Abstract
The aim of this study is to investigate how many leiomyoma patients are exposed to bisphenol-A (BPA) , an endocrine disruptor, and whether the serum concentration of BPA is related to leiomyoma growth. Initially, 128 patients were divided into one control and three leiomyoma groups (mild, moderate and severe) according to the size of the leiomyomas. Serum BPA concentrations were measured by liquid chromatography and mass spectrometry (LC/MS) . Nearly two-thirds of leiomyoma patients were exposed to BPA and the range of BPA was from non-detection to 2.603 ng/ml. The mean BPA concentrations in the groups were 1.015 ± 0.775 ng/ml (control) , 0.774 ± 0.834 ng/ml (mild) , 1.261 ± 0.797 ng/ml (moderate) and 1.244 ± 0.860 ng/ml (severe) (p = 0.158) . After recombination into two group, Group 1 (control plus mild) vs. Group 2 (moderate plus severe) , higher level was found in Group 2 even with no statistical significance (p = 0.06) . In conclusion, about two-thirds of leiomyoma patients were exposed to BPA, but it may not have growth promoting effect on leiomyoma.
Keywords: Bisphenol Bisphenol-A (BPA) , Endocrine disruptor, Leiomyoma, Liquid chromatography and mass spectrometry (LC/MS)
INTRODUCTION
Uterine leiomyomas (fibroids or myomas) are female benign neoplasms originating from smooth muscle cells of the uterus. They are found in 25% of reproductive age women and nearly 80% of surgically excised uteri (Buttram and Reiter, 1981). Although myoma growth is dependent on estrogen secreted from the ovary, the exact pathogenetic mechanisms are not yet elucidated.
An endocrine disruptor is defined by United States Environmental Protection Agency (EPA) as “an exogenous chemical substance or mixture that alters the structure or function (s) of the endocrine system and causes adverse effects”. Bisphenol-A (BPA) is a well-known endocrine disruptor that has been used primarily in the production of polycarbonate plastic products and in epoxy resins used to line the interior of metal cans. Recently, it has been reported that human serum levels are generally in the range of 0.2~20 ng/ml, with children having higher levels (Calafat et al., 2008; Vandenberg et al., 2007). Concern about population health risks related to BPA exposure have been raised because BPA could function as a synthetic estrogen and have a potential influence on reproduction. Actually, many animal studies have shown the deleterious effects of fetal exposure. However, no definite conclusions have been drawn to date linking BPA exposure to adverse reproductive health (Maffini et al., 2006). Moreover, there are no data to support serum BPA levels being related to the development and growth of leiomyomas. There have been only a few reports that a xenoestrogen might influence the occurrence of female reproductive diseases such as uterine adenocarcinoma or leiomyomas (Hodges et al., 2001; Newbold et al., 2006).
The techniques used to measure BPA in serum have included liquid chromatography-mass spectrometry (LC/MS) , gas chromatography-mass spectrometry (GC/MS) , high performance liquid chromatography and ELISA. Among them, it is known that mass spectrometry is considered the most accurate and precise method for measuring trace levels of BPA in biological samples (Vandenberg et al., 2007). LC/MS was used to measure BPA in this study.
Leiomyoma growth may be related to the duration of estrogen exposure. While early menarche increases the developing leiomyoma, estrogen deprivation, such as occurs postmenopausally or with depot GnRH agonist treatment, decreases leiomyoma size (Schwartz, 2001). This phenomenon raises the hypothesis that BPA with estrogen-like activity might involved in the growth of leiomyoma. Although experimental animal studies have shown that BPA causes the occurrence of leiomyomas if the exposure occurs during fetal life (Newbold et al., 2007), there are no human studies to document BPA’s adverse health effect so far. Therefore, in the present study, we tried to evaluate whether the serum BPA levels are correlated with variable leiomyoma size.
MATERIALS AND METHODS
Study subjects.
The present study was performed at the Department of Obstetrics and Gynecology, Dong-A University hospital. After obtaining informed consent, a total of 128 women were divided into control and myoma group, and had serum BPA levels obtained by venipuncture. The plasma samples were separated and frozen at -70℃ until BPA was measured through liquid chromatography-mass spectrometry (LC/MS) . The control group (n = 28) was defined as the absence of uterine leiomyomas on transvaginal ultrasonography examination. The subjects were divided into three subgroups: mild (n = 38) , moderate (n = 32) and severe (n = 30) , according to the number and size of leiomyomas measured by transvaginal ultrasonography. Namely, the mild group was defined as the presence of one or two leiomyomas smaller than 3 cm in diameter. The moderate group was defined as the presence of one or two leiomyomas measuring 3 to 5 cm in diameter. The severe group was defined as the presence of any leiomyoma greater than 5 cm in diameter. This protocol for the research project has been approved by a suitably constituted Ethics Committee of the institution within which the work was undertaken and that it conforms to the provisions of the Declaration of Helsinki (as revised in Tokyo 2004) .
