Abstract
Lipid peroxidation is a free radical oxidation of polyunsaturated fatty acids such as linoleic acid or arachidonic acid. This process has been related with various pathologies and disease status mainly because of the oxidation products formed during the process. The oxidation products include reactive aldehydes such as malondialdehyde and 4-hydroxynonenal. These reactive aldehydes can form adducts with DNAs and proteins, leading to the alterations in their functions to cause various diseases. This review will provide a short summary on the implication of lipid peroxidation on cancer, atherosclerosis, and neurodegeneration as well as chemical and biochemical mechanisms by which these adducts affect the pathological conditions. In addition, select examples will be presented where antioxidants were used to counteract oxidative damage caused by lipid peroxidation. At the end, isoprostanes are discussed as a gold standard for the assessment of oxidative damages.
Keywords: Lipid peroxidation, Malondialdehyde, 4-Hydroxy-2-nonenal, Antioxidant, Isoprostane
INTRODUCTION
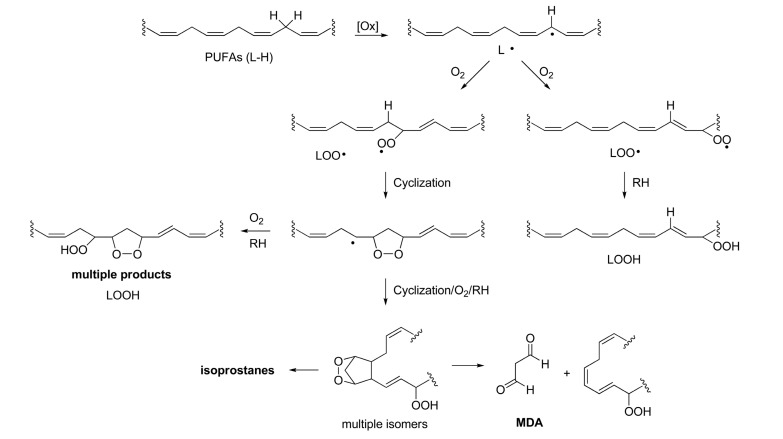
Lipid peroxidation is a free radical oxidation of polyunsaturated fatty acids (PUFAs) such as linoleic acid or arachidonic acid. The basic mechanism of lipid peroxidation is found in the radical chain reaction observed in typical autoxidation process, an oxidation by molecular oxygen (O2) : initiation-propagation-termination (Porter, 1986). Therefore, lipid peroxidation is self-propagating and will proceed until substrate is consumed or termination occurs. In this way, lipid peroxidation is fundamentally different from other forms of free radical injury in that it is a self-sustaining process capable of extensive tissue damage (Porter et al., 1995). In initiation step, abstraction of hydrogen at bisallylic position afforded lipid pentadienyl radical, from which various regioisomeric and stereoisomeric peroxyl radicals are formed leading to a complex mixture of peroxyl products (Scheme 1) (Marnett, 1999). The primary products of lipid peroxidation are lipid hydroperoxides (LOOH). They provide sources of a variety of reactive oxygen species (ROS) that cause oxidative stress along with other ROS such as superoxide (O2·-) , ozone (O3) , and hydroxyl radical (HO·) etc. There are also examples of enzyme-catalyzed oxidation of lipids where lipoxygenases (LOXs) (Brash, 1999) and cyclooxygenases (COXs) (Rouzer and Marnett, 2003; Rouzer and Marnett, 2009) are involved. When arachidonic acid (AA) is a substrate, isomers of HpETEs (hydroperoxy eicosatetraenoic acids) and HETEs (hydroxy eicosatetraenoic acids) are formed as primary lipid peroxidation products from LOX activity. COXs catalyze the formation of a rather specific hydroperoxide (prostaglandin (PG) G2) and hydroxy product (PGH2) from AA. In this review, non-enzymatic lipid peroxidation and the related toxicology will be discussed.
