Abstract
Dissociation of superoxide dismutase 1 dimers is enhanced by glutathionylation, although the dissociation constants reported to date are imprecise. We have quantified the discreet dissociation constants for wild-type superoxide dismutase 1 and six naturally occurring sequence variants, in their unmodified and glutathionylated forms, at the ratios expressed. Unmodified superoxide dismutase 1 variants that shared similar dissociation constants with SOD1WT had disproportionately increased dissociation constants when glutathionylated. This defines a key role for glutathionylation in superoxide dismutase 1 associated familial amyotrophic lateral sclerosis.
Approximately one in five cases of familial amyotrophic lateral sclerosis (fALS) is due to naturally occurring mutations in the gene encoding superoxide dismutase 1 (SOD1)1. Over 150 such mutations have been reported to occur, and these mutations can occur at virtually any residue of the primary sequence. Many of these mutations confer a significant level of instability upon the native dimer, which may ultimately unfold and form insoluble cytoplasmic aggregates - a hallmark of SOD1 associated fALS pathology2. Recently, evidence has emerged to suggest that SOD1WT aggregation is also involved in some cases of the considerably more prevalent sporadic ALS3,4,5.
The prevailing hypothesis of SOD1 aggregation suggests that dissociation of the native homodimer is a key step, as SOD1 monomers have been identified as a common misfolding intermediate6,7. This mechanism is supported by the extraordinary difference in dissociation constant (KD) reported for the SOD1A4V dimer (3 μM)8, compared with SOD1WT (2.25 nM)9; i.e. the dissociation of SOD1A4V into monomers is approximately 1000 times more facile at concentrations found in vivo. Furthermore, chemical cross-linking of the aggregation prone variant, SOD1A4V can prevent its aggregation in vitro8, while cross-linking of other mutants conferred greater thermostability10.
A compounding factor in SOD1 dissociation is the post-translational modification (PTM) of Cys111 by the ubiquitous antioxidant, glutathione (GSH), which has been demonstrated as being the dominant PTM in human tissue9 (Fig. 1a; Fig. S1a). Similar to many residues in the primary chain of SOD1, Cys11111 is proximal to the dimer interface and, not surprisingly, the introduction of GSH has been proposed to augment dissociation11. We explored this hypothesis for a set of human SOD1 variants with well-defined and broad ranging rates of disease progression; from 1.2 years survival post-onset for SOD1A4V, to approximately 17.6 years for SOD1H46R.
Figure 1. Mass spectrometry analysis of SOD1 variants.
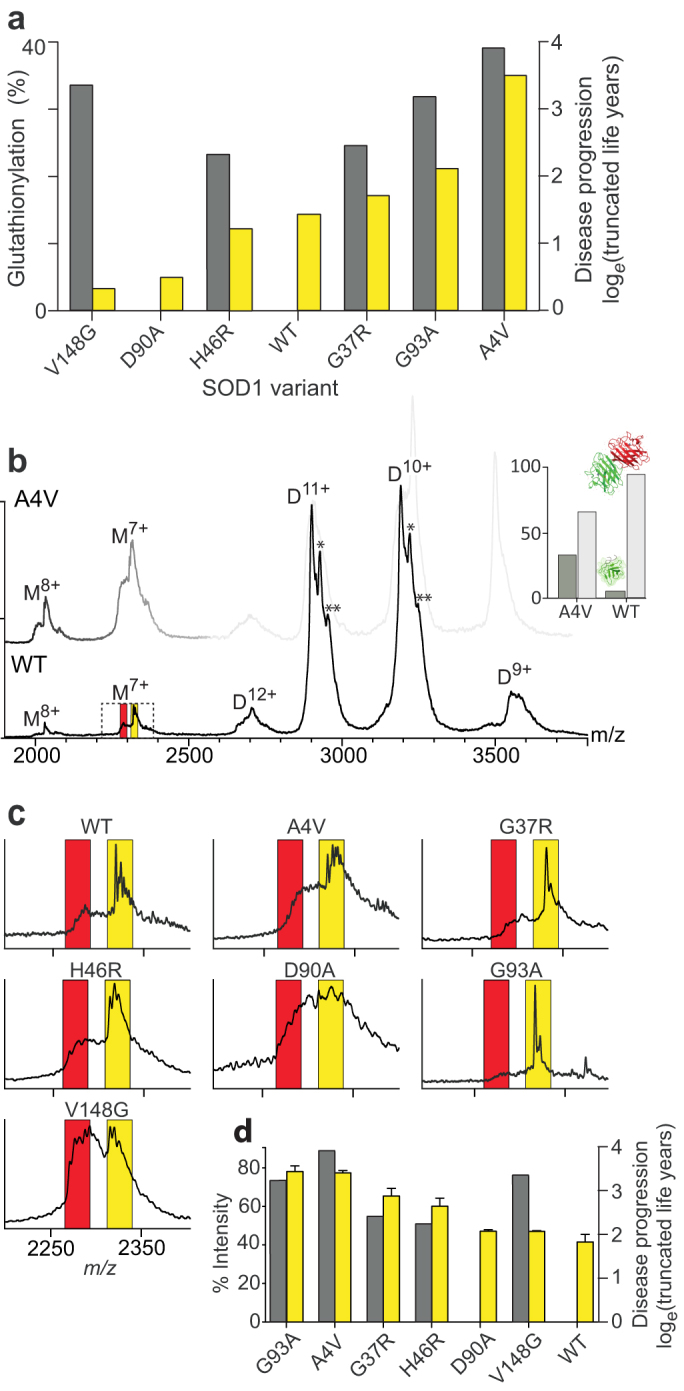
