Abstract
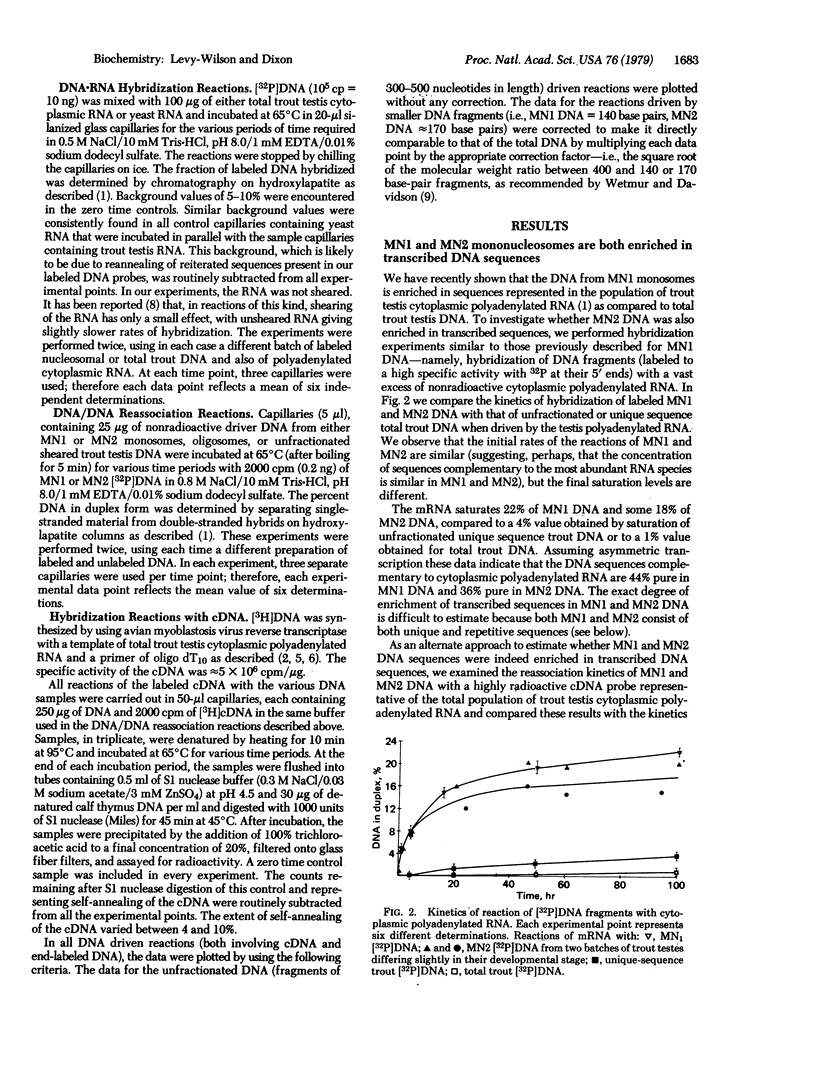
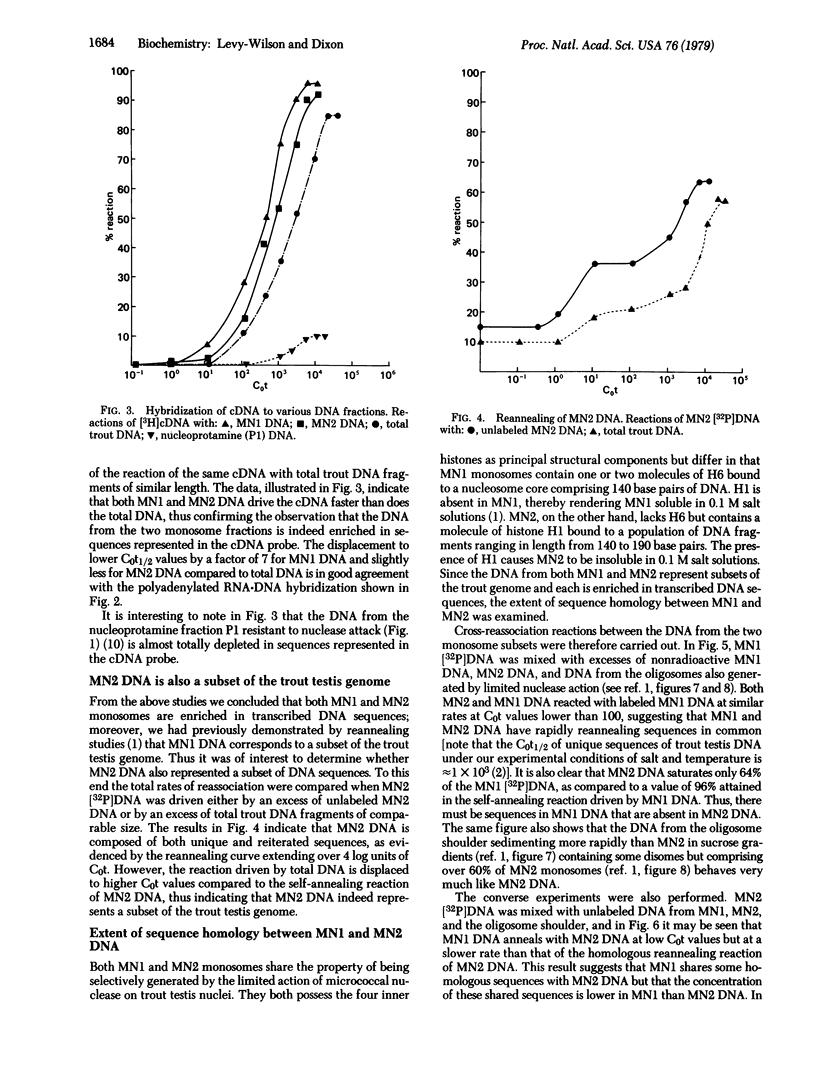
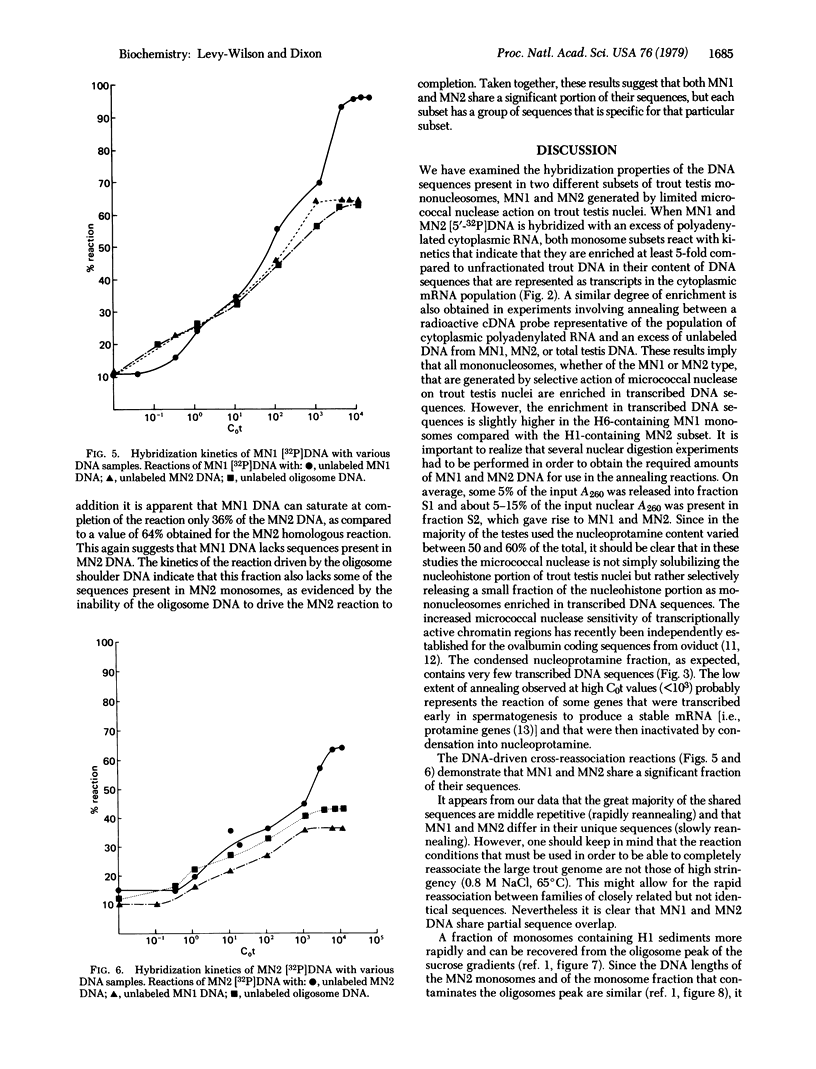
Hybridization experiments show that DNA extracted from two distinct subsets of mononucleosomes (MNI and MN2) generated by a limited action of micrococcal nuclease on trout testis nuclei is enriched approximately 7-fold in sequences that are transcribed into cytoplasmic polyadenylated RNA in trout testis cells. Both subsets of mononucleosomes contain eight core histones, but MNI also possesses one or two molecules of a small, basic, high-mobility-group (HMG) protein H6 [Levy W., B., Connor, W. & Dixon, G. H. (1979) J. Biol. Chem. 254, 609-620], bound to a DNA fragment of 140 base pairs. In contrast, MN2 contains 1 molecule of H1 but no H6, and its DNA length is somewhat longer at 140-190 base pairs. The preferential release of these two subsets of mononucleosomes is correlated with the presence of a second larger HMG protein, HMG-T, in the linker regions flanking both types of mononucleosomes. The HMG-T-containing linker regions appear to be considerably more susceptible to attack by micrococcal nuclease than H1-containing linkers. Cross-reassociation reactions between the DNA from MN1 and MN2 subsets indicate that they share a significant extent of sequence overlap but also that each subset contains specific sequences that are absent in the other subset.