Summary
Great strides have been made in improving the outcome of patients with metastatic colorectal cancer and targeted agents are an important part of the treatment arsenal. The approved monoclonal antibodies, bevacizumab, cetuximab and panitumumab, are part of the standard of care, yet only recently have we begun to define which patients benefit from these therapies using predictive tumor biomarkers. More recently, novel agents including aflibercept and regorafenib have had promising results and may become approved therapies. In addition, agents targeting the mTOR pathway and the TNF pathway have demonstrated early evidence of benefit. In the coming years, we may experience an influx of new therapies, possibly leading to further prolongation of patient survival or even, for some, a cure.
Colorectal cancer (CRC) results in nearly 500,000 deaths annually in the world and, after lung cancer, it is the second cause of cancer-related death in the USA [1]. However, in the last 15 years there has been significant improvement in outcomes in the setting of advanced disease, particularly for patients with metastatic CRC (mCRC). In a meta-analysis published by Thirion et al. in 2004, the addition of leucovorin to 5-FU resulted in an approximate 12-month median overall survival (OS) of mCRC patients. This regimen and its variations became the backbone for development of new drug combinations in CRC [2]. Subsequently, the incorporation of oxaliplatin and irinotecan were found to improve median OS by up to approximately 16–18 months [3,4]. New targeted agents have been added to this core selection of chemotherapeutic drugs, resulting in a median OS as high as 24–30 months [5,6]. The benefit of incorporating targeted biological agents into treatment regimens has since spurred a number of ongoing efforts in basic, translational and clinical research. While monoclonal antibodies (mAbs) that inhibit angiogenesis or the EGF receptor (EGFR) have a proven role in mCRC, a number of small-molecule kinase inhibitors that target intracellular signaling pathways are showing great promise, and may enhance the therapeutic armamentarium for mCRC.
This article focuses on molecular targeted therapy with established and approved drugs, new agents in the advanced stages of development, and novel compounds that appear promising in Phase I and II trials.
Antiangiogenic agents
Angiogenesis, a process that involves the recruitment of new blood vessels, does not occur in normal healthy adults except as a normal part of wound repair, tissue remodeling and inflammation [7]. However, angiogenesis is critical for the growth of tumors, in which this normally highly complex and regulated process is altered, leading to leaky, excessively branching, tortuous vessels, with an abnormal endothelial lining. Angiogenesis is regulated by a number of signaling proteins, including the VEGFs, FGF, PDGF, TGF-α, and their respective transmembrane receptors [8].
VEGF signaling occurs in an endocrine, paracrine and autocrine fashion. Tumor cells and stromal tissue, including endothelial and fibroblast cells, produce VEGFs; the VEGF family is comprised of six subtypes (A–F), as well as PGFs 1 and 2. It is believed that VEGF-A is the most prominent mediator of angiogenesis in the VEGF family. VEGFs act by binding extracellularly to their cognate transmembrane tyrosine kinase receptors (VEGFRs). VEGFR activation initiates a signal transduction cascade that leads to expression of genes related to cell proliferation, survival, migration, and differentiation capabilities (Figure 1) [9]. This process increases vascular permeability and causes the branching and formation of new blood vessels. There is evidence that mCRC progression and overall disease prognosis can be correlated with levels of endogenous VEGFs [10].
Figure 1. VEGF signaling pathway.
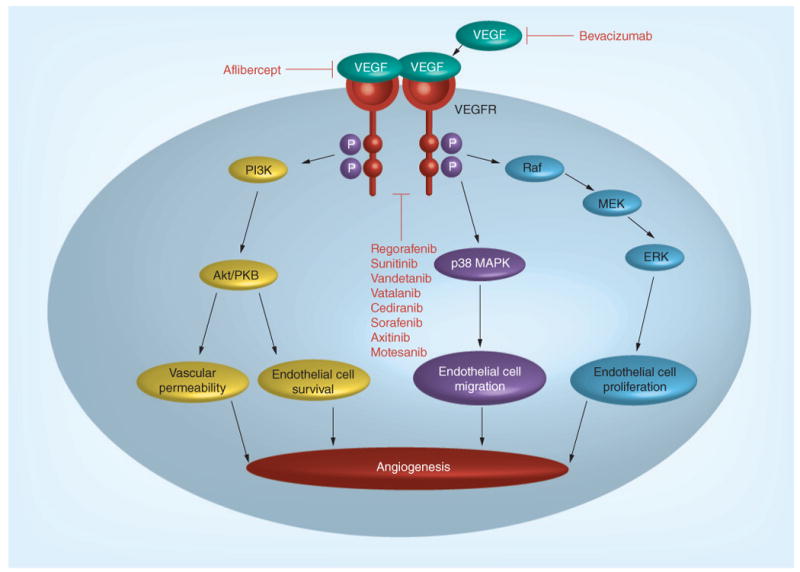
Adapted with permission from [69].