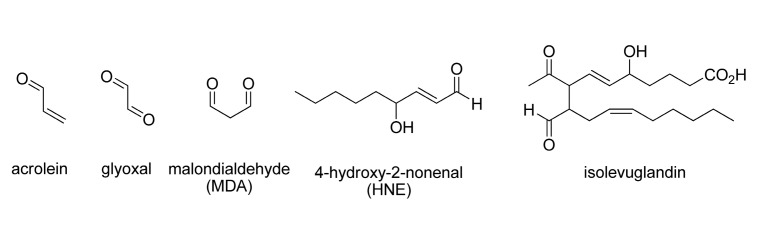
Fig. 1. Reactive aldehydes generated from lipid peroxidation.
Lipid peroxidation has been implicated in various diseases and pathological conditions (Negre-Salvayre et al., 2010) such as carcinogenesis (Marnett, 2000), cardiovascular diseases (Steinberg et al., 1989; Glass and Witztum, 2001; Lee and Blair, 2001), neurodegeneration, (Bradley et al., 2010; Markesbery et al., 2005) and aging (Muller et al., 2007).
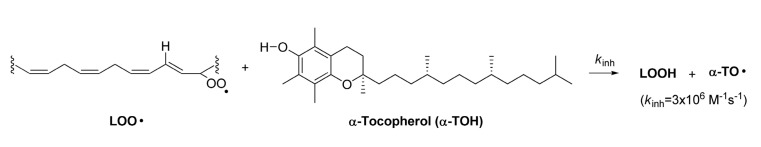
Phenolic antioxidants are typically used to inhibit lipid peroxidation. This class of antioxidants have a weak O-H bond dissociation enthalpy (BDE) such that it can transfer its hydrogen atom to lipid peroxyl radical to perturb a radical chain reaction. α-tocopherol (α-TOH) is the most active phenolic chain-breaking antioxidants found in nature. Its OH BDE (78.3 kcal/mol) is low enough to inhibit lipid peroxyl radical (LOO·) , a chain propagating species, from propagating the chain reaction (Scheme 2) .
Scheme 2. α-Tocopherol inhibits lipid peroxyl radical propagation.
Many toxicological aspects of lipid peroxidation are attributed to various reactive aldehydes generated from oxidized lipids. These aldehydes react with cellular nucleophiles such as proteins, nucleic acids and glutathione (GSH) to form a variety of adducts. The formation of those adducts are related to various pathologies. The reactive aldehydes make up a large part of endogenous electrophile repertoires that include malondialdehyde (MDA) , 4-hydroxy-2-alkanals (i.e., 4-hydoxy-2-nonenal (4-HNE) ) and γ-ketoaldehydes (i.e., levuglandins) . With tremendous advances in mass spectroscopy techniques coupled with reverse phase HPLC, detections and measurements of DNA adduct with lipid peroxidation products have been significantly progressed (Blair, 2008).
Scheme 1. Pathways of lipid peroxidation.
DNA adduct formation and carcinogenesis.
Oxidative stress is well known to play an important role in carcinogenesis (Klaunig and Kamendulis, 2004; Jacobs and Marnett, 2010). Endogenous DNA adducts with reactive aldehydes generated from lipid peroxidation provide an important etiology of human genetic diseases and cancer. The DNA adducts lead to the alterations in DNA structure such as DNA base modifications and frame shifts. Pyrimido[1,2α]purin-10 (3H) -one (M1G) , a major DNA (dG) -MDA adduct, is one of the most important adducts and is found to be a strong mutagenic agent (Chaudhary et al., 1996; Niedernhofer et al., 2003). M1G induced frame shift mutations (-1 and -2) when positioned in a reiterated (CpG) 4 sequence but not when positioned in a nonreiterated sequence in Escherichia coli and in COS-7 cells (VanderVeen et al., 2003). The spectrum of mutations induced by the naturally occurring M1G was determined by site-specific approaches using M13 vectors replicated in Escherichia coli (Fink et al., 1997). In E. coli, M1G-modified genomes containing a cytosine opposite to M1G resulted in roughly equal numbers of M1G→A and M1G→T mutations with few M1G→C mutations. The total mutation frequency was approximately 1%, which represents a 500-fold increase in mutations compared with unmodified M13MB102 vector. Transformation of modified genomes containing a thymine opposite M1G allowed an estimate to be made of the ability of M1G to block replication (Fink et al., 1997). Interestingly, this adduct can be even found in normal healthy individuals. It was found that DNA from healthy individuals contain about 5,400 MDA-dG adducts per cell in liver (Chaudhary et al., 1994). This study demonstrated the normal endogenous level of DNA adduct formation caused by lipid peroxidation in disease-free subjects, and the crucial role of chemical carcinogenesis of endogenous source, antioxidants and DNA repair system. DNA adduct with 4-HNE (Minko et al., 2009) was also found to generate p53 gene mutation (Chung et al., 2003) which can play a role in carcinogenesis.