(a) Percentage of recombinantly expressed SOD1 that was glutathionylated (yellow), as determined by the area under the peaks of MS spectra, compared to disease progression (grey), expressed in truncated life years as loge[(75 − (25 + d)], where d equals the reported average disease duration, assumed life expectancy is 75 years and age at onset of disease is set to 50 years. (b) Mass spectrum of native SOD1WT (main) and SOD1A4V (faint) with species charge states labeled (D = dimer, M = monomer). Additional maxima within the peaks are the result of glutathionylation of one or both subunits to give a mixed (*) or gsSOD1(**) dimer. Inset shows the ratio of dimer to monomer for the two proteins, derived from charge state peak areas in the spectrum. (c) Expansion of the M7+ region showing peaks corresponding to uSOD1 (red) and gsSOD1 (yellow). (d) The percentage of gsSOD1 monomer (yellow) compared to disease progression (grey) for all SOD1 variants. The gsSOD1 measurements were performed in triplicate and error bars represent standard error of the mean.
Results
Our initial assay, using mass spectrometry, revealed that all variants were glutathionylated to some extent when produced in E. coli, from a low of 3% for SOD1V148G to 36% for SOD1A4V (Fig. 1a). When these data were arranged in order of disease progression, a plausible correlation was evident for SOD1A4V, SOD1G93A, SOD1G37R and SOD1H46R; however substantial glutathionylation of non-pathogenic SOD1WT compared to the aggressive variant SOD1V148G, suggested that this modification alone is not predictive of disease progression. Indeed, while the sequence of these SOD1 variants may determine their susceptibility to glutathionylation, further work is required to confirm this in ALS patients.
To compare the relative dissociation propensity of our SOD1 variants, we performed nanoelectrospray-ionization mass spectrometry, with conditions optimized to preserve solution phase structures during ionization and subsequent in vacuo detection12 (Fig. 1b; Fig. S2). Each of the SOD1 variant spectra exhibited peaks arising from two dominant ion populations at ~2900 m/z and ~3200 m/z, corresponding to the 10+ and 11+ ions of their respective dimers. Only minor peaks were observed for the 9+ and 12+ charge states, indicating that the dimers had negligible variation in their tertiary and quaternary structures. On closer inspection, a series of three maxima were identified within the 10+ and 11+ peaks, arising from dimers composed of; (i) two unmodified SOD1 (uSOD1) monomers; (ii) uSOD1 paired with gsSOD1; (iii) two gsSOD1 monomers. With the exception of SOD1A4V, monomers were significantly less abundant than dimers, but similarly confined to two (7+ and 8+) charge states (Fig. 1b & inset). For all variants, the most abundant monomer observed was gsSOD1 (Fig. 1c), even though uSOD1 constituted the majority of total protein.