The first and only approved angiogenesis-targeting agent in mCRC is bevacizumab. Bevacizumab is a mAb that binds to VEGF-A, interfering with the interaction of VEGF-A with its receptor (VEGFR) and preventing receptor activation. Initially, it was hoped that bevacizumab would prevent tumor growth by inhibiting angiogenesis, but when used as a single agent, bevacizumab produced minimal response rates (RRs) [11]. However, when bevacizumab was combined with cytotoxic chemotherapies, there was significant improvement in outcome compared with cytotoxic chemotherapy treatment alone, perhaps due to an additive suppression of tumor cell growth and induction of apoptosis. In the landmark trial by Hurwitz et al., chemotherapy-naive patients with mCRC were treated with a combination of irinotecan, 5-FU bolus, leucovorin (IFL) and bevacizumab. This combination not only increased RRs, but also prolonged progression-free survival (PFS) and OS when compared with patients treated with the IFL regimen alone [12]. Moreover, bevacizumab has been shown to add efficacy to other modern combinations of cytotoxic drugs, such as oxaliplatin, 5-FU bolus and continuous infusion, and leucovorin (FOLFOX) in second- and even third-line settings [13]. The large observational BRiTE cohort study, which compared the survival of patients treated with or without bevacizumab beyond first-line therapy, demonstrated an advantage in OS when continuing bevacizumab treatment until disease progression (31.8 vs 19.9 months; hazard ratio [HR]: 0.48; p < 0.001) [14]. More recently, the TML trial, a definitive, prospective Phase III study involving 820 patients, confirmed a survival benefit with the use of bevacizumab in the second-line setting [15]. Patients were randomized to receive oxaliplatin- or irinotecan-based therapy with or without bevacizumab. The addition of bevacizumab to chemotherapy improved OS from 9.8 months (chemotherapy alone) to 11.2 months. It is anticipated that this trial will lead to the approval of bevacizumab in the second-line setting.
However, the addition of bevacizumab has not always resulted in improved outcomes. In the first-line setting, the combination of FOLFOX and bevacizumab did not improve OS when compared with FOLFOX alone [13]. Moreover, in the adjuvant setting, a large Phase III trial, adding bevacizumab to FOLFOX in patients with resected stage III CRC, failed to demonstrate an improvement in OS, possibly reflecting that the neoangiogenesis signal is not as critical in the setting of micrometastatic disease [16,17].
A newer antiangiogenic agent likely to gain approval in the treatment of mCRC is aflibercept, a fully humanized recombinant fusion molecule of the human VEGF receptor extracellular domain 3 and the fragment crystallizable (Fc) region of human IgG1. This molecule specifically binds to circulating VEGF-A, VEGF-B and PLGF-1 and -2, thus preventing activation of their respective receptors [18]. In a Phase II trial of 51 heavily pretreated mCRC patients, aflibercept produced a disease control rate (DCR) of approximately 30% at 4 months in patients who had previously received bevacizumab [19]. The VELOUR trial is a Phase III study comparing irinotecan and 5-FU with and without aflibercept in patients whose disease had progressed on an oxaliplatin-based regimen [20]. Initial results were presented at the 2011 meeting of the European Society for Medical Oncology World Congress on Gastrointestinal Cancer. The aflibercept group had a longer OS (13.50 vs 12.06 months; HR: 0.817; p < 0.005) and PFS (6.90 vs 4.67 months; HR: 0.758; p = 0.0001) compared with the placebo group. The RR in the aflibercept group was 19.8% compared with 11.1% in the placebo group (p = 0.0001).