Not only these DNA damages, i.e., mismatch or frame shift in DNA replication, caused by adduct formation, but also intracellular signal transduction can be caused by those reactive aldehydes. In particular, it is reported that 4-HNE inhibits the activation of NF-κB in human colorectal cancer cell line (RKO) and human lung cancer cell line (H1299) .At doses that inhibit IkBα degradation, HNE inhibits IκB kinase (IKK) activity by direct reaction with IKK (Ji et al.,2001). It was also found that activation of the heat shock response by HNE is dependent on the expression and nuclear translocation of heat shock factor 1 (HSF1) , which promotes the expression of heat shock protein 40 (Hsp40) and Hsp70-1. HNE disrupts the inhibitory interaction between Hsp70-1 and HSF1, leading to the activation heat shock gene expression (Jacobs and Marnett, 2007). With increasing amount of data accumulated, there are attempts to deconvolute the biological activity and metabolic fates of these reactive aldehydes in systemic view. For example, oxidative damages on proteins by HNE (Codreanu et al.,2009) and global gene expression change by HNE (West and Marnett, 2005) were investigated.
Lipid peroxidation in LDL and chain-breaking antioxidants.
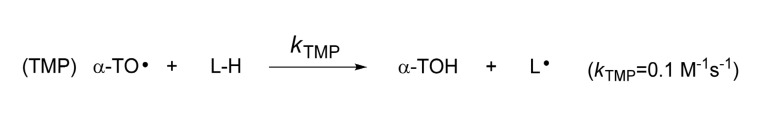
One of the most biologically important lipid peroxidations occurs in low-density lipoprotein (LDL) . LDL is the major carrier of cholesterol and cholesteryl esters of fatty acids. Thus, the oxidation of LDL is accompanied by the extensive oxidation of linoleic acids and arachidonic acids (720 and 180 molecules per LDL, respectively) in LDL particle. Inference from epidemiological and biochemical studies suggested that the oxidation of LDL can lead to the generation of fatty streak, which has been believed an initial event of atherosclerosis. This oxidation theory suggested retentive and oxidative events trigger biochemical changes in artery wall that lead to lipid deposition. Since antioxidants such as vitamin E (α-tocopherol) defend against oxidation of lipids (lipid peroxidation) , vitamin E has been the focus of several large supplementation studies against atherosclerosis, but there is no clear link found between the vitamin and disease progression (Upston et al., 2003). It was later found that α-TOH is not a particularly good antioxidant in LDL. Indeed, under some conditions, α-TOH in LDL acts as a prooxidant to increase the oxidation of cholesteryl linoleate and phospholipids. α-TOH can mediate lipid peroxidation through tocopherol-mediated peroxidation (TMP) mechanism (Bowry et al., 1992) where tocopheroxyl radical (α-TO·) abstracts hydrogen atom from lipid molecule (L-H) to regenerate L•, the chain carrying radical, albeit slowly (Scheme 3) . In fact, additional supplementation of α-TOH from 18 μM to 83 μM resulted in about two-fold increase in the oxidation of both cholesteryl linoleate and phospholipids in the absence of co-antioxidants (Bowry et al., 1992). Thus, can not provide protection against lipid peroxidation in atherosclerosis condition where extensive oxidative stress possibly deplete endogenous co-antioxidants that coordinate with lipophilic α-TOH for the effective protection in LDL particles.
Scheme 3. Mechanism of α-Tocopherol-mediated peroxidation (TMP) .