The mixture of SOD1 dimers was further interrogated using tandem mass spectrometry to isolate an ion population exclusive to the dimer (D11+), prior to collision-induced dissociation (CID). At the minimum accelerating voltage (6 V) required to traverse an argon-filled CID region, the spectrum contained a single large peak of isolated D11+ ions (Fig. S3a). At an accelerating potential of 20 V, two additional peaks arose at ~2700 m/z and 3200 m/z, being due to the dissociation of D11+ into charge-complementary M5+ and M6+ monomers. These peaks increased in intensity at 40 V and 60 V, after which the more asymmetric dissociation pathway via M4+ and M7+ monomer ions became competitive. This hierarchical gas-phase molecular division, based on charge, was also observed for gsSOD1 (Fig. S3b). We compared the M6+ abundance for uSOD1 and gsSOD1 under low (10 V) versus high (70 V) energy CID conditions: at 10 V, gsSOD1A4V, gsSOD1G93A and gsSOD1G37R had a greater proportion of M6+ than uSOD1 indicating that less energy is required to remove gsSOD1 monomer from the dimer than uSOD1. A comparison of the spectra at 70 V versus 10 V shows that gsSOD1:uSOD1 is larger at low energy CID for all seven proteins, which supports the hypothesis that modification by glutathione destabilizes the dimer.
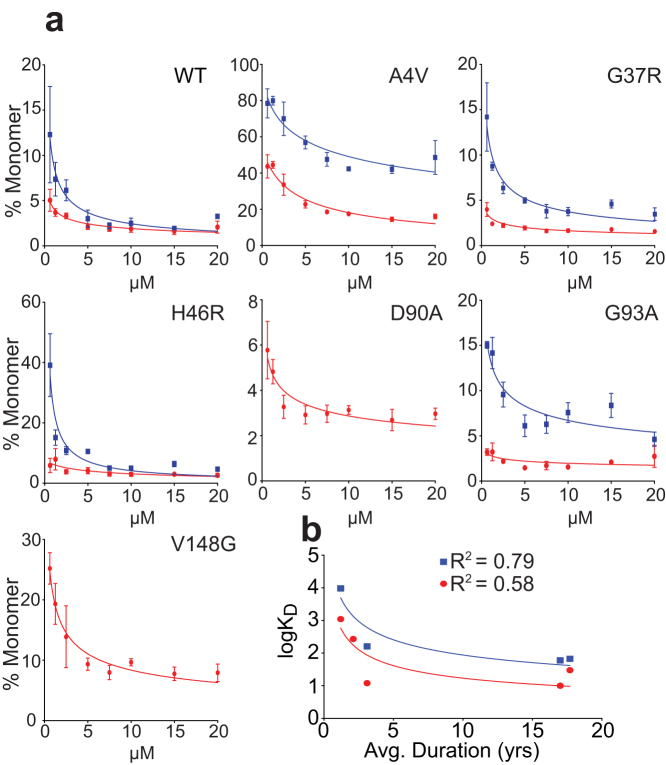
A caveat to the gas-phase analysis of molecular interactions is the absence of bulk water which, to varying degrees, can affect the strength of non-covalent association between proteins13. Thus, it was possible that the more facile removal of gsSOD1 from the gas-phase dimer (Fig. 1b&c) is not necessarily reflective of the aqueous environment found in vivo. To address this uncertainty, we prepared dilution series of the SOD1 variants ranging from 20 μM down to 625 nM, prior to mass analysis using identical instrument conditions for all samples. The spectra acquired at each concentration provided a snapshot of all species present in solution and so, by plotting the proportion of monomer versus concentration (Fig. 2a; Fig. S4), we were able to calculate discreet dissociation constants for (uSOD1)2 and (gsSOD1)2 (Table 1; Fig. 2b). Unlike the homodimers, which dissociate into their respective and unique monomer pairs, products of the mixed dimer (uSOD1:gsSOD1) contributed to both populations of monomer, and were not so readily estimated. In an effort to account for monomers originating from the mixed dimer, we monitored the decline in (uSOD1:gsSOD1) 11+ ion intensity with successive dilution, and subtracted an equivalent quantity from each monomer signal. Although imprecise, we believe this correction attenuated, to some extent, the confounding effects of the mixed dimer species.
Figure 2. SOD1 monomer abundance increases with dilution.
