Abstract
This study investigated the antioxidative action of Corni Fructus aqueous extract on kidneys of diabetic mice. The electron donating abilities of Corni Fructus aqueous extract and its antioxidant activities (XO, SOD, CAT, GST, eNOS) in kidneys of C57BL/6 or db/db mice were evaluated. For in vivo study, seven week-old male mice were divided into normal control group (NC, C57BL/6 mice) , diabetic control group (DC, db/db mice) and Corni Fructus (500 mg/kg/day for 8 weeks) treated diabetic group (DCF, db/db mice) . The electron donating abilities of Corni Fructus aqueous extract exhibited 7%, 24.4%, and 42.7% at concentrations of 100, 500, and 1000 μg/ml, respectively. The activity of XO in the DCF group was significantly lower than the DC group by 35% (p < 0.05) . The SOD activity was significantly higher in the DCF group than the DC group by 26% (p < 0.05) . The activities of CAT and GST were lowered in the DCF group than the DC group by 26% (p < 0.05) and 7.6%, respectively. The mRNA expression of eNOS in kidneys was lower in the DCF group than the DC group by 24%. These results indicate that Corni Fructus reduced oxidation stress as evidenced by the restoration of the enzymatic antioxidative defense system in renal tissues of db/db mice. It is suggested that these antioxidative actions of Corni Fructus on renal tissues in db/db mice could contribute to its renoprotective effects on diabetic nephropathy.
Keywords: Corni Fructus, Antioxidative ability, eNOS, Renoprotective effect
INTRODUCTION
Hyperglycemia generates reactive oxygen species (ROS) that in turn cause lipid peroxidation and membrane damage (Hunt et al., 1988; Morel and Chisolm, 1989) . Previous studies (Venkateswaran and Pari, 2002; Latha and Pari, 2003) have reported that lipid peroxidation in the liver, kidney, and brain of diabetic rats was increased. Oxidative stress has been defined as a disturbance in the balance between the production of reactive oxygen species and antioxidant defenses (Lawrence et al., 2001) .
Recently, attention has been drawn to the theory that oxidative stress is involved in the development of complications associated with chronic diabetes mellitus (Maritim et al., 2003) . Oxidative stress has been considered as a common pathogenetic factor in diabetic nephropathy and other complications (Baynes, 1991; Larkins and Dunlop, 1992) .
Fructus of Cornus officinalis Sieb. et Zucc. (Corni Fructus) has been used as Korean traditional medicine. It represents one of the seven-component herbs in Yukmi-jihangtang that has been used for the treatment of diabetes mellitus or diabetic complications in Korean traditional medicine (Jin et al., 2006) . Major chemical constituents which have been identified by many investigators are saponins, phenolic acid (gallic acid, tannic acid) and loganin (Li et al., 1994) . Saponins and phenolic acid are known to have antioxidant activities (Rong et al., 1995) . Recently, it has been reported that Corni Fructus has beneficial effect on advanced glycation end product-mediated renal injury in STZ-treated diabetic rats (Yamabe et al., 2007) and db/db mice (Kim et al., 2009, Kim and Kim, 2010) .
But the antioxidative effect of Corni Fructus extract in vivo has not yet reported. Thus, the aim of the current study was to investigate the antioxidative action of Corni Fructus on kidneys of diabetic mice.
MATERIALS AND METHODS
Corni fructus aqueous extraction.
The fruits of Cornus officinalis were purchased from Gunwi, Gyeongbuk, Korea and authenticated by a Doctor of Oriental Medicine in the Department of Oriental Medicine, College of Oriental Medicine, Sangji University (Gangwon-Do, Korea) . The Corni Fructus (Fruits of Cornus officinalis, 600 g) was boiled with six liters of distilled water for 2 hours in a heating extractor (COSMOS-660, Kyungseo Machine Co., Korea) .The aqueous extract was concentrated to 3 l, and then dispensed into pouches containing daily volume each and stored at 4℃ until use. The yield of the aqueous extract was calculated as 25% (w/w) by a lyophilization method.