This finding shed a new light on the strategy for the development of novel chain-breaking antioxidant for the treatment of atherosclerosis. It is required that new antioxidant not only should have excellent antioxidant activity but also should not mediate TMP process. Indeed there are a few examples of remarkably successful antioxidants where pyridinol-based antioxidants inhibit the oxidation of cholesteryl linoleate in isolated human LDL, and do not participate antioxidant-mediated peroxidation (AMP) (Nam et al., 2007; Kim et al., 2005; Serwa et al., 2010). Additional supplementation of lipophilic pyridinol antioxidants from 75 μM to 150 μM afforded about two-fold decrease in the oxidation of cholesteryl linoleate in the absence of co-antioxidants.
Neurodegeneration.
Due to the high concentration of polyunsaturated fatty acids in brain relative to other organs, lipid peroxidation is one of the major outcomes of free radical-mediated injury to brain. Biochemical studies have demonstrated increased concentrations of reactive products from lipid peroxidation in diseased regions of Alzheimer’s disease (AD) brain. For example, an elevated level of 4-HNE was found in ventricular fluid in AD (Lovell et al., 1997) and acrolein (Lovell et al., 2001) was also found at high level in AD brain, with toxic effect toward hippocampus.HNE was also found to bind to histones that provide a protective shield for DNA against oxidative stress, probably because of their abundant lysine residues, and this binding affects the conformation of the histone. This histone-HNE adduct affects the ability of the histone to bind to DNA leading to the vulnerability of DNA toward oxidation in AD brain (Drake et al., 2004). Immunohistochemical studies have localized protein adducts of these aldehydes to lewy bodies in Parkingson Disease (PD) and dementia with lewy bodies (DLB) , and to neurofibrillary tangles and some components of senile plaques in AD, DLB, and mouse models of Aβ cerebral amyloidogenesis (Montine et al., 2004). The enzymatic product of the COXs, PGH2, rearranges in part to highly reactive γ-ketoaldehydes, levuglandin (LG) E (2) and LGD (2) . These γ-ketoaldehydes react with free amines on proteins to form a covalent adduct. It was found that LGE enhances oligomerization of amyloid β (1-42) thus increases neurotoxicity on primary cultures of cerebral neuron of mice (Boutaud et al., 2006). The formation of LG adducts of protein (levuglandinyl-lysine adducts) in brains of AD patients showed that this post-translational modification is increased significantly in the hippocampus in Alzheimer’s disease. The magnitude of the increase was correlated with the pathological evidence of severity (Zagol-Ikapitte et al., 2005).
There is growing evidence that oxidative stress (Adams and Odunze, 1991) and mitochondrial respiratory failure with attendant decrease in energy output are implicated in nigral neuronal death in Parkinson disease (PD) (Fessel and Jackson Roberts, 2005). Using polyclonal antibody to detect HNE-protein adduct in post-mortem brain, average of 58%of nigral neurons were positively stained for HNE-modified proteins in PD. In contrast, only 9% of substantia nigral neurons were positive in the control subjects. These results indicate the presence of oxidative stress within nigral neurons in PD, and this oxidative stress may contribute to nigral cell death. HNE-protein adducts are accumulated in age-related manner in the neuron (Yoritaka et al., 1996).
A number of methods exist to quantify free radicals and their oxidation products although many of these techniques suffer from a lack of sensitivity and specificity, especially when used to assess oxidant stress status in vivo. Similarly,many oxidation products are not very good in vivo biomarkers for the assessment of oxidative damage mainly because of their chemical instability in vivo environment. More stable and quantitiatve biomarkers are isoprostanes (IsoPs), (Morrow et al., 1990) isofuranes (IsoFs) (Fessel et al., 2002) and nueroprostanes (NeuroPs) (Roberts et al., 1998).
Isoprostanes as a gold standard to assess oxidative stress.