(a) of SOD1 variants showing changes in the proportion of monomeric uSOD1 (red) and gsSOD1 (blue) as a function of protein concentration: (i) SODWT, (ii) SOD1A4V, (iii) SOD1G37R, (iv) SOD1H46R, (v) SOD1D90A, (vi) SOD1G93A, (vii) SOD1V148G. Analyses were performed in triplicate at each concentration and error bars represent standard deviation about the mean. (b) Relationship between disease duration and KD for uSOD1 (red) and gsSOD1 (blue).
Table 1. SOD1 dissociation constants as determined by native mass spectrometry. The dissociation constants are means ± standard error from 3 distinct data sets with each containing 5 data points (see supplementary figure 4).
| Variant | WT | A4V | G37R | H46R | D90A | G93A | V148G |
|---|---|---|---|---|---|---|---|
| SOD1 KD (nM) | 9 ± 1 | 1100 ± 100 | 10 ± 1 | 30 ± 4 | 25 ± 4 | 12 ± 1 | 271 ± 40 |
| GS-SOD1 KD (nM) | 34 ± 5 | 9600 ± 900 | 60 ± 9 | 97 ± 16 | - | 160 ± 32 | - |
All uSOD1 variants had KD values greater than uSOD1WT (9 ± 1 nM), implying that these single residue substitutions are sufficient to disturb the quaternary structure (Table 1). The most substantial augmentation was observed for uSOD1A4V (1.1 ± 0.1 μM), being approximately 100-fold greater than uSOD1WT, whereas uSOD1G93A (12 ± 1 nM) had a KD only marginally higher than uSOD1WT. Significantly, the KD value of each gsSOD1 species was higher than the corresponding uSOD1, with the largest (13-fold) increase observed for gsSOD1G93A, followed by gsSOD1A4V (9-fold), gsSOD1G37R (6-fold) and gsSOD1WT (4-fold) (Table 1). There was insufficient gsSOD1V148G and gsSOD1D90A to accurately calculate their KD values.
Discussion
Glutathionylation of SOD1 expressed in E. coli, has previously been reported to occur at each of the four cysteine residues (Cys6, Cys57, Cys111, and Cys146) as detected by mass spectrometry14. Contrastingly, our analysis of recombinant SOD1WT demonstrated that no more than one glutathione was bound per subunit, and this was the case for all SOD1 variants in this study (Fig. S1). This discrepancy stems from the protocol used by Furukawa and coworkers14, which directed expression of SOD1 into inclusion bodies or soluble apo-proteins followed by refolding and metal enrichment after extraction from bacterial cells15. In the present work, SOD1 was co-expressed with the yeast-copper-chaperone for SOD1 (yCCS) in the presence of copper and zinc, with subsequent purification of soluble SOD1 from the bacterial cytosol16. The yCCS facilitates folding of the nascent chain, and formation of the disulfide bond between Cys57 and Cys14614. This disulfide effectively locks the β-barrel structure, rendering these Cys residues inaccessible to larger molecules; ipso facto, unlikely sites of glutathionylation. Furthermore, it has been demonstrated that Cys6 is buried inside the β-barrel of catalytically active SOD1, i.e., inaccessible to aqueous reductants17. We assessed the dismutase activity of our variants using in-gel zymography, which yielded positive results for all but the metal-binding site mutant, SOD1H46R (Fig. S1b). Therefore, although we did not explicitly demonstrate Cys111 to be the site of our observed glutathionylation, it is inferable that the only cysteine residue sufficiently exposed and therefore substantially modified by glutathione was Cys111.