A number of small-molecule kinase inhibitors that inhibit VEGFRs have been tested in mCRC. The potential advantages of small-molecule kinase inhibitors compared with mAbs include their oral bioavailability, their low risk of causing infusion reactions, their theoretical increased tumor penetration and their broader spectrum of cell signaling pathway inhibition. However, until recently, despite a number of large clinical trials involving thousands of patients, no small-molecule kinase inhibitor has been shown to improve outcome in the treatment of CRC. For example, vatalanib (PTK/ZK222584), which specifically acts against VEGFR-1, VEGFR-2, PDGFR-B and c-kit, has been tested in the Phase III CONFIRM-1 trial. However, there was no improvement in PFS and OS in patients treated with FOLFOX plus vatalanib compared with FOLFOX alone [21]. Another Phase III trial, CONFIRM-2, compared the efficacy of FOLFOX with that of FOLFOX combined with vatalanib in irinotecan-refractory mCRC. While there was an improvement in PFS of 1.4 months in the FOLFOX/vatalanib-treated cohort, the OS remained unchanged [22]. Sunitinib is a TKI that targets the VEGFRs, PDGFR, c-KIT, RET and LT3. This drug is approved and widely used in patients with renal cell cancer [23]. However, in a Phase II trial of sunitinib in refractory mCRC patients, the outcomes were disappointing with a RR of only 1.1% and 13 patients experiencing stable disease for 6 months [24]. In addition, a Phase II study of irinotecan, 5-FU bolus and continuous infusion, and leucovorin (FOLFIRI), with or without vandetanib, revealed no significant benefit with the addition of vandetanib to a chemotherapy regimen [25]. Finally, cediranib (AZD2171), another TKI that blocks VEGFR, was tested in patients with mCRC in a randomized Phase III trial (HORIZON III) as first-line therapy in combination with FOLFOX and bevacizumab [26]. Results from this trial have yet to be published as a manuscript or abstract but a press release by AstraZeneca confirmed that there was no improvement in OS with the addition of cediranib [101].
Nevertheless, there are still several ongoing clinical trials studying other small-molecule kinase inhibitors. Axitinib (AX-013736), a selective inhibitor of VEGFR-1, -2 and -3, is being examined in a Phase II study, in combination with FOLFOX, or FOLFOX and bevacizumab. Motesanib (AMG 706) is a multikinase inhibitor that is being evaluated for safety and pharmacokinetic characteristics, in combination with panitumumab and either FOLFIRI or FOLFOX. Sorafenib (BAY-43-9006) is a potent inhibitor of VEGFR-2 and has the added advantage of inhibiting Raf kinases, which are downstream of the VEGFR and EGFR pathways. Phase II trials are studying sorafenib in combination with cetuximab, in addition to FOLFOX6, in patients with mCRC.
Finally, one TKI has shown promise for patients with mCRC. Regorafenib (BAY73-4506) is a new, broad spectrum TKI that blocks multiple kinase signaling pathways. These pathways involve not only angiogenic (VEGFR-1–3 and TIE2) but also stromal (PDGFR-b and FGFR) and oncogenic (KIT and RET) pathways [27]. A Phase I trial of 38 patients with mCRC, treated with regorafenib monotherapy, showed a DCR of 74% (partial response [PR]: 4%; stable disease [SD] ≥7 weeks: 70%) [28]. The interim results of a Phase III trial were recently presented. In this randomized study, 760 mCRC patients who had received all standard therapies, including cetuximab and bevacizumab, were assigned to receive regorafenib or placebo. The DCR was 44.8% in the regorafenib cohort and only 15.3% in the placebo group. There was also an improvement in median OS of 6.4 months in the regorafenib arm compared with 5 months in the placebo arm (p > 0.005). This trial is the first positive Phase III trial using TKIs in CRC [29].
EGFR antagonists
EGFR antagonists target the EGFR family, also known as the ErbB protein family, which is comprised of four receptor tyrosine kinases (ErbB1/EGFR/HER1, ErbB2/HER2/neu, ErbB3/HER3 and ErbB4/HER4) [30]. These proteins are membrane-bound receptors that are activated by ligands including EGF and TGF-β. Ligand binding results in receptor homo- or hetero-dimerization and autophosphorylation of the c-terminal tyrosine residues (Figure 2). This enables docking of cytoplasmic proteins and activation of several signaling pathways including the Ras–Raf–MAPK, PI3K–Akt, protein kinase C, STAT and Src kinase pathways. Activation of these signal transduction cascades results in tumor cell growth and migration, cell invasion, and impaired apoptosis [31].