Among the three biomarkers mentioned above, IsoPs are known to give the most reliable and robust results (Milne et al., 2005, 2008). IsoPs are prostaglandin-like compounds formed in vivo by non-enzymatic free radical oxidation of AA. In a recent multi-investigator study, termed the Biomarkers of Oxidative Stress (BOSS) Study, sponsored by the National Institutes of Health (USA) , it was found that the most accurate method to assess in vivo oxidant stress status is the quantification of plasma or urinary IsoPs, and thus, currently, quantification of these compounds provides the “gold standard” to assess oxidative injury in vivo (Kadiiska et al., 2005). Defining normal levels of F2-IsoPs in healthy humans (Milne et al., 2007) is particularly important in that it allows for an assessment of the effects of diseases on endogenous oxidant tone and allows for the determination of the extent to which various therapeutic interventions affect levels of oxidant stress. Elevations of IsoPs in human body fluids and tissues have been found in a various human disorders, including atherosclerosis (Morrow, 2005; Pratico et al., 1998; Davies and Roberts, 2011), diabetes (Davi et al., 2003), obesity (Keaney et al., 2003), cigarette smoking (Morrow et al., 1995), neurodegenerative diseases (Montine et al., 2004), and many others. Usefulness of IsoPs as biomarker can be originated from their biological activity in various oxidative stress settings. IsoPs strongly reduce the inflammatory response in macrophage by inhibiting lipopolysaccharide-stimulated IκBα degradation and subsequent NF-κB nuclear translocation and transcriptional activity (Milne et al., 2005). Further, treatments for some of these conditions, including antioxidant supplementation, antidiabetic treatments, cessation of smoking, and even weight loss, have been shown to decrease production of F2-IsoPs (Davi et al., 1999; Roberts et al., 2007). As an example for the assessment of oxidative damage, it was shown that doses of α-TOH of 1600 IU/day or greater are required to statistically affect plasma F2-IsoP levels. Interestingly, vitamin C supplementation does not alter IsoP levels in humans (Roberts et al., 2007; Levine et al., 2001).
CONCLUSIONS
Mechanism of lipid peroxidation process and formation of the oxidation products from oxidized lipids are discussed as well as their toxicological roles in various pathologies. Among the oxidation products, malondialdehyde and 4-hydroxynanenal have been a central theme not only because they extensively form adducts with DNA and proteins to alter their normal functions but also because they are found to be correlated with disease status in vivo. Their roles in controlling signal transduction pathways and gene expression profiles are being studied in global networking view, highlighting the importance of oxidative damages. As shown in atherosclerosis case study, supplementation of antioxidants might not be enough to ameliorate pathologies caused by oxidative damages. In spite of increasing body of biochemical and toxicological mechanisms and evidences in clinical pharmacology, there are not many successful therapeutic strategies developed to reduce oxidative damages other than antioxidant supplementation. Further investigations on the chemical, biochemical and biological mechanisms underneath the oxidative damages warrant the novel strategy to treat or prevent the oxidative damages.
Acknowledgments
This work is partly supported by Gyeonggi-do, Korea.
References
- 1.Adams J.D. Jr. Odunze I.N. Oxygen free radicals andParkinson’s disease. Free Radic. Biol. Med. 1991;10:161–169. doi: 10.1016/0891-5849(91)90009-R. [DOI] [PubMed] [Google Scholar]