DPPH (1, 1-diphenyl-2-picrylhydrazyl) free radical scavenging assay.
The DPPH radical scavenging activity was evaluated according to the method of Blois (1958) with a slight modification. Four milliliters of various concentrations of the extracts in methanol (100, 500, 1000 μg/ml) were added to a 1 ml solution of DPPH radical in methanol of 1.5 × 10-4 M. The mixture was shaken vigorously and allowed to stand for 30 min. The absorbance of the resulting solution was measured at 520 nm with a spectrophotometer (Shimadzu UV-1601, Japan) . The free radical-scavenging activity of each solution was then calculated as percent inhibition according to the following equation:
%inhibition = (Ablank - Asample) /Ablank × 100
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound) , and Asample is the absorbance of the test compound reaction. BHT (butylated hydroxy toluene) was used as the positive control.
Animal study and collection of kidney.
The experiment was performed using 7-week-old male C57BL/KsJ-db/db mice and C57BL/6 mice (non-diabetic mice) (Central Lab. Animal Inc., Seoul, Korea) . Animals were housed in plastic cages in a controlled environment (22 ± 2℃, humidity of 50 ± 10%, 12 h light/dark cycle, ad libitum feeding and watering) . Animal study was carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Keimyung University. After acclimation, the animals were randomly assigned to three groups (seven animals each) , for the 8-week experiment. The experimental groups were non-diabetic control group (NC) of C57BL/6 mice, diabetic control group (DC) of db/db mice, and group of diabetic db/db mice fed Corni Fructus extract (500 mg/10 ml/kg) once daily for 8 weeks (DCF) . After necropsy, the kidneys were weighed and stored at-80℃ for enzymatic and genetic analyses.
Antioxidant enzyme activities in kidneys.
The kidney tissue was homogenized in 0.25 M sucrose solution using a tissue homogenizer with a Teflon pestle at 4℃ to give 20%homogenate (w/v) . The homogenate was centrifuged at 600 ×g for 10 min to remove any cell debris and then the supernatant was further centrifuged at 10,000 ×g for 20 min to remove the mitochondria pellet. Finally, the supernatant was ultracentrifuged at 105,000 ×g for 60 min to obtain the cytosol supernatant. The amounts of protein in the mitochondria and cytosolic fractions were measured by the method of Lowry et al. (1951) with bovine serum albumin as the standard. The activity of Catalase (CAT) was assayed by measuring the hydrogen peroxide (H2O2) decreasing rate at 240 nm by spectrophotometer by the methods of Aebi (1984) . The activity of superoxide dismutase (SOD) was measured spectrophotometrically by the inhibition rate of auto-oxidation of hematoxylin at 560 nm by spectrophotometer by the methods of Martin et al. (1987) . The activities of glutathione S-transferase (GST) and xanthine oxidase (XO) were measured at 340 nm and 292 nm by spectrophotometer by the methods of Habig et al. (1974) and Stripe and Della (1969) , respectively.
Isolation of total RNA and cDNA synthesis.
Total RNA was isolated from kidneys of mice using the High Pure RNA Isolation Kit (Roche Applied Science, Penzberg, Germany) following the manufacturer’s protocol. The quantity and quality of the isolated total RNA were determined by the UV/Vis spectrophotometer (Mecasys Co., Korea) . Only samples with 2.0 > OD260/280 > 1.8 were processed. cDNA was synthesized from 2 μg of total RNA, using AccuPower CycleScript RT PreMix Kit (Bioneer, Korea) in a final volume of 20 μl.
Real-time quantitative RT-PCR.