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellard M., Gannon F., Chambon P. Nucleosome structure III: the structure and transcriptional activity of the chromatin containing the ovalbumin and globin genes in chick oviduct nuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):779–791. doi: 10.1101/sqb.1978.042.01.078. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A., Mobbs J. D., Marshall A. J. Chromatin structure: a property of the higher structures of chromatin and in the time course of its formation during chromatin replication. Nucleic Acids Res. 1976 Dec;3(12):3293–3304. doi: 10.1093/nar/3.12.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Garrard W. T., Bagi G., Wilson R. F., Bonner J. Partial purification of the template-active fraction of chromatin: a preliminary report. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2193–2197. doi: 10.1073/pnas.71.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda B. M., Baillie D. L., Candido E. P. The subunit structure of chromatin: characteristics of nucleohistone and nucleoprotamine from developing trout testis. FEBS Lett. 1974 Nov 1;48(1):156–159. doi: 10.1016/0014-5793(74)81086-0. [DOI] [PubMed] [Google Scholar]
- Iatrou K., Spira A. W., Dixon G. H. Protamine messenger RNA: evidence for early synthesis and accumulation during spermatogenesis in rainbow trout. Dev Biol. 1978 May;64(1):82–98. doi: 10.1016/0012-1606(78)90062-3. [DOI] [PubMed] [Google Scholar]