- 2.Blair I.A. DNA adducts with lipid peroxidation products. J. Biol. Chem. 2008;283:15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutaud O. Montine T.J. Chang L. Klein W.L. Oates J.A. PGH2-derived levuglandin adducts increase the neurotoxicityof amyloid beta1-42. J. Neurochem. 2006;96:917–923. doi: 10.1111/j.1471-4159.2005.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowry V.W. Ingold K.U. Stocker R. Vitamin E inhuman low-density lipoprotein. When and how this antioxidantbecomes a pro-oxidant. Biochem. J. 1992;288:341–344. doi: 10.1042/bj2880341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley M.A. Markesbery W.R. Lovell M.A. Increased levels of 4-hydroxynonenal and acrolein in the brainin preclinical Alzheimer disease. Free Radic. Biol. Med. 2010;48:1570–1576. doi: 10.1016/j.freeradbiomed.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brash A.R. Lipoxygenases: occurrence functions catalysisand acquisition of substrate. J. Biol. Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary A.K. Nokubo M. Reddy G.R. Yeola S.N. Morrow J.D. Blair I.A. Marnett L.J. Detection of endogenousmalondialdehyde-deoxyguanosine adducts in human liver. Science. 1994;265:1580–1582. doi: 10.1126/science.8079172. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary A.K. Reddy G.R. Blair I.A. Marnett L.J. Characterization of an N6-oxopropenyl-2'-deoxyadenosineadduct in malondialdehyde-modified DNA using liquidchromatography/electrospray ionization tandem mass spectrometry. Carcinogenesis. 1996;17:1167–1170. doi: 10.1093/carcin/17.5.1167. [DOI] [PubMed] [Google Scholar]
- 9.Chung F.L. Pan J. Choudhury S. Roy R. Hu W. Tang M.S. Formation of trans-4-hydroxy-2-nonenal- andother enal-derived cyclic DNA adducts from omega-3 andomega-6 polyunsaturated fatty acids and their roles in DNArepair and human p53 gene mutation. Mutat. Res. 2003;531:25–36. doi: 10.1016/j.mrfmmm.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Codreanu S.G. Zhang B. Sobecki S.M. Billheimer D.D. Liebler D.C. Global analysis of protein damage by thelipid electrophile 4-hydroxy-2-nonenal. Mol. Cell. Proteomics. 2009;8:670–680. doi: 10.1074/mcp.M800070-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davi G. Ciabattoni G. Consoli A. Mezzetti A. Falco A. Santarone S. Pennese E. Vitacolonna E. Bucciarelli T. Costantini F. Capani F. Patrono C. In vivo formation of8-iso-prostaglandin f2alpha and platelet activation in diabetesmellitus: effects of improved metabolic control and vitamin Esupplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 12.Davi G. Chiarelli F. Santilli F. Pomilio M. Vigneri S. Falco A. Basili S. Ciabattoni G. Patrono C. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: role of interleukin-6 and disease duration. Circulation. 2003;107:3199–3203. doi: 10.1161/01.CIR.0000074205.17807.D0. [DOI] [PubMed] [Google Scholar]
- 13.Davies S.S. Roberts L.J. 2nd. F (2) -isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic. Biol. Med. 2011;50:559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drake J. Petroze R. Castegna A. Ding Q. Keller J.N. Markesbery W.R. Lovell M.A. Butterfield D.A. 4-Hydroxynonenal oxidatively modifies histones: implications for Alzheimer’s disease. Neurosci. Lett. 2004;356:155–158. doi: 10.1016/j.neulet.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Fessel J.P. Porter N.A. Moore K.P. Sheller J.R. Roberts L.J. 2nd. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (iso-furans) that are favored by increased oxygen tension. Proc.Natl. Acad. Sci. U S A. 2002;99:16713–16718. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fessel J.P. Jackson Roberts L. Isofurans: novel products of lipid peroxidation that define the occurrence of oxidant injury in settings of elevated oxygen tension. Antioxid RedoxSignal. 7:202–209. doi: 10.1089/ars.2005.7.202. [DOI] [PubMed] [Google Scholar]
- 17.Fink S.P. Reddy G.R. Marnett L.J. Mutagenicity in Escherichia coli of the major DNA adduct derived from the endogenous mutagen malondialdehyde. Proc. Natl. Acad. Sci.U S A. 1997;94:8652–8657. doi: 10.1073/pnas.94.16.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glass C.K. Witztum J.L. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/S0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs A.T. Marnett L.J. Heat shock factor 1 attenuates 4-Hydroxynonenal-mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J.Biol. Chem. 2007;282:33412–33420. doi: 10.1074/jbc.M706799200. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs A.T. Marnett L.J. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc. Chem. Res. 2010;43:673–683. doi: 10.1021/ar900286y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji C. Kozak K.R. Marnett L.J. IkappaB kinase a molecular target for inhibition by 4-hydroxy-2-nonenal. J. Biol.Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- 22.Kadiiska M.B. Gladen B.C. Baird D.D. Germolec D. Graham L.B. Parker C.E. Nyska A. Wachsman J.T. Ames B.N. Basu S. Brot N. Fitzgerald G.A. Floyd R.A. George M. Heinecke J.W. Hatch G.E. Hensley K. Lawson J.A. Marnett L.J. Morrow J.D. Murray D.M. Plastaras J. Roberts L J. 2nd. Rokach J. Shigenaga M.K. Sohal R.S. Sun J. Tice R.R. Van Thiel D.H. Wellner D. Walter P.B. Tomer K.B. Mason R.P. Barrett J.C. Biomarkers of oxidativestress study II: are oxidation products of lipids proteins and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Keaney J.F. Jr. Larson M.G. Vasan R.S. Wilson P.W. Lipinska I. Corey D. Massaro J.M. Sutherland P. Vita J.A. Benjamin E.J. Obesity and systemic oxidative stress:clinical correlates of oxidative stress in the framingham study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 24.Kim H.Y. Pratt D.A. Seal J.R. Wijtmans M. Porter N.A. Lipid-soluble 3-pyridinol antioxidants spare alpha-tocopheroland do not efficiently mediate peroxidation of cholesterolesters in human low-density lipoprotein. J. Med. Chem. 2005;48:6787–6789. doi: 10.1021/jm0507173. [DOI] [PubMed] [Google Scholar]
- 25.Klaunig J.E. Kamendulis L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.H. Blair I.A. Oxidative DNA damage and cardiovasculardisease. Trends Cardiovasc Med. 2001;11:148–155. doi: 10.1016/S1050-1738(01)00094-9. [DOI] [PubMed] [Google Scholar]
- 27.Levine M. Wang Y. Padayatty S.J. Morrow J. a new recommended dietary allowance of vitamin C for healthyyoung women. Proc. Natl. Acad. Sci. U S A. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovell M.A. Ehmann W.D. Mattson M.P. Markesbery W.R. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol Aging. 1997;18:457–461. doi: 10.1016/S0197-4580(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 29.Lovell M.A. Xie C. Markesbery W.R. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging. 2001;22:187–194. doi: 10.1016/S0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 30.Markesbery W.R. Kryscio R.J. Lovell M.A. Morrow J.D. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann. Neurol. 2005;58:730–735. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- 31.Marnett L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999;424:83–95. doi: 10.1016/S0027-5107(99)00010-X. [DOI] [PubMed] [Google Scholar]
- 32.Marnett L.J. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 33.Milne G.L. Musiek E.S. Morrow J.D. F2-isoprostanesas markers of oxidative stress in vivo: An overview. Biomarkers. 2005;10:S10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- 34.Milne G.L. Yin H. Brooks J.D. Sanchez S. Jackson Roberts L. 2nd. Morrow J.D. Quantification of F2-isoprostanesin biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 35.Milne G.L. Yin H. Morrow J.D. Human biochemistryof the isoprostane pathway. J. Biol. Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minko I.G. Kozekov I.D. Harris T.M. Rizzo C.J. Lloyd R.S. Stone M.P. Chemistry and biology of DNA containing 1N (2) -deoxyguanosine adducts of the alphabeta-unsaturated aldehydes acrolein crotonaldehyde and 4-hydroxynonenal. Chem. Res. Toxicol. 22:759–778. doi: 10.1021/tx9000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montine K.S. Quinn J.F. Zhang J. Fessel J.P. Roberts L.J.2nd. Morrow J.D. Montine T.J. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem. Phys. Lipids. 2004;128:117–124. doi: 10.1016/j.chemphyslip.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Morrow J.D. Hill K.E. Burk R.F. Nammour T.M. Badr K.F. Roberts L.J. 2nd. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase free radical-catalyzed mechanism. Proc. Natl. Acad.Sci. U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrow J.D. Frei B. Longmire A.W. Gaziano J.M. Lynch S.M. Shyr Y. Strauss W.E. Oates J.A. Roberts L.J. 2nd. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 40.Morrow J.D. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 41.Muller, F.L. Lustgarten M.S. Jang Y. Richardson A. Van Remmen H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007;43:477–503. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 42.Nam T.G. Rector C.L. Kim H.Y. Sonnen A.F. Meyer R. Nau W.M. Atkinson J. Rintoul J. Pratt D.A. Porter N.A. Tetrahydro-18-naphthyridinol analogues of alpha-tocopherol as antioxidants in lipid membranes and low-density lipoproteins. J. Am. Chem. Soc. 2007;129:10211–10219. doi: 10.1021/ja072371m. [DOI] [PubMed] [Google Scholar]
- 43.Negre-Salvayre A. Auge N. Ayala V. Basaga H. Boada J. Brenke R. Chapple S. Cohen G. Feher J. Grune T. Lengyel G. Mann G.E. Pamplona R. Poli G. Portero-Otin M. Riahi Y. Salvayre R. Sasson S. Serrano J. Shamni O. Siems W. Siow R.C. Wiswedel I. Zarkovic K. Zarkovic 2003. Pathological aspects of lipid peroxidation. FreeRadic. Res. 2010;44:1125–1171. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- 44.Niedernhofer L.J. Daniels J.S. Rouzer C.A. Greene R.E. Marnett L.J. Malondialdehyde a product of lipid peroxidation is mutagenic in human cells. J. Biol. Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- 45.Porter N.A. Mechanisms for the autoxidation of polyunsaturated fatty acids. Acc. Chem. Res. 1986;19:262–268. doi: 10.1021/ar00129a001. [DOI] [Google Scholar]
- 46.Porter N.A. Caldwell S.E. Mills K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 47.Pratico D. Tangirala R.K. Roberts D.J. Rokach J. FitzGerald G.A. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat. Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 48.Roberts L.J. 2nd. Montine T.J. Markesbery W.R. Tapper A.R. Hardy P. Chemtob S. Dettbarn W.D. Morrow J.D. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 49.Roberts L.J. 2nd. Oates J.A. Linton M.F. Fazio S. Meador B.P. Gross M.D. Shyr Y. Morrow J.D. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic. Biol. Med. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouzer C.A. Marnett L.J. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 2003;103:2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 51.Rouzer C.A. Marnett L.J. Cyclooxygenases: structural and functional insights. J. Lipid Res. 2009;50:S29–34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serwa R. Nam T.G. Valgimigli L. Culbertson S. Rector C.L. Jeong B.S. Pratt D.A. Porter N.A. Preparation and investigation of vitamin B6-derived aminopyridinol antioxidants. Chem. Eur. J. 2010;16:14106–14114. doi: 10.1002/chem.201001382. [DOI] [PubMed] [Google Scholar]
- 53.Steinberg D. Parthasarathy S. Carew T.E. Khoo J.C. Witztum J.L. Beyond cholesterol. Modifications of lowdensity lipoprotein that increase its atherogenicity. N. Engl. J.Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 54.Upston J.M. Kritharides L. Stocker R. The role of vitamin E in atherosclerosis. Prog. Lipid. Res. 2003;42:405–422. doi: 10.1016/S0163-7827(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 55.VanderVeen L.A. Hashim M.F. Shyr Y. Marnett L.J. Induction of frameshift and base pair substitution mutations by the major DNA adduct of the endogenous carcinogenmalondialdehyde. Proc. Natl. Acad. Sci. U S A. 2003;100:14247–14252. doi: 10.1073/pnas.2332176100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West J.D. Marnett L.J. Alterations in gene expression induced by the lipid peroxidation product 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005;18:1642–1653. doi: 10.1021/tx050211n. [DOI] [PubMed] [Google Scholar]
- 57.Yoritaka A. Hattori N. Uchida K. Tanaka M. Stadtman E.R. Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc.Natl. Acad. Sci. U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zagol-Ikapitte I. Masterson T.S. Amarnath V. Montine T.J. Andreasson K.I. Boutaud O. Oates J.A. Prostaglandin H (2) -derived adducts of proteins correlate with Alzheimer’sdisease severity. J. Neurochem. 2005;94:1140–1145. doi: 10.1111/j.1471-4159.2005.03264.x. [DOI] [PubMed] [Google Scholar]