Quantitative real-time RT-PCR was used to quantify the amounts of eNOS gene mRNA. cDNA was diluted 1 : 10 with autoclaved deionized water, and 2 μl of the diluted cDNA was added to 10 μl of iQTM SYBR Green Supermix (BioRad Laboratories, Inc., USA) and 7 pmol/l of specific primer. The Primer sequences used were as follows: β-actin (forward: 5'-CCCAGGCATTGCTGACAGG-3' and reverse: 5'-TGGAAGGTGGACAGTGAGGC-3') , eNOS (forward: 5'-CAACGCTACCACGAGGACA-3' and reverse: 5'-CTCCTGCAAAGAAAAGCTCTGG-3') . This reaction mixture was filled up to a final volume of 20 μl with water. PCR was carried out in a real-time PCR cycler (iCycler IQ System, BioRad, USA) . The program was optimized and performed finally with denaturation at 95℃ for 5 min followed by 40 cycles of amplification (95℃ for 20 s, annealing Tm for 20 s, 72℃ for 30 s) . After completion of the PCR, the melting curve of the product was measured by temperature gradient from 60 to 95℃ at 0.5℃ per second with continuous fluorescence monitoring to produce a melting profile of the primers. The relative abundance of mRNA was standardized with β-actin mRNA as the invariant control. The amplification curves for Ct (threshold cycle) determination and melting curves for temperature estimations at the peak of the curves were analyzed using the Gene Expression Analysis for iCycler iQ Real-Time PCR Detection System from BioRad.
Statistical analysis.
Values were given as mean ± SD of 7 mice in each group. The data of antioxidant enzyme activities in kidneys were analyzed by Student’s t-test using SPSS-12.0. The limit of statistical significance was set at p < 0.05.
RESULTS
DPPH free radical scavenging activity.
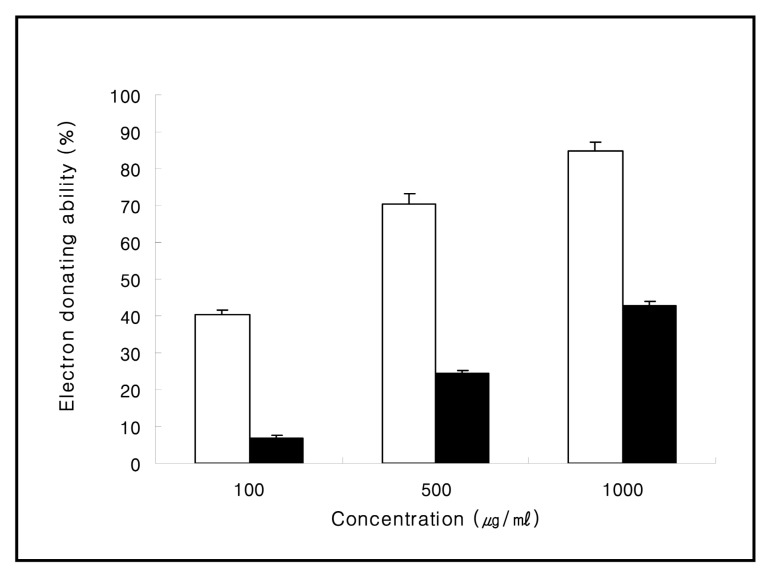
The electron donating abilities of aqueous extract obtained from Corni Fructus exhibited 7.0%, 24.4%, and 42.7% at concentrations of 100, 500, and 1000 μg/ml, respectively. The electron donating abilities of BHT (butylated hydroxy toluene) , which was used as the positive control, exhibited 40.3%, 70.6%, and 84.7% at the same concentrations, respectively (Fig. 1) .
Fig. 1. The electron donating abilities of aqueous extract obtained from Corni Fructus. BHT (butylated hydroxy toluene) was used as the positive control. □; BHT (butylated hydroxy toluene) , ■; Corni Fructus aqueous extract. Values are mean ± SD of 3 experiments.
