leading to cell survival and cell proliferation (Song et al., 2005). A recent study showed that Akt is frequently active in human nonsmall lung cancer (Tang et al., 2006). Additionally, the inhibitor of Akt signaling treatment induces anti-tumor activity in cancer (Yang et al., 2004). It is generally thought that the mTOR functions were downstream of the Akt pathway in cancer cells. The mTOR is a key controller of cell growth, and protein translation, primarily through two distinct pathways: ribosomal p70 S6 kinase (p70S6K) and the eukaryotic translation initiation factor 4E (eIF4E) binding proteins (4E-BPs) (Papadimitrakopoulou and Adjei 2006). Our findings showed that inhalation of PMs-Arsenic increased an expression of p-Akt and p-mTOR at the high concentration group, but the crucial exchange was not found in the un-phosphorylated form of the two molecules, Akt and mTOR (Fig. 3) . These results indicate that inhalation of PMs-Arsenic have led to increased the activity of Akt/mTOR signaling pathway. Thus, these results suggest that PMs-Arsenic could affect Akt signaling pathway in the lung. However, we have limited information of interaction between PMs and arsenic because of weakness in study design. Nevertheless, this study should provide information for potential toxicity of PMs-Arsenic in arsenic used workplace,such as semiconductor production, manufacturing of pigments and glass.
In summary, this study suggests that inhaled PMs-Arsenic could increase genotoxicity and could affect Akt signaling pathway in the lung. In genotoxicity, increase of aberrant cells was dose-dependent and induction of CA by PMs-Arsenic was observed with the clastogenic effect occurring at the high concentration group. Also, PMs-Arsenic induced the increase in micronucleus frequency at high concentration in the peripheral blood. Taken together, the present study demonstrates that inhaled PMs-Arsenic could induce genotoxicity and affect Akt signaling pathway in the lung.Therefore, the inhalation of PMs-Arsenic needs for a careful risk assessment in the workplace.
Acknowledgments
This work was supported in part by a grant from Korea Research Foundation (KRF, grant # 2010-0000784) . J.T.K., A.M.T., and S.K.H. are grateful for financial supported from BK21 program. MHC was also partially supported by the Research Institute for Veterinary Science, Seoul National University.
References
- 1.Beck B.D. Slayton T.M. Farr C.H. Sved D.W. Crecelius E.A. Holson J.F. Systemic uptake of inhaled arsenic in rabbits. Hum. Exp. Toxicol. 2002;21:205–215. doi: 10.1191/0960327102ht237oa. [DOI] [PubMed] [Google Scholar]
- 2.de Kok T.M. Hogervorst J.G. Briede J.J. van Herwijnen M.H. Maas L.M. Moonen E.J. Driece H.A. Kleinjans J.C. Genotoxicity and physicochemical characteristics of traffic-related ambient particulate matter. Environ. Mol. Mutagen. 2005;46:71–80. doi: 10.1002/em.20133. [DOI] [PubMed] [Google Scholar]
- 3.Dockery D.W. Pope C.A. 3rd. Acute respiratory effects of particulate air pollution. Annu. Rev. Public Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson K. MacNee W. Potential mechanisms of adverse pulmonary and cardiovascular effects of particulate air pollution (PM10) . Int. J. Hyg. Environ. Health. 2001;203:411–415. doi: 10.1078/1438-4639-00059. [DOI] [PubMed] [Google Scholar]
- 5.Dong Z. The molecular mechanisms of arsenic-induced cell transformation and apoptosis. Environ. Health Perspect. 2002;110:757–759. doi: 10.1289/ehp.02110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enterline P.E. Day R. Marsh G.M. Cancers related to exposure to arsenic at a copper smelter. Occup. Environ. Med. 1995;52:28–32. doi: 10.1136/oem.52.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hathaway G.J. Proctor N.H. Hughes J.P. Fischman M.L. Chemical Hazards of the workplace. 2nd edition VanNostrand Reinhold Co; New York: 1991. [Google Scholar]
- 8.Hayashi H. Kanisawa M. Yamanaka K. Ito T. Udaka N. Ohji H. Okudela K. Okada S. Kitamura H. Dimethylarsinic acid a main metabolite of inorganic arsenics has tumorigenicity and progression effects in the pulmonary tumors of A/J mice. Cancer Lett. 1998;125:83–88. doi: 10.1016/S0304-3835(97)00484-9. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi M. MacGregor J.T. Gatehouse D.G. Adler I.D. Blakey D.H. Dertinger S.D. Krishna G. Morita T. Russo A. Sutou S. In vivo rodent erythrocyte micronucleus assay. II. Some aspects of protocol design including repeated treatments integration with toxicity testing and auto matedscoring. Environ. Mol. Mutagen. 2000;35:234–252. [PubMed] [Google Scholar]