Figure 2. EGF receptor signaling pathway.
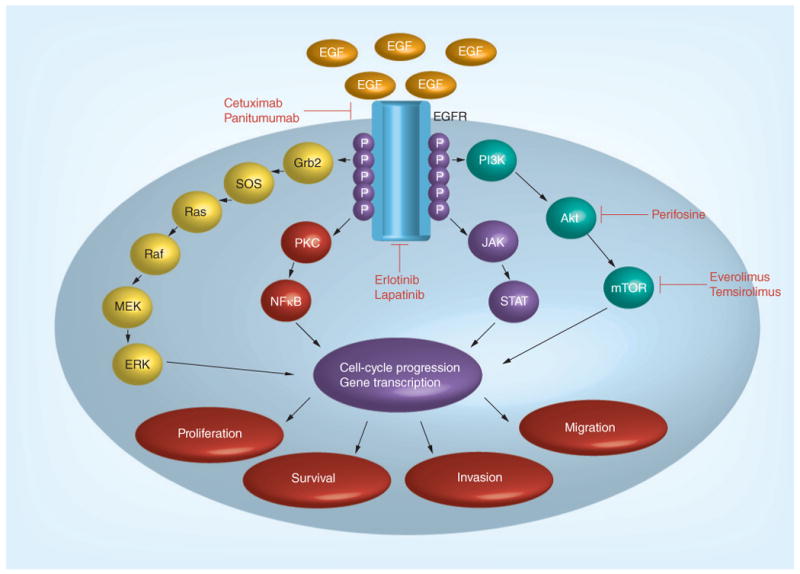
Adapted with permission from [69].
As with inhibition of angiogenesis, EGFR inhibition can be achieved through the use of TKIs and mAbs. Cetuximab is a mAb that prevents EGFR signaling by impairing receptor dimerization, promoting receptor internalization and degradation, and activating antibody-dependent cell-mediated cytotoxicity and complement fixation [32]. US FDA approval of cetuximab was based on the pivotal BOND 1 study, a Phase III trial in which irinotecan-refractory mCRC patients were randomized to receive cetuximab alone, or cetuximab plus irinotecan. Patients who received cetuximab alone had a RR of 11% and a median time to progression of 1.5 months. Moreover, patients who received cetuximab plus irinotecan had a RR of 23% with a time to progression of 4.1 months, demonstrating for the first time that a targeted therapy can re-sensitize some patients previously resistant to a cytotoxic chemotherapy [33]. In a refractory patient population, cetuximab monotherapy showed benefit over placebo, with a RR of 10% [34].
In the first-line setting, the CRYSTAL trial showed a benefit when combining cetuximab with FOLFIRI compared with FOLFIRI alone, with an improvement in PFS (8.9 vs 8.0 months). There was no difference in OS. This was the first study to show an interaction between KRAS mutation status and response to cetuximab, as discussed later in this review [35]. In two large Phase III randomized trials, the addition of cetuximab to oxaliplatin-based chemotherapy did not show any benefit in the first-line setting. In the Medical Research Council COIN trial, the addition of cetuximab to oxaliplatin plus 5-FU, versus oxaliplatin plus 5-FU alone did not improve the OS (17 vs 17.9 months) nor the PFS (8.6 months in both groups), although the RR was higher (64%) with cetuximab than without (57%; p = 0.05) [36]. Similarly, the Phase III NORDIC trial, involving 571 patients randomized to receive 5-FU bolus administration plus oxaliplatin (FLOX) alone or FLOX plus cetuximab demonstrated no differences in PFS or OS between the two treatment arms [37]. Thus, the role of cetuximab in the first-line setting is still unclear; there is apparent PFS (but no OS) benefit when combined with irinotecan-based therapy, but no benefit when added to oxaliplatin-based therapy.