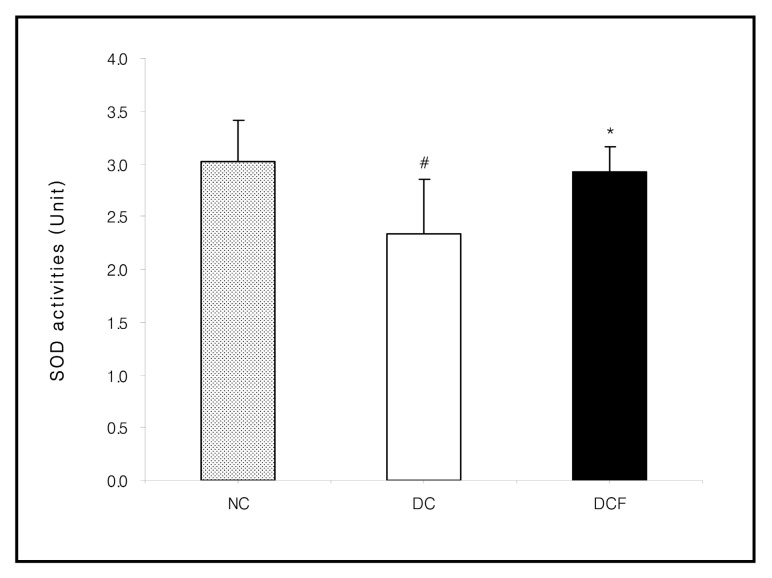
Fig. 3. Effect of Corni Fructus on kidney SOD activities in C57BL/6 and db/db mice fed the Corni Fructus extract for 8 weeks. Unit: U (50% inhibition of autoxidation of hematoxylin) /mg protein/min. NC: Non-diabetic control group. DC: Untreated diabetic group. DCF: Diabetic group fed Corni Fructus extract. Values are mean ± SD of 7 mice. The value with a sharp-note is significantly different from NC group by t-test (#; p < 0.05) . The value with an asterisk is significantly different from DC group by t-test (*; p < 0.05) .
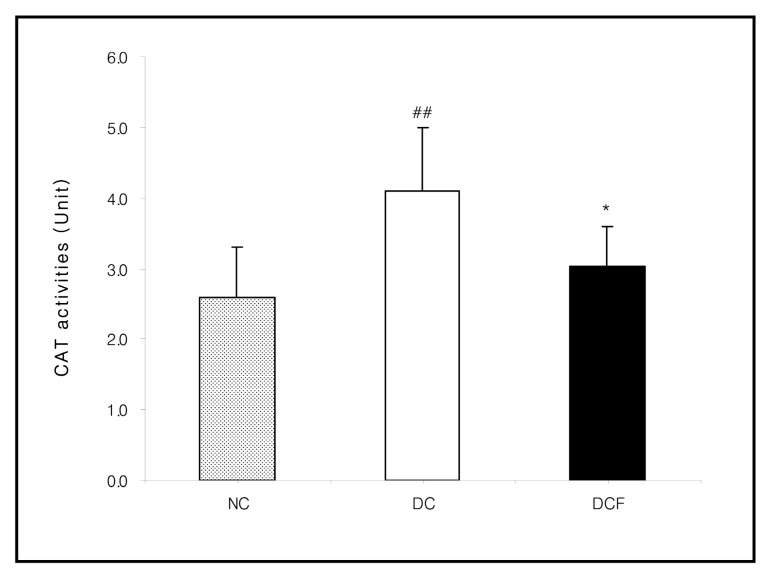
Fig. 4. Effect of Corni Fructus on kidney CAT activities in C57BL/6 and db/db mice fed the Corni Fructus extract for 8 weeks. Unit: nmole H2O2 reduced/mg protein/min. NC: Non-diabetic control group. DC: Untreated diabetic group. DCF: Diabetic group fed Corni Fructus extract. Values are mean ± SD of 7 mice. The value with a sharp-note is significantly different from NC group by t-test (##; p < 0.01) . The value with an asterisk is significantly different from DC group by t-test (*; p < 0.05) .
Antioxidant enzyme activities in kidney.