- 10.Holson J.F. Stump D.G. Ulrich C.E. Farr C.H. Absence of prenatal developmental toxicity from inhaled arsenic trioxide in rats. Toxicol. Sci. 1999;51:87–97. doi: 10.1093/toxsci/51.1.87. [DOI] [PubMed] [Google Scholar]
- 11.Hopenhayn-Rich C. Biggs M.L. Smith A.H. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba Argentina. Int. J. Epidemiol. 1998;27:561–569. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- 12.Kim M.Y. Kim Y.C. Cho M.H. Combined treatment with 4- (N-methyl-N-nitrosamino) -1- (3-pyridyl) -1-butanone and dibutyl phthalate enhances ozone-induced genotoxicity in B6C3F1 mice. Mutagenesis. 2002;17:331–336. doi: 10.1093/mutage/17.4.331. [DOI] [PubMed] [Google Scholar]
- 13.Kunzli N. Kaiser R. Medina S. Studnicka M. Chanel O. Filliger P. Herry M. Horak F. Jr. Puybonnieux-Texier V. Quénel P. Schneider J. Seethaler R. Vergnaud J.C. Sommer H. Public-health impact of outdoor and trafficrelated air pollution: a European assessment. Lancet. 2000;356:795–801. doi: 10.1016/S0140-6736(00)02653-2. [DOI] [PubMed] [Google Scholar]
- 14.Noda Y. Suzuki T. Kohara A. Hasegawa A. Yotsuyanagi T. Hayashi M. Sofuni T. Yamanaka K. Okada S. In vivo genotoxicity evaluation of dimethylarsinic acid in Muta-Mouse. Mutat. Res. 2002;513:205–212. doi: 10.1016/s1383-5718(01)00313-8. [DOI] [PubMed] [Google Scholar]
- 15.Oberdörster G. Oberdörster E. Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadimitrakopoulou V. Adjei A.A. The Akt/mTOR and mitogen-activated protein kinase pathways in lung cancer therapy. J. Thorac. Oncol. 2006;1:749–751. doi: 10.1097/01243894-200609000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Park J.H. Han K.T. Eu K.J. Kim J.S. Chung K.H. Park B. Yang G.S. Lee K.H. Cho M.H. Diffusion flamederived fine particulate matters doped with iron caused genotoxicity in B6C3F1 mice. Toxicol. Ind. Health. 2005;21:57–65. doi: 10.1191/0748233705th215oa. [DOI] [PubMed] [Google Scholar]
- 18.Partridge M.A. Huang S.X. Hernandez-Rosa E. Davidson M.M. Hei T.K. Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: implications for genotoxic mechanisms in mammalian cells. Cancer Res. 2007;67:5239–5247. doi: 10.1158/0008-5472.CAN-07-0074. [DOI] [PubMed] [Google Scholar]
- 19.Petrick J.S. Ayala-Fierro F. Cullen W.R. Carter D.E. Vasken Aposhian H. Monomethylarsonous acid (MMA (III) ) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- 20.Pope C.A. 3rd. Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who’s at risk? Environ. Health Perspect. 2000;108:713–723. doi: 10.2307/3454408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pope C.A. 3rd. Burnett R.T. Thun M.J. Calle E.E. Krewski D. Ito K. Thurston G.D. Lung cancer cardiopulmonary mortality and long-term exposure to fine particulate air pollution. JAMA. 2002;28:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roubicek D.A. Gutierrez-Castillo M.E. Sordo M. Cebrian-Garcia M.E. Ostrosky-Wegman P. Micronuclei induced by airborne particulate matter from Mexico City. Mutat. Res. 2007;631:9–15. doi: 10.1016/j.mrgentox.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Rudel R. Slayton T.M. Beck B.D. Implications of arsenic genotoxicity for dose response of carcinogenic effects. Regul. Toxicol. Pharmacol. 1996;23:87–105. doi: 10.1006/rtph.1996.0031. [DOI] [PubMed] [Google Scholar]
- 24.Schins R.P. Lightbody J.H. Borm P.J. Shi T. Donaldson K. Stone V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol. Appl. Pharmacol. 2004;195:1–11. doi: 10.1016/j.taap.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Song G. Ouyang G. Bao S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang J.M. He Q.Y. Guo R.X. Chang X.J. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Tchounwou P.B. Wilson B. Ishaque A. Important considerations in the development of public health advisories for arsenic and arsenic-containing compounds in drinking water. Rev. Environ. Health. 1999;14:211–229. doi: 10.1515/reveh.1999.14.4.211. [DOI] [PubMed] [Google Scholar]
- 28.Vega L. Styblo M. Patterson R. Cullen W. Wang C. Germolec D. Differential effects of trivalent and pentavalent arsenicals on cell proliferation and cytokine secretion in normal human epidermal keratinocytes. Toxicol. Appl. Pharmacol. 2001;172:225–232. doi: 10.1006/taap.2001.9152. [DOI] [PubMed] [Google Scholar]
- 29.Yang L. Dan H.C. Sun M. Liu Q. Sun X.M. Feldman R.I. Hamilton A.D. Polokoff M. Nicosia S.V. Herlyn M. Sebti S.M. Cheng J.Q. Akt/protein kinase B signaling inhibitor-2 a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]