Panitumumab is another anti-EGFR mAb that is FDA approved for the treatment of refractory mCRC. In patients whose disease was progressing on standard therapy, panitumumab demonstrated an 8% RR [38]. In the PRIME study, the addition of panitumumab to FOLFOX as first-line therapy did increase PFS when compared with FOLFOX alone (9.6 vs 8.0 months; p = 0.02), with a nonsignificant trend towards improvement in OS (23.9 vs 19.7 months; p = 0.07) [39]. Similarly, in the second-line setting, the addition of panitumumab to FOLFIRI significantly improved PFS, when compared with FOLFIRI alone (5.9 vs 3.9 months; p = 0.004) [40].
The protein k-ras (encoded by the KRAS gene) is active downstream of the EGFR and it has been shown that constitutively active mutations in the KRAS gene impact the efficacy of anti-EGFR therapy in patients with mCRC. K-ras is a small serine–threonine kinase that is farnesylated and inserted into the cell membrane. It is activated just downstream of the EGFR and propagates further signaling events. A constitutively active k-ras protein promotes tumor cell growth, even when EGFR signaling is blocked. Cancer growth continues due to the downstream signaling of mutant KRAS [41]. Based on this, researchers have analyzed colon cancer specimens from two large clinical trials using cetuximab and panitumumab. In the cetuximab study, Lievre et al. identified a KRAS gene mutation in 27% of patients, with a RR of 0% in tumors with mutated KRAS versus 40% in tumors with wild-type KRAS, and a median OS of 10.1 versus 14.3 months, respectively [42]. Similarly, Amado et al. identified KRAS mutations in 43% of patients, again with 0% of patients with mutated KRAS responding versus 17% in the wild-type KRAS group [43]. As a result of these findings, we are now routinely assessing KRAS mutational status to guide therapeutic decisions. Furthermore, for the 20–40% of patients for whom anti-EGFR mAbs are unlikely to help, a number of newer agents that target pathways downstream of the EGFR are being assessed in clinical trials. Some of the promising, yet early, examples are discussed below.
The NORDIC trial results [37] have complicated our understanding of the relationship between KRAS gene status and anti-EGFR mAb therapy. In this large Phase III study, there was no correlation between KRAS mutational status (wild-type or mutated) and PFS for patients treated with cetuximab. In an excellent editorial written by Grothey and Lenz, the predictive value of KRAS mutations for patients treated with anti-EGFR mAbs was discussed [44]. One primary conclusion was that the chemotherapy backbone could be a critical determinant. Thus, the outcome of irinotecan-based therapies was improved with anti-EGFR mAbs. For oxaliplatin-based combinations, the manner in which fluorouracil is administered (bolus vs infusional) may determine whether or not there is a benefit to the addition of anti-EGFR mAbs. Therefore, more studies are needed to understand the interaction between KRAS gene mutational status, the chemotherapy backbone and the addition of an anti-EGFR mAb.
There have been attempts to combine EGFR inhibitors with bevacizumab, based on the hypothesized advantage of blocking both VEGF and EGFR signaling. However, two large trials have demonstrated worse outcome for patients treated with dual antibody therapy in the first-line setting. In the PACCE study, patients were administered FOLFOX or FOLFIRI plus bevacizumab, with or without panitumumab; the addition of panitumumab resulted in a decreased PFS (10.0 vs 11.4 months) and OS (19.4 vs 24.5 months), along with greater toxicity [45]. Similarly, in the CAIRO-2 study, the addition of cetuximab to a regimen of capecitabine, oxaliplatin and bevacizumab in previously untreated mCRC patients resulted in a decreased PFS when compared with capecitabine, oxaliplatin and bevacizumab alone (9.4 vs 10.7 months) [46].
Despite the clinical activity and safety data of the anti-EGFR antibodies in CRC, anti-EGFR TKIs as single agents showed minimal activity in mCRC. Erlotinib is a TKI that has been approved for lung and pancreatic cancer treatment. In a Phase II trial as second-line therapy in patients with mCRC, erlotinib combined with capecitabine plus oxaliplatin (XELOX) resulted in a RR of 25%. However, 78% of patients had grade 3 or 4 toxicities, mainly involving diarrhea [47]. Lapatinib, a dual inhibitor of EGFR and HER2/neu, was well tolerated as a single agent, but demonstrated little activity in a Phase II trial as second-line treatment for mCRC [48]. The first encouraging results with a TKI targeting the EGFR in mCRC were seen in a recent Phase II trial, using a combination of cetuximab and erlotinib as a dual approach to targeting the EGFR. The DUX trial involved 50 patients with refractory mCRC and the primary end point of the study was RR, evaluated separately in KRAS wild-type versus KRAS mutant tumors. The overall RR was 31%, which increased to 41% when considered in KRAS wild-type tumors only [49]. Thus, while the TKIs may have a limited role in the treatment of mCRC, there may be benefit when used in combination with anti-EGFR mAbs.