Preparation of serum samples.
To measure the total concentration of BPA in serum, the serum samples were pretreated with β-glucuronidase for extracting the free form of BPA. Briefly, 100 μl of serum sample was mixed with 200 μl of acetic acid buffer solution (pH 5.0; sodium acetate 1.2 g, acetic acid 1 ml, L-ascorbic acid 0.15 g, EDTA-2Na 0.01 g, distilled water 100 ml) and 10 μl of β-glucuronidase (22,000 U/ml; Sigma-Aldrich, St. Louis, USA) and incubated for 18 hours at 37℃. The sample mixture was then passed through the SPE column (Isolute M-M car-tridge; Sorbent mass: 500 mg, Reservior volume: 3 ml; Biotage, Uppsala, Sweden) , and the column was washed with 6 ml of 35% methanol and eluted with 2.5 ml of 100% methanol. The eluted samples were dried under a stream of nitrogen at 40℃ and dissolved with 100 μl of 10% methanol. Samples were then subjected to LC-MS.
Liquid chromatography-mass spectrometry.
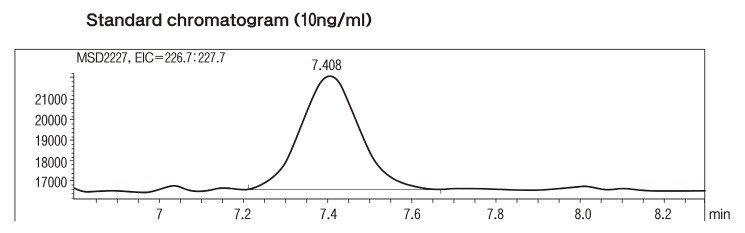
LC-MS was performed using a Zorbax Eclipse XDB-C18 (5 ㎛, 4.6 × 150 mm) reversed-phase column in an Agilent 1100 LC/MSD system linked to an electrospray (ES) ionization interface (Agilent Technologies, Palo Alto, USA) . The injection volume was 5 μl and the column oven was maintained at 40℃ for LC. This separation was performed using a mobile phase from 0.01% acetic acid in distilled water (Mobile phase A) and 55% acetonitrile (Mobile phase B) . The flow-rate was 0.4 ml/min. The working conditions for electrospray ionization MS were as follows: the drying nitrogen gas temperature was set at 350℃ and the gas introduced into the capillary region at a flow-rate of 10 l/min; the capillary was held at a potential of 3,500 V relative to the counter electrode for the negative-ion mode. The fragmentor voltage was 120 V during the chromatographic run. Standard solutions of BPA were prepared in methanol and added to a fixed concentration of BPA; this resulted in a calibration curve covering the concentration range 2~500 pg. BPA concentrations were calculated relative to BPA standards that were added to the samples prior to extraction, giving a final extract concentration of 100 ng/ml. Fig. 1 is 10 ng/ml standard chromatogram graph.
Fig. 1. Standard chromatogram (10 ng/ml) .
Statistical analysis.
All the statistical analyses were performed by using statistical software package SPSS 11.0 (SPSS Inc., Chicago, IL, USA) . Parametric statistics were presented as mean values ± SD, unless stated otherwise. Statistical analyses among the groups were performed by analysis of variance (ANOVA) and independent samples t-test. Significance was determined as p < 0.05.
RESULTS
Detection rate and concentrations of BPA.
Of 28 control patients, 18 showed BPA detection, as did 60 out of the 100 leiomyoma patients. The highest detection rate was in the severe group (70%) , and the lowest detection was in the mild group (47.4%) . The control group had a mean age ± SD of 43.9 ± 10.1 and the mild, moderate and severe group were 42.6 ± 9.0, 43.4 ± 9.1, 44.7 ± 7.6, respectively.
The mean ± SD BPA concentrations (ng/ml) in controls was 1.015 ± 0.775 and the mild, moderate and severe concentrations were 0.774 ± 0.834, 1.261 ± 0.797, and 1.244 ± 0.860 respectively. There was no statistical significance between groups (P = 0.158) (Table 1) .
Table 1.