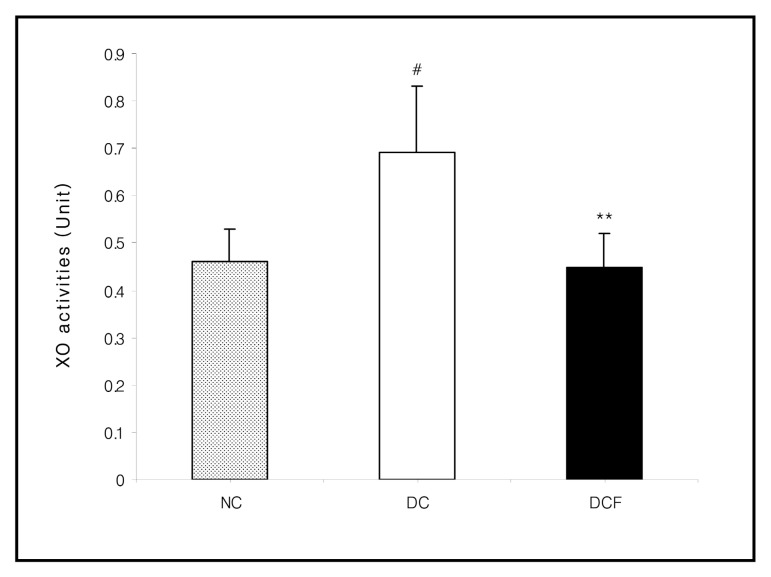
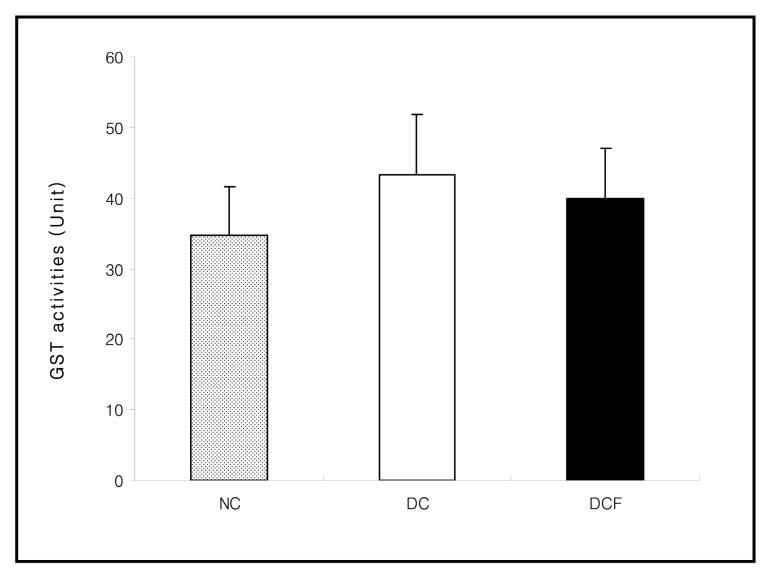
The activities of XO (p < 0.05) , CAT (p < 0.01) and GST in the DC group were higher than the NC group. However, they were lowered in the DCF group, compared to the DC group by 35% (p < 0.01) , 26% (p < 0.05) and 7.6%, respectively. The activity of SOD in the DC group was significantly lower than the NC group (p < 0.05) and was significantly higher in the DCF group than the DC group by 26% (p < 0.05) (Figs. 2~5) .
Fig. 2. Effect of Corni Fructus on kidney XO activities in C57BL/6 and db/db mice fed the Corni Fructus extract for 8 weeks. Unit: nmole uric acid formed/mg protein/min. NC: Non-diabetic control group. DC: Untreated diabetic group. DCF: Diabetic group fed Corni Fructus extract. Values are mean ± SD of 7 mice. The value with a sharp-note is significantly different from NC group by t-test (#; p < 0.05) . The value with an asterisk is significantly different from DC group by t-test (**; p < 0.01) .