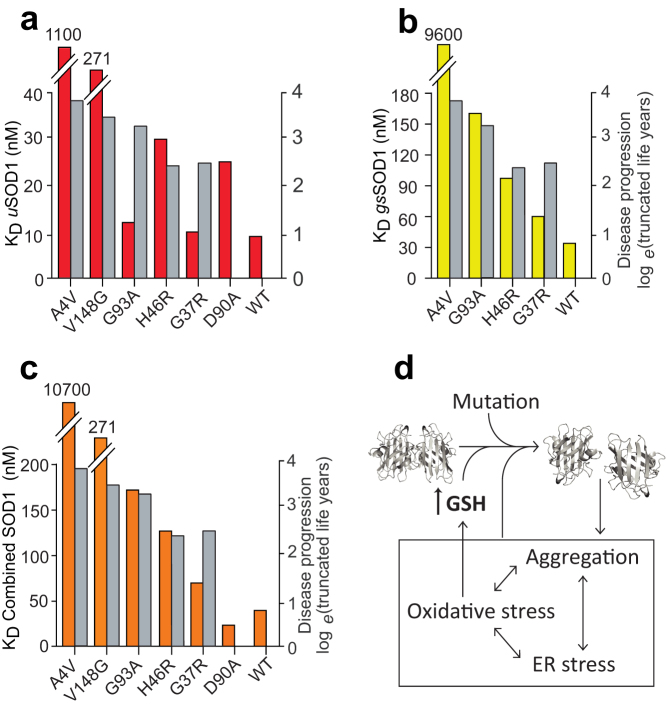
It is well documented that patients suffering from SOD1-associated fALS have variable survival times, dependent upon the particular amino acid mutation18. Considering the role played by dimer dissociation, as an entrée to aggregation, we scrutinized our data for evidence of a link between patient survival and KD (Fig. 2b). For uSOD1, the KD values were poorly associated with disease duration (R2 = 0.58), primarily due to the outlier characteristics of uSOD1G93A. In contrast, KD values for gsSOD1 showed a much stronger correlation (R2 = 0.79), indicating that glutathionylation may be responsible for the disparity between perceived SOD1 stability and observed patient survival. Consider the G93A mutation. This is reported as having little effect upon dimer dissociation19, and our results for the KD of uSOD1G93A confirm this (Fig. 3a); yet the survival time of patients carrying this mutation is, on average, a mere 2.2 years. Our ability to distinguish between, and calculate separate KD values for uSOD1 and gsSOD1 resolves this apparent inconsistency - the KD of gsSOD1G93A is 13 times higher than that of uSOD1 (Fig. 3b; Table 1) - highlighting the significance of this methodology.
Figure 3. The profound effect of glutathionylation on SOD1 dissociation constants.
Histograms comparing KD values of (a) uSOD1, (b) gsSOD1, and (c) uSOD1 + gsSOD1, to disease progression (grey). (d) A scheme of the proposed factors affecting SOD1 dimer dissociation. Briefly, dimer dissociation occurs due to mutation, glutathionylation, and cellular stresses. The increased monomer population is more likely to unfold and increase cellular stress. Glutathione (GSH) levels in the cell are elevated during periods of oxidative stress, thereby increasing the chances of SOD1 becoming glutathionylated.
Our data can be used to reframe the original hypothesis in more specific terms: i.e., it is not only (the extent of) SOD1 glutathionylation that influences the rate of disease progression, but also the KD of the glutathionylated species. Glutathionylation, however, is not a requirement for pathogenicity, as sequence modification alone of SOD1V148G causes a 30-fold increase in KD over SOD1WT. Similarly, our extracted KD value for uSOD1A4V reflects the considerable instability of this non-glutathionylated species. These mutations are located deep in the inter-subunit cleft, where small molecular volume changes are able to disrupt the dimer interface leading to more facile dissociation20 (Fig. S5).
In contrast to Val148 and Ala4, Gly37 and Gly93 are spatially remote to the dimer interface, being situated in the β3/β4 Greek key loop and β5/β6 inter-strand loop respectively (Fig. S5). It is notable that these loops are proximal to each other and the Leu38 cork, while Cys111 is within the β6/β7 Greek key loop at the opposite end of the β-barrel. Prior to glutathionylation, the individual mutations G37R and G93A impart negligible effect upon the KD of SOD1; i.e., in isolation, these mutations are not pathologically destabilizing (Table 1). Rather, it is the synergy of glutathionylation and point mutation at the β-barrel ‘poles' that accounts for the significantly higher KD values reported in this study, and presumably, the malignance of these variants in vivo. It stands to reason then, that glutathionylation of SOD1 alters the KD of a multitude of individual variants in a unique, allosteric-like fashion, and that it is the combination of KD(uSOD1) and KD(gsSOD1) (Fig. 3c) which impact the pathogenicity of the protein. Therefore, we propose an elaboration to the contemporary notion in which SOD1 dissociation is the primary underlying cause of SOD1 fALS.