Statistical comparisons of four groups
| Control (n = 28) | Myoma groups | P-value | |||
|---|---|---|---|---|---|
|
| |||||
| Mild (n = 38) | Moderate (n = 32) | Severe (n = 30) | |||
|
| |||||
| Age (years) | 43.9 ± 10.1 | 42.6 ± 9.0 | 43.4 ± 9.1 | 44.7 ± 7.6 | 0.825 |
| Range of age | 24 - 55 | 26 - 59 | 26 - 70 | 29 - 61 | |
| BMI (kg/m2) | 21.2 ± 2.9 | 22.4 ± 2.8 | 21.8 ± 2.5 | 21.5 ± 2.7 | 0.853 |
| No. of detection (%) | 18 (64.3) | 18 (47.4) | 21 (65.6) | 21 (70.0) | |
| Mean BPA concentrations (ng/ml) | 1.015 ± 0.775 | 0.774 ± 0.834 | 1.261 ± 0.797 | 1.244 ± 0.860 | 0.158 |
| Range of BPA (ng/ml) | ND - 1.771 | ND - 2.073 | ND - 2.155 | ND - 2.549 | |
Values are mean ± SD.
ND = non detection.
Comparison of control plus mild group versus moderate plus severe group.
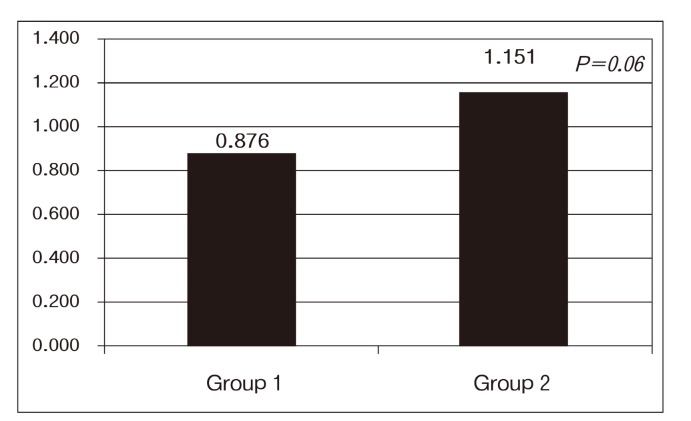
After recombination of the four groups into two groups, control plus mild (Group 1) and moderate plus severe (Group 2) , the data were analyzed. The mean BPA concentration of Group 1 was 0.876 ± 0.813 ng/ml, and that of Group 2 was 1.151 ± 0.826 ng/ml (P =0.06) (Fig. 2) .
Fig. 2. Statistical comparison of control plus mild group (Group 1) with moderate plus severe group (Group 2) .
Discussion
Uterine leiomyomas are one of the most common gynecologic neoplasms arising from the myometrial compartment of the uterus in reproductive age women. It is wellknown that the growth of leiomyomas is dependent on estrogen stimulation secreted from the ovary, as verified by the regression of these tumors during menopause or GnRH analogue treatment. In spite of many studies of leiomyoma growth, the exact molecular mechanisms are not yet established.Moreover, it is uncertain whether a xenoestrogen, which possesses the possibility of disrupting normal estrogen function, has a direct effect on the growth of leiomyomas. In this study, we tried to measure the extent of BPA, a xenoestrogen, exposure to the patients with or without leiomyomas and determine if the serum BPA concentrations are related to leiomyoma growth.
BPA is one of the largest-volume chemicals produced worldwide, with more than 6 billion pounds produced per year, and it is the building block of polycarbonate plastic. Many studies have found that BPA leaches from polycarbonate baby bottles and reusable water bottles (Vandenberg et al., 2007, 2009). Other reusable food containers, along with some paper and card-board food containers, have been examined for their BPA content, as well (Vandenberg et al., 2007).
Although BPA could act as a synthetic estrogen, it has not been considered a great risk to either wild life or humans due to its low affinity for the estrogen receptors (ER-α and ER-β) . It has been reported that BPA has 10,000-fold weaker affinity than that of estradiol or diethylstilbesterol (Gould et al., 1998; Kuiper et al., 1998). However, numerous studies have shown that BPA can affect reproductive physiology and rodent behavior at doses lower than the‘safe’ human exposure limit, 50 μg/kg/day (Vandenberg et al., 2009).
While there has been an increase in the reported evidences for the effects of BPA using animal models, studies documenting a direct relationship between BPA exposure and adverse human health effects are very rare. There have only been studies that measure the BPA concentrations in maternal and fetal plasma, amniotic fluid or urine samples among populations so far. For instance, serum BPA was measured by ELISA method in adult men and women with mean concentrations of 1.49 ± 0.11 and 0.64 ± 0.10 ng/ml (Takeuchi and Tsutsumi, 2002). Other studies have reported, the presence of BPA in fetal and maternal plasma (Inoue et al., 2000; Tan and Ali Mohd, 2003) and five times higher levels in amniotic fluid than maternal plasma (Maffini et al., 2006). In an animal study, leiomyomas were seen in all BPA treated groups (Newbold et al., 2007), which suggest that the endocrine disrupting chemicals with estrogen-like activity are contributing to the development of various human diseases (Olden and Newbold, 2000).