Fig. 5. Effect of Corni Fructus on kidney GST activities in C57BL/6 and db/db mice fed the Corni Fructus extract for 8 weeks. Unit: nmole 2 4-dinitrobenzene-glutathione conjugate/mg protein/min. NC: Non-diabetic control group. DC: Untreated diabetic group. DCF: Diabetic group fed Corni Fructus extract. Values are mean ± SD of 7 mice.
eNOS expression in kidney by real-time quantitative RT-PCR.
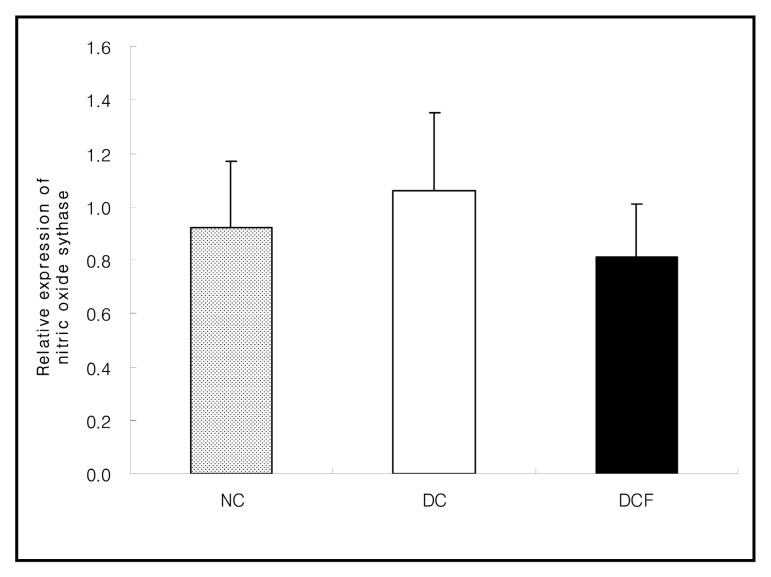
The mRNA expression in eNOS (endothelial nitric oxide synthase) in the DC group was slightly higher than the NC group (1.06 ± 0.29 vs. 0.92 ± 0.25) and it was lower in the DCF group than the DC group (0.81 ± 0.19 vs. 1.06 ± 0.29) (Fig. 6) .
Fig. 6. eNOS expression in the kidney of C57BL/6 and db/db mice fed the Corni Fructus extract for 8 weeks. These values are shown relative to those of the housekeeping gene β-actin by real-time RT-PCR. Values are mean ± SD of 5 mice.
DISCUSSION
This study intended to find out the electron donating abilities of the aqueous extract from Corni Fructus and its antioxidant action on kidney in C57BL/6 or db/db mice. Antioxidant enzymes are capable of eliminating reactive oxygen species (ROS) and lipid peroxidation products, thereby protecting cells and tissues from oxidative damage. Recent studies have indicated that ROS plays an intermediate but key role in the development of diabetic nephropathy. High glucose level directly increases hydrogen peroxide production of mesangial cells and lipid peroxidation of glomerular mesangial cells (Butler et al., 2000) . XO has been proposed to be a major source of ROS in diabetes mellitus. Out of oxygen free radicals generated by this enzyme, superoxide anion radical (O2·-) is known to be generated as intracellular respiratory byproduct and is also direct etiologic factor of cellular injury. SOD accelerates dismutation of superoxide anion radical to hydrogen peroxide that in turn is removed by CAT and GPx (Del Maestro, 1980) . Moreover, GST is also known to be associated with the detoxication of oxygen free radicals.
Moreover, components of reactive nitrogen species (RNS) , including nitric oxide (NO) and peroxynitrite, have important biological activities. ROS and RNS are continuously produced under physiological conditions (Taghizadeh Afshari et al., 2007) . Generally, the development of oxidative stress is due to the excessive formation and/or insufficient removal of highly reactive molecules such as, reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Karasu, 2010) .