- Levy W B., Wong N. C., Dixon G. H. Selective association of the trout-specific H6 protein with chromatin regions susceptible to DNase I and DNase II: possible location of HMG-T in the spacer region between core nucleosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2810–2814. doi: 10.1073/pnas.74.7.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Diversity of sequences of polyadenylated cytoplasmic RNA from rainbow trout (Salmo gairdnerii) testis and liver. Biochemistry. 1977 Mar 8;16(5):958–964. doi: 10.1021/bi00624a023. [DOI] [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Renaturation kinetics of cDNA complementary to cytoplamic polyadenylated RNA from rainbow trout testis. Accessibility of transcribed genes to pancreatic DNase. Nucleic Acids Res. 1977 Apr;4(4):883–898. doi: 10.1093/nar/4.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B., Wong N. C., Watson D. C., Peters E. H., Dixon G. H. Structure and function of the low-salt extractable chromosomal proteins. Preferential association of trout testis proteins H6 and HMG-T with chromatin regions selectively sensitive to nucleases. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):793–801. doi: 10.1101/sqb.1978.042.01.079. [DOI] [PubMed] [Google Scholar]
- Watson D. C., Peters E. H., Dixon G. H. The purification, characterization and partial sequence determination of a trout testis non-histone protein, HMG-T. Eur J Biochem. 1977 Mar 15;74(1):53–60. doi: 10.1111/j.1432-1033.1977.tb11365.x. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]