The present study is the first trial to measure the serum BPA concentrations from patients with and without uterine leiomyomas and identify the relationship between the serum BPA levels and leiomyoma numbers and sizes. We found that BPA was detected in approximately two-thirds of patients overall, but found no statistical differences between case and control groups. Although there was no statistical difference, we could find higher BPA levels in patients with larger leiomyomas (Group 2) than smaller group (Group 1) . This result may imply that larger-scale studies would be needed to clarify the growth promoting effects of BPA on leiomyomas. In this study, the leiomyoma sizes were measured by transvaginal ultrasound subjectively. So, there would be a lack of precision to measure the presence of leiomyomas or their sizes. Therefore, we decided that the comparison between the two groups would be more meaningful than the four group comparisons.
In conclusion, we found that nearly two-thirds of leiomyoma patients were exposed to BPA and there was no statistical significance of BPA levels between control and leiomyoma groups. Although we could not find the BPA’s growth promoting effect on leiomyoma, more studies intended for larger populations would be needed.
Acknowledgments
This research was supported by a grant (08162KFDA443) from Korea Food & Drug Administration in 2008 and this study was supported by the research fund of Dong-A University Medical Center in Korea.
References
- 1.Buttram V.J. Reiter R. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil. Steril. 1981;36:433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 2.Calafat A.M. Ye X. Wong L.Y. Reidy J.A. Needham L.L. Exposure of U.S. population to bisphenol-A and 4-tertiary-octyl phenol: 2003-2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould J.C. Leonardo L.S. Maness S.C. Wagner B.L. Conner K. Zacharewski T. Safe S. McDonnell D.P. Gaido K.W. Bisphenol A interacts with estrogen receptor alpha in a distinct manner from estradiol. Mol. Cell. Endocrinol. 1998;142:203–214. doi: 10.1016/S0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 4.Hodges L.C. Hunter D.S. Bergerson J.S. Fuchs-Young R. Walker C.L. An in vivo/in vitro model to assess endocrine disrupting activity of xenoestrogens in uterine leiomyoma. Ann. N. Y. Acad. Sci. 2001;948:100–111. doi: 10.1111/j.1749-6632.2001.tb03991.x. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K. Kato K. Yoshimura Y. Makino T. Nakazawa H. Determination of bisphenol A in human serum by highperformance liquid chromatography with multi-electrode electrochemical detection. J. Chromatogr. B. 2000;749:17–23. doi: 10.1016/S0378-4347(00)00351-0. [DOI] [PubMed] [Google Scholar]
- 6.Kuiper G.G. Lemmen J.G. Carlsson B. Corton J.C. Safe S.H. van der Saag P.T. van der Burg B. Gustaffsson J.A. Interaction of estrogenic chemicals and phytoestrogens wwith estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/en.139.10.4252. [DOI] [PubMed] [Google Scholar]
- 7.Maffini M.V. Rubin B.S. Sonnenschein C. Soto A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell. Endocrinol. 2006;254-255:179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Newbold R.R. Jefferson W.N. Padilla-Banks E. Long-term adverse effect of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 2007;24:253–258. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newbold R.R. Padilla-Banks E. Jefferson W.N. Adverse effects of the model environmental estrogen diethylstilbesterol are transmitted to subsequent generations. Endocrinology. 2006;147:S11–S17. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 10.Olden K. Newbold R.R. Women’s health and the environment in the 21st century. Environ. Health Perspect. 2000;108:767–768. doi: 10.2307/3454303. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz S.M. Epidemiology of uterine leiomyomata. Clin. Obstet. Gynecol. 2001;44:316–326. doi: 10.1097/00003081-200106000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi T. Tsutsumi O. Serum Bisphenol A concentrations showed gender differences, possibly linked to androgen levels. Biochem. Biophys. Res. Commun. 2002;291:76–78. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 13.Tan B.L. Ali Mohd M. Analysis of selected pesticides and alkylphenols in human cord blood by gas chromatograph-mass spectrometer. Talanta. 2003;61:385–391. doi: 10.1016/S0039-9140(03)00281-9. [DOI] [PubMed] [Google Scholar]
- 14.Vandenberg L.N. Hauser R. Marcus M. Olea N. Welshons W.V. Human exposure to bisphenol A (BPA) . Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Vandenberg L.N. Maffini M.V. Sonnenschein C. Rubin B.S. Soto A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]