Nitric oxide (NO) , a family of RNS, arises from the guanidino group of L-arginine in an NADPH-dependent reaction catalyzed by a family of NO synthases (NOS) . The three isoforms of NOS are neuronal NOS (nNOS) , inducible NOS (iNOS) and endothelial NOS (eNOS) (Lowenstein and Snyder, 1992) . Over-generated NO in morbid conditions reacts with superoxide anion radical leading to cellular injury or death. It is known that NO is associated with the hemodynamic changes and glomerulus filtration (Xia et al., 1996) . Especially NO arised from eNOS can cause vascular endothelial cell dysfunction (Karasu, 2010) . In this study, we assessed the expression of eNOS to evaluate the cause of vascular endothelial cell dysfunction in kidney, and the results of decreased eNOS expression by Corni Fructus treatment indicate Corni Fructus shows antioxidative action.
High XO activity and NOS mRNA expression in renal tissues of DC group in comparison with the NC group indicate that oxygen free radicals were overproduced from kidneys due to hyperglycemia, in support of previous studies suggesting that oxidative stress is associated with onset of microvascular complications. However, the activity of XO in DCF group was significantly lower than the DC group and similar to the NC group, which estimates that Corni Fructus acts upon decreasing in production of oxygen free radicals. The activity of SOD in DC group was significantly lower than the NC group, which could be due to the increased utilization of the enzyme for scavenging of oxygen free radicals overproduced in DC group. The activities of CAT and GST in DC group showed significantly higher or much higher than the NC group, which could be due to the de novo synthesis of the enzymes for detoxification of oxygen free radicals in DC group. Data presented in our investigation indicate that the progression of diabetes results in augmentation of oxidative stress accompanied by impaired enzymatic antioxidative defense system in the kidneys of diabetic rats. On the contrary, the activities of SOD, CAT and GST in DCF group appeared to be similar to those of the NC group.
These results indicate that treatment with Corni Fructus reduced oxidation stress as evidenced by the restoration of the enzymatic antioxidative defense system in renal tissues of db/db mice. And renoprotective effects of Corni Fructus on diabetic nephropathy in db/db mice in our previous study (Kim and Kim, 2010) could be attributed to its antioxidative action on renal tissues.
References
- 1.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 2.Baynes J.W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diabetes.40.4.405. [DOI] [PubMed] [Google Scholar]
- 3.Blois M.S. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 4.Butler R. Morris A.D. Belch J.J.F. Hill A. Struthers A.D. Allopurinol normalizes endothelial dysfunction in type2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 5.Del Maestro R.F. An approach to free radicals in medicine and biology. Acta Physiol. Scand. Suppl. 1980;492:153–168. [PubMed] [Google Scholar]
- 6.Habig W.H. Pabst M.J. Jakoby W.B. Glutathione Stransferase. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 7.Hunt J.V. Dean R.T. Wolff S.P. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes and aging. Biochem. J. 1988;256:205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin U.H. Kim D.I. Lee T.K. Lee D.N. Kim J.K. Lee I.S. Kim C.H. Herbal formulation Yukmi-jihang-tang-Jahage regulates bone resorption by inhibition of phosphorylation mediated by tyrosine kinase Src and cyclooxygenase expression. J. Ethnopharmacol. 2006;106:333–343. doi: 10.1016/j.jep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Karasu C. Glycoxidative stress and cardiovascular complications in experimentally-induced diabetes: effects of antioxidant treatment. Open Cardiovasc. Med. J. 2010;4:240–256. doi: 10.2174/1874192401004010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H.J. Kim Y.C. Antidiabetic and renoprotective effects of Corni Fructus extract in db/db mice. Mol. Cell Toxicol. 2010;6:135–142. doi: 10.1007/s13273-010-0020-7. [DOI] [Google Scholar]