Specifically, dissociation of the SOD1 dimer is a biophysical consequence of either, (i) a potent destabilizing mutation such as A4V, or (ii) glutathionylation of a weak or non-destabilizing mutation such as G93A. Consequently, there is a twofold increase in the abundance of monomers, which are more susceptible to misfold into toxic conformations compared to the dimer6,7 (Fig. 3d). If the cellular clearance rate of misfolded SOD1 is inadequate, increasingly frequent hydrophobic interactions ultimately give way to aggregation, forming larger amorphous structures that stress the cellular machinery21. Many neurodegenerative diseases, including ALS are also associated with oxidative stress22, which in turn can lead to an up-regulation of glutathione23. Indeed, an increase in GSH has been observed in ALS patient CSF24. Analysis of human tissue from ALS patients, and in cell experiments, are currently under way to further investigate the role of glutathionylation in SOD1 associated fALS.
Methods
Materials
All solutions were prepared using Milli-Q ultra-purified water using the Millipore purification system (Massachusetts, USA). All materials were of analytical grade. Tryptone, Tris-base, DTT, β-mercaptoethanol, bromophenol blue, brilliant blue, ammonium persulphate, SDS, ampicillin sodium salt, and IPTG were from Amresco (Ohio, USA). NaCl, ammonium sulphate, agar, acetonitrile, Tris-HCl, tetramethylethylenediamine, ammonium acetate and RNase were from Sigma-Aldrich (Missouri, USA). DNase was from Roche Diagnostics (NSW, Australia). Carbenicillin was from Carbenicillin Direct (UK). Copper sulphate pentahydrate was from Ace Chemical Company (SA, Australia). Zinc sulphate heptahydrate was from Hopkin and Williams LTD (Birmingham, UK). Formic acid and acetic acid were from Univar (NSW, Australia). Methanol was from Ajax Finechem (NSW, Australia). Gold coated borosilicate electrospray capillaries were made in-house.
Expression and purification of recombinant SOD1
Plasmids encoding SOD1WT, SOD1A4V, SOD1G37R, SOD1H46R, SOD1D90A, SOD1G93A, and SOD1V148G were a gift from the Oliveberg Group (Sweden). Protein expression and purification were performed according to16. Briefly, the SOD1 plasmids were transformed into chemically competent BL21* E. coli using heat shock. Following transformation, the protein was expressed in the presence of copper and zinc ions with the addition of IPTG. The expressed SOD1 was purified via size exclusion chromatography (Hiload 16/60 Superdex 75 PG, GE USA) and anion exchange chromatography (Hiscreen Capto-Q, GE USA).
Sample preparation for mass spectrometry
SOD1 samples were desalted and buffer exchanged into 200 mM NH4OAc (pH 7), using gel filtration chromatography (Superdex 200 10/300 GL, GE USA). Briefly, SOD1 samples were concentrated to 20 mg/ml and 100 μl was loaded onto the column at a flowrate of 0.4 mg/ml. The concentration of SOD1 was determined at 265 nm using a UV/Vis spectrophotometer, and a molar extinction coefficient of 18 700 M−1cm−1 25.
Mass spectrometry
SOD1 samples in 200 mM NH4OAc were denatured using formic acid and acetonitrile with the final concentration of each being 10% and 40% respectively. Mass analyses were performed on a Q-ToF ULTIMA mass spectrometer (Waters, UK) in positive ion mode with nanoelectrospray source. Instrument parameters included: capillary 1.5 kV, sample cone 200 V, desolvation gas flow 180 L/h. Native protein analyses were performed in 200 mM NH4OAc on a SYNAPT G2 HDMS (Waters, UK) in positive ion mode. Instrument parameters were similar to Ruotolo et al. (2008)26 with the exceptions: capillary voltage 1.5 kV, sample cone 30 V, cone gas 70 L/h, trap collision voltage 6 V, ion-transfer stage pressure 4.15 e−1 mbar, ToF analyzer pressure 2.38 e−6 mbar. All SOD1 variants were analyzed at a concentration of 20 μM. For MS/MS experiments, the trap collision energy was increased from 10 to 100 V in increments of 10 V with 30 scans at each increment. The dilution series mass spectra were acquired at concentrations of 20, 15, 10, 7.5, 5, 2.5, 1.25 and 0.625 μM with the following instrument parameters: capillary voltage 1.52 kV, sample cone 137 V, cone gas 78 L/h, trap collision voltage 16 V, transfer CE 8 V, ion transfer stage pressure 3.72 e−4 mbar, ToF analyser pressure 9.01 e−7 mbar, backing pressure 4.0 e0 mbar. All mass spectra in this study were externally calibrated using a solution of cesium iodide (10 mg/ml in water) and were processed using Masslynx 4.1 software (Waters, UK).
Dissociation constants
Dissociation constants were derived from the mass spectrometry data using the method of Rose et al., (2011)27. Briefly, the intensity, as determined by the area under the peak, of all peaks relating to SOD1 monomers (Mi) and dimers (Di) were summed to give total signal intensity (ITS):
 |
The proportion of monomer signal (PM) was determined by dividing the intensity of the monomeric species (IM) by the total signal intensity (ITS):
 |
The concentration of monomer at equilibrium ([M]eq) was determined by multiplying the proportion of monomer signal (PM) by the total protein concentration in μM as determined by UV/Vis spectroscopy ([Po]):
 |
The concentration of dimer at equilibrium ([D]eq) is defined as:
 |
The KD was derived from the gradient of the plot of [D]eq against [M]2eq (fitting performed with Prism 5.0, GraphPad Software, Inc.) In each case at least 5 data points were plotted with repeat experiments being performed if the line of best fit gave least-squares-regression, R2 < 0.75.
Statistical analysis
Statistical analyses were carried out using Prism 5.0 software (Graphpad). All mass analyses were performed in triplicate (n = 3) with error bars representing the standard error of the mean. The dissociation plots (Figure 2) were fitted with a Log (Gaussian) function. The KD plots (Fig. S4) were fitted using linear regression.
Author Contributions
JAA and JJY conceptualized, designed and supervised the study. LM performed experiments, analyzed data, and wrote the initial manuscript draft. JAA revised and finalized the manuscript for submission.
Supplementary Material
Supplementary information
Acknowledgments
We thank the Oliveberg Group, (Stockholm University, Sweden) for kindly providing the SOD1 expression vectors. JJY is supported by the Australian Research Council in the form of a DECRA Fellowship, and by NHMRC project grant 1003032. LM is supported by a University of Wollongong Matching Scholarship. Research facilities were provided by the University of Wollongong Illawarra Health and Medical Research Institute.
References
- Valentine J. S., Doucette P. A. & Potter S. Z. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu. Rev. Biochem 74, 563–593 (2005). [DOI] [PubMed] [Google Scholar]
- Sibata N., Asayama K., Hirano A. & Kobayashi M. Immunohistochemical study on superoxide dismutases in spinal cords from autopsied patients with amyotrophic lateral sclerosis. Dev. Neurosci. 18, 492–498 (1996). [DOI] [PubMed] [Google Scholar]
- Bosco D. A. et al. Wild-Type and Mutant SOD1 Share an Aberrant Conformation and a Common Pathogenic Pathway in ALS. Nat. Neurosci 13, 1396–1403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg K. et al. Novel antibodies reveal inclusions containing non-native SOD1 in sporadic ALS patients. PLoS ONE 5, e11552 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrishevsky E. et al. Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in Amyotrophic lateral sclerosis. PLoS ONE 7, e35050 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhit R. et al. Monomeric Cu,Zn-superoxide Dismutase Is a Common Misfolding Intermediate in the Oxidation Models of Sporadic and Familial Amyotrophic Lateral Sclerosis. J. Biol. Chem 279, 15499–15504 (2004). [DOI] [PubMed] [Google Scholar]
- Svensson A. E. et al. Metal-free ALS variants of dimeric human Cu,Zn-superoxide dismutase have enhanced populations of monomeric species. PLoS ONE 5, 1–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S. S. et al. An intersubunit disulfide bond prevents in vitro aggregation of a superoxide dismutase-1 mutant linked to familial Amyotrophic lateral sclerosis. Biochem 43, 4899–4905 (2004). [DOI] [PubMed] [Google Scholar]
- Wilcox K. C. et al. Modifications of Superoxide Disumutase (SOD1) in Human Erythrocytes: a Possible Role in Amyotrophic Lateral Sclerosis. J. Biol. Chem 284, 13940–13947 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair J. R., Boggio K. J., Petsko G. A., Ringe D. & Agar J. N. Strategies for stabilizing superoxide dismutase (SOD1), the protein destabilized in the most common form of familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 107, 21394–21399 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redler R. et al. Glutathionylation at Cys-111 Induces Dissocation of Wild Type and FALS Mutant SOD1 Dimers. Biochem 50, 7057–7066 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobott F., Hernandez H., McCammon M. G., Tito M. A. & Robinson C. V. A tandem mass spectrometer for improved transmission and analysis of large macromolecular assemblies. Anal. Chem. 74 (2002). [DOI] [PubMed] [Google Scholar]
- Rostom A. A., Tame J. R. H., Ladbury J. E. & Robinson C. V. Specificity and interactions of the protein OppA: Partioning solvent binding effects using mass spectrometry. J. Mol. Biol. 296, 269–279 (2000). [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Torres A. S. & O'Halloran T. V. Oxygen-induced maturation of SOD1: A key role for disulfide formation by the copper chaperone (CCS). EMBO J. 23, 2872–2881 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinweber B. et al. Aggregation of ALS mutant superoxide dismutase expressed in Escherichia coli. Free Radic. Biol. Med 36, 911–918 (2004). [DOI] [PubMed] [Google Scholar]
- Lindberg M. J., Normark J., Holmgren A. & Oliveberg M. Folding of human superoxide dismutase: disulfide reduction prevents dimerization and produces marginally stable monomers. Proc. Natl. Acad. Sci. USA 101, 15893–15898 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Beem K. M., Richardson J. S. & Richardson D. C. Determination and analysis of the 2 Å structure of copper, zinc superoxide dismutase. J. Mol. Bio 160, 181–217 (1982). [DOI] [PubMed] [Google Scholar]
- Wang Q., Johnson J. L. & Agar N. Y. R. N. A. J. Protein Aggregation and Protein Instability Govern Familial Amyotrophic Lateral Sclerosis Patient Survival. PLoS Bio 6, 1508–1526 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumfeldt J. A. O., Stathopulos P. B., Chakrabartty A., Lepock J. R. & Meiering E. M. Mechanism and thermodynamics of guanidinium cholride-induced denaturation of ALS-associated mutant Cu,Zn superoxide dismutases. J. Mol. Bio 355, 106–123 (2006). [DOI] [PubMed] [Google Scholar]
- Perry J. J. P., Shin D. S., Getzoff E. D. & Tainer J. A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 1804, 245–262 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S. J. et al. Compartmentalization of superoxide dismutase 1 (SOD1G93A) aggregates determines their toxicity. Proc. Natl. Acad. Sci. 109, 15811–15816 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber S. C. & Shaw P. J. Oxidative stress in ALS: Key role in motor neuron injury and therapeutic target. Free. Radic. Biol. Med. 48, 629–641 (2010). [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Rossi R., Giustarini D., Colombo R. & Milzani A. S-glutathionylation in protein redox regulation. Free. Radic. Biol. Med 43, 883–898 (2007). [DOI] [PubMed] [Google Scholar]
- Tohgi H. et al. Increase in oxidized NO products and reduction in oxidized glutathione in cerebrospinal fluid from patients with sporadic form of amyotrophic lateral sclerosis. Neurosci. Lett. 260, 204–206 (1999). [DOI] [PubMed] [Google Scholar]
- Stansell M. J. & Deutsch H. F. The levels of catalase and of erythrocuprein in human erythrocytes. Clin. Chim. Acta 14, 598–607 (1966). [DOI] [PubMed] [Google Scholar]
- Ruotolo B. T., Benesch J. L. P., Sandercock A. M., Hyung S. & Robinson C. V. Ion mobility-mass spectrometry analysis of large protein complexes. Nat. Protoc 3, 1139–1152 (2008). [DOI] [PubMed] [Google Scholar]
- Rose R. J. et al. Quantitative analysis of the interaction strength and dynamics of human IgG4 half molecules by native mass spectrometry. Structure 19, 1274–1282 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information