Novel therapies
Akt/perifosine
It has been demonstrated that activation of the PI3K–Akt–mTOR signaling cascade contributes to cancer cell growth, and clinical progression and metastasis of CRC [50]. Akt is a serine–threonine kinase that promotes tumor growth and indirectly activates NF-κB, which contributes to chemotherapy resistance. Perifosine is an alkylphospholipid that inhibits constitutive and inducible Akt phosphorylation in a dose- dependent manner that thus could inhibit tumor progression [51]. 5-fluorouracil inactivates NF-κB and demonstrates synergistic activity when combined with perifosine in vitro [52]. A placebo-controlled Phase II trial of perifosine plus capecitabine involved 38 patients with mCRC, randomized to receive capecitabine plus perifosine or capecitabine alone. An impressive OS of 17.7 months in the perifosine arm was demonstrated, compared with 7.6 months in the group treated with capecitabine alone. The overall RRs were 20 and 7%, respectively, with one complete response (CR) in the capecitabine plus perifosine arm [53]. These results inspired the X-PECT trial, a highly anticipated Phase III study of perifosine plus capecitabine versus capecitabine alone, which was expected to lead to fast-track approval of perifosine. Enrollment of 468 patients was completed in August 2011 and the Phase III results were surprisingly negative. Patients were randomized into one of the two treatment arms (234 patients in each) and the median OS was 6.4 months in patients receiving perifosine plus capecitabine compared with 6.8 months in those receiving capecitabine alone [54]. Nevertheless, several ongoing trials are targeting the PI3K–Akt pathway using small-molecule targeted inhibitors. One promising approach appears to be dual targeting of the PI3K–Akt pathway concurrently with the MEK pathway, particularly in KRAS-mutant CRC patients. While no definitive data are yet available, it is hoped that inhibition of these two primary pathways downstream of the EGFR will result in greater disease control.
Apoptosis/TNF
One of the hallmarks of CRC is an impaired apoptotic pathway [55]. One strategy for restoring apoptosis is to activate the extrinsic pathway. In this regard, induction of TNF – a known agonist that triggers apoptosis – could play a role in the treatment of mCRC. However, clinical experience with TNF modulators has demonstrated severe toxicity, discouraging further development [56]. NGR–hTNF is a compound derived from human TNF (hTNF) and the tumor homing peptide NGR; the mechanism of action of NGR–hTNF incorporates the antitumor properties of TNF as well as increased vascular permeability. NGR–hTNF is an attractive molecule to develop with the intention of being combined with traditional chemotherapy. It has been studied in a Phase I trial [57] and the established safe dose has been used in two Phase II trials for patients with mCRC. One of these trials included 33 previously treated mCRC patients treated with NGR–hTNF. The DCR was 39.4%, including one PR, with a median PFS and OS of 2.5 and 13.1 months, respectively [58]. The second study combined NGR–hTNF with XELOX. Thus, 24 patients were treated in two cohorts, one cohort with a low dose and the other with a high dose of NGR–hTNF. PFS rates of 50% (low-dose cohort) and 33% (high-dose cohort) were observed at 3 months [59]. This drug is likely to be incorporated in more definitive trials for patients with mCRC.
IGF-1
The IGF-1 pathway has been implicated in the carcinogenesis of many solid tumors. The IGF-1 receptor belongs to the insulin receptor family. IGF-1 binding induces IGF receptor (IGFR) dimerization, and autophosphorylation of tyrosine residues. In mCRC, induction of the IGF-1 pathway is believed to be a mechanism of secondary resistance to EGFR inhibitors, making IGF-1, or the IGFR, a promising target. However, the development of mAbs against the IGFR has been disappointing. IMC-A12 is a human monoclonal IgG1 antibody that binds with high affinity to the IGFR1, and one Phase II trial randomized 64 patients with mCRC to IMC-A12 alone or IMC-A12 plus cetuximab. There was only one PR reported, which was in the combination arm [60]. Nevertheless, with better patient selection, dual antagonism of the EGFR and the IGFR may have a role in mCRC.