- 11.Kim H.J. Kim K.S. Lee T.J. Kim Y.C. Antidiabetic effects of Corni Fructus extract on blood glucose and insulin resistance in db/db mice. Toxicol. Res. 2009;25:93–99. doi: 10.5487/TR.2009.25.2.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkins R.G. Dunlop M.E. The link between hyperglycemia and diabetic nephropathy. Diabetologia. 1992;35:499–504. doi: 10.1007/BF00400475. [DOI] [PubMed] [Google Scholar]
- 13.Latha M. Pari L. Preventive effects of Cassia auriculata L. flowers on brain lipid peroxidation in rats treated with streptozotocin. Mol. Cell. Biochem. 2003;243:23–28. doi: 10.1023/A:1021697311150. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence J.C. Jill S.G. Eric P.D. Joyce A.D. Donald D.L. Mark A.Y. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001;50:1927–1937. doi: 10.2337/diabetes.50.8.1927. [DOI] [PubMed] [Google Scholar]
- 15.Li K.M. Yang X.J. Yu M.Q. Xie C. Xu L.Z. Determination of loganin in Cornus officinalis Sieb. et Zucc. by TLC scanner. Zhongguo Zhong Yao Za Zhi. 1994;19:738–763. [PubMed] [Google Scholar]
- 16.Lowenstein C.J. Snyder S.J. Nitric oxide, a novel biologic messenger. Cell. 1992;70:705–707. doi: 10.1016/0092-8674(92)90301-R. [DOI] [PubMed] [Google Scholar]
- 17.Lowry O.H. Rosenbrough N.J. Far A.L. Randall R.J. Protein measurement with the folin phenol reagent. J.Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Maritim A.C. Sanders R.A. Watkins J.B. Effect of alpha lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2003;14:288–294. doi: 10.1016/S0955-2863(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 19.Martin J.P. Dailey M. Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch. Biochem. Biophys. 1987;255:329–336. doi: 10.1016/0003-9861(87)90400-0. [DOI] [PubMed] [Google Scholar]
- 20.Morel D.W. Chisolm G.M. Antioxidative treatment of diabetic rats inhibits lipoprotein oxidation and cytotoxicity. J.Lipid Res. 1989;30:1827–1834. [PubMed] [Google Scholar]
- 21.Rong Y. Li L. Shah V. Lau B.H.S. Pycnogenol protects vascular endothelial cells from t-butyl hydroperoxideinduced oxidant injury. Biotechnol. Ther. 1995;5:117–126. [PubMed] [Google Scholar]
- 22.Stirpe F. Della C.E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O) . J. Biol. Chem. 1969;244:3855–3863. [PubMed] [Google Scholar]
- 23.Taghizadeh Afshari A. Shirpoor A. Farshid A. Saadatian R. Rasmi Y. Saboory E. Ilkhanizadeh B. Allameh A. The effect of ginger on diabetic nephropathy plasma antioxidant capacity and lipid peroxidation in rats. Food Chem. 2007;101:148–153. doi: 10.1016/j.foodchem.2006.01.013. [DOI] [Google Scholar]
- 24.Venkateswaran S. Pari L. Antioxidant effect of Phaseolus vulgaris in streptozotocin-induced diabetic rats. AsiaPac. J. Clin. Nutr. 2002;11:206–209. doi: 10.1046/j.1440-6047.2002.00292.x. [DOI] [PubMed] [Google Scholar]
- 25.Xia Y. Dawson V.L. Dawson T.M. Snyder S.H. Zweier J.L. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitritemediated cellular injury. Proc. Natl. Acad. Sci. USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamabe N. Kang K.S. Goto E. Tanaka T. Yokozawa T. Beneficial effect of Corni Fructus a constituent of Hachimi-jio-gan, on advanced glycation end-product-mediated renal injury in streptozotocin-treated diabetic rats. Biol. Pharm.Bull. 2007;30:520–526. doi: 10.1248/bpb.30.520. [DOI] [PubMed] [Google Scholar]