Abstract
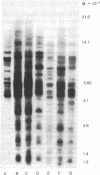
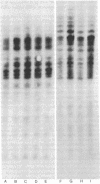
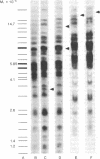
The topography of endogenous type C viral sequences in mouse cellular DNA was investigated by EcoRI nuclease restriction and application of the Southern blotting technique. The DNAs from one outbred and five inbred strains were resolved into 20-35 fragments containing viral sequences, distributed in unique, though related, patterns for each mouse strain. Different normal tissues from the same animal were indistinguishable in their DNA patterns, suggesting that tissue differentiation is not associated with gross alteration in the topography of endogenous type C virus sequences. Tumor tissues from spontaneous lymphomas of AKR/J mice were similarly analyzed. In four out of seven individual tumors we detected the emergence of one or two new virus-containing DNA fragments. The mass of these fragment varied, indicating different insertion sites of the new viral sequences. The detection of these new viral sequences suggests that each tumor was composed of descendents of only one or a few cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns A., Jaenisch R. Increase of AKR-specific sequences in tumor tissues of leukemic AKR mice. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2448–2452. doi: 10.1073/pnas.73.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Lieber M. M., Todaro G. J. Nucleic acid homology of murine type-C viral genes. J Virol. 1974 Dec;14(6):1394–1403. doi: 10.1128/jvi.14.6.1394-1403.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Qualitative and quantitative studies of AKR-type murine leukemia virus sequences in mouse DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1085–1101. doi: 10.1101/sqb.1974.039.01.124. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M. A very large repeating unit of mouse DNA containing the 18S, 28S and 5.8S rRNA genes. Cell. 1977 Aug;11(4):795–805. doi: 10.1016/0092-8674(77)90292-6. [DOI] [PubMed] [Google Scholar]
- GROSS L. Pathogenic properties, and "vertical" transmission of the mouse leukemia agent. Proc Soc Exp Biol Med. 1951 Oct;78(1):342–348. doi: 10.3181/00379727-78-19068. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Milstien J. B., Martin M. A., Aaronson S. A. Characterization of murine leukaemia virus-specific DNA present in normal mouse cells. Nat New Biol. 1973 Jul 18;244(133):76–79. doi: 10.1038/newbio244076a0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard-Schuller R., Hohn B., Brack C., Hirama M., Tonegawa S. DNA clones containing mouse immunoglobulin kappa chain genes isolated by in vitro packaging into phage lambda coats. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4709–4713. doi: 10.1073/pnas.75.10.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Lilly F., Duran-Reynals M. L., Rowe W. P. Correlation of early murine leukemia virus titer and H-2 type with spontaneous leukemia in mice of the BALB/c times AKR cross: a genetic analysis. J Exp Med. 1975 Apr 1;141(4):882–889. [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Alter B. P., Altay C., Mahoney M. J., Lazarus H., Hobbins J. C., Nathan D. G. Application of endonuclease mapping to the analysis and prenatal diagnosis of thalassemias caused by globin-gene deletion. N Engl J Med. 1978 Jul 27;299(4):166–172. doi: 10.1056/NEJM197807272990403. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Greenberger J. S., Aaronson S. A. Oncogenicity of an endogenous C-type virus chemically activated from mouse cells in culture. J Virol. 1974 Jan;13(1):237–240. doi: 10.1128/jvi.13.1.237-240.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]