mTOR inhibitors
mTOR is a serine–threonine kinase member of the PI3K–Akt family [61]. The activation of mTOR in response to mitogenic stimuli results in phosphorylation of eukaryotic initiation factor 4E-binding protein and p70s6 kinase, resulting in increased cell proliferation, decreased apoptosis and neoangiogenesis. mTOR also integrates signaling through the VEGF–VEGFR, HIF-1 and HER family receptors, by acting as a common mediator. mTOR inhibition leads to cell cycle arrest and suppression of VEGF production, and also blocks proliferation of endothelial and vascular smooth muscle cells. mTOR inhibitors are thought to have the ability to overcome the over-activation of VEGFR and Akt signaling that is observed following EGFR inhibition [62]. Thus, the addition of an mTOR inhibitor may overcome resistance to EGFR inhibitors. Two mTOR inhibitors are now approved for use, with several more in development. The first of these drugs was temsirolimus, which is FDA approved for the treatment of advanced renal cell cancer [63]. Everolimus (RAD-001) is FDA approved for the treatment of renal cell cancer and pancreatic neuroendocrine tumors. In a Phase I trial of everolimus (RAD-001) as a single agent, there was one PR [64]. However, it is believed that mTOR inhibitors will be more effective in CRC when combined with traditional chemotherapy. For example, a Phase I trial of temsirolimus with 5-FU in refractory mCRC showed one PR lasting 7.4 months [65]. We have recently completed a Phase I trial combining temsirolimus with capecitabine, in which 25 out of 36 patients had highly refractory CRC. We demonstrated a 2-month DCR (CR, PR or SD) of 59% for the entire population, with 22% of patients exhibiting disease control for more than 6 months [66]. In addition, mTOR inhibitors have also been combined with bevacizumab and the EGFR mAbs. For example, in a Phase II trial of everolimus combined with bevacizumab in previously treated mCRC patients, a minor response in 16% of patients was observed, although 8% of patients exhibited grade 3–4 toxicity [67]. Howard et al. presented a Phase I trial of bevacizumab, everolimus and panitumumab in patients with advanced solid tumors [68]. While there were notable toxicities, including grade 3/4 rash and mucositis, nine out of 18 patients had SD for at least 2 months as a best response, with a median PFS of 5.8 months; some patients experienced SD for over a year. In addition, two PRs were seen. Therefore, the combination of mTOR inhibitors and other mAbs appears to have significant efficacy, but with concomitant toxicity that could be responsive to dose and schedule modifications.
Conclusion
The present review describes only published, or publicly presented, clinical trial results of promising targeted agents for patients with mCRC. However, only a small fraction of the novel approaches under investigation are represented here. The targeting of EGFR and VEGF pathways has been a success and, with increasing comprehension of the need for personalized medicine, additional biomarkers may further define the patients who will benefit the most from any particular therapy. A dependence on tumor biomarkers will increase our dependence on valid tumor tissue samples, cell signaling pathway analysis, and the determination of which signaling pathways tumors are dependent upon (oncogene addiction). Furthermore, an increase in the understanding of the molecular biology of tumors will result in the incorporation of a number of promising new drugs into the therapeutic armamentarium. Only a combined effort to discover new biomarkers, design better translational studies, and consider the specific characteristics of a tumor and the patient will enable the development of innovative treatments with the hope of not only controlling tumor growth but also attempting to cure cancers in their advanced stages. We conclude as follows:
Improvement has been made in the last 15 years in the treatment of mCRC. Targeted therapy is now commonly used in first-, second- and third-line treatments;
Bevacizumab is the only antiangiogenesis therapy that is FDA approved. Bevacizumab targets VEGF directly. Two newer agents are likely to gain FDA approval in 2012: regorafenib (a broad-spectrum TKI) and aflibercept (a fusion molecule that targets VEGF-A and -B and PDGF-1 and -2). Both of these agents showed promising results in Phase III trials: the global Phase III CORRECT (regorafenib) [29] and the VELOUR study (aflibercept) [20];
Cetuximab and panitumumab are mAbs that target the EGFR and both have been FDA approved for use in mCRC. KRAS is a predictive biomarker that guides the use of these drugs. New data suggest the need to revise the value of current biomarkers;
There are a number of other promising novel targeted agents currently being developed for the treatment of mCRC.
Future perspective
We believe that the development and use of targeted agents in mCRC is a key to improving the outcome of this disease. mCRC clinical research is evolving towards more rational trial design – targeted agents need to show their biological effects at early stages in their development. This is today's Phase 0 study paradigm.
We conceive that the use of biomarkers for mCRC will increase in the years to come; we will understand the molecular subtypes of mCRC in more detail and have a better grasp on the primary and secondary mechanisms of resistance to targeted agents.
It is envisaged that pharmacogenomics will play an increasingly more important role in the design of the best treatment for each patient as an individual.
Finally, the exciting new field of cancer genomics and sequencing will assist in the discovery of new signaling pathways or networks that play a part in the growth and spread of mCRC.
Practice points.
Metastatic colorectal cancer is an evolving field that requires permanent attention to the latest advances related to prognosis, predictive factors and new treatments.
The targeted agents currently approved for metastatic colorectal cancer include the monoclonal antibodies bevacizumab, which targets tumor angiogenesis, and cetuximab and panitumumab, which block the EGF receptor pathway.
Regarding bevacizumab, there is no validated biomarker that predicts positive or negative antitumor efficacy. The use of this drug is based on the benefit demonstrated in clinical trials, involving the general metastatic colorectal cancer population. However, any physician considering patient treatment with bevacizumab cannot ignore its adverse effects.
In relation to cetuximab and panitumumab, the current recommendation is to check for the mutation status of KRAS. If the patient's tumor expresses the wild-type gene, there is potential benefit to be gained from the use of these drugs, whereas if KRAS is mutated there is no benefit expected.
New targeted agents that are undergoing US FDA review with the possibility of approval in the near future include regorafenib, a tyrosine kinase inhibitor with a broad spectrum of action, and aflibercept, a recombinant fusion molecule that blocks VEGF.
Acknowledgments
The authors would like to thank Katya Selinevich for providing the illustrations.
Footnotes
Financial & competing interests disclosure: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Thirion P, Michiels S, Pignon JP, et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22(18):3766–3775. doi: 10.1200/JCO.2004.03.104. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26(4):689–690. doi: 10.1200/JCO.2007.15.5390. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 7.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Excellent review article about the role of angiogenesis in cancer.
- 8.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 9.Cascinu S, Staccioli MP, Gasparini G, et al. Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin Cancer Res. 2000;6(7):2803–2807. [PubMed] [Google Scholar]
- 10.Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36(6):748–753. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 11.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19(3):843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]; ▪▪ First Phase III trial to demonstrate an improvement in overall survival with a targeted agent in metastatic colorectal cancer (mCRC). Based on this trial, the US FDA approved bevacizumab in mCRC.
- 13.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized Phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 14.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26(33):5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 15.Arnold D, Andre T, Bennouna J, et al. Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first progression in patients with metastatic colorectal cancer (mCRC) previously treated with BEV plus CT: results of a randomized Phase III intergroup study (TML study) J Clin Oncol. 2012;30(Suppl) Abstract CRA3503. [Google Scholar]; ▪ This trial, recently presented at the American Society of Clinical Oncology 2012, will likely lead to FDA approval of bevacizumab in the second-line setting.
- 16.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29(1):11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Lambrechts D, Prenen H, et al. Lessons from the adjuvant bevacizumab trial on colon cancer: what next? J Clin Oncol. 2011;29(1):1–4. doi: 10.1200/JCO.2010.32.2701. [DOI] [PubMed] [Google Scholar]
- 18.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang P, Cohen SJ, Bjarnason GA, et al. Phase II trial of aflibercept (VEGF Trap) in previously treated patients with metastatic colorectal cancer (MCRC): a PMH Phase II consortium trial. J Clin Oncol. 2008;26(Suppl) Abstract 4027. [Google Scholar]
- 20.Van Cutsem E, Tabernero J, Lakomý R, et al. Intravenous (IF) aflibercept versus placebo in combination with irinotecan/5-FU (FOLFIRI) for second-line treatment of metastatic colorectal cancer (MCC): results of a multinational Phase III trial (EFC10262-VELOUR). ESMO 13th World Congress on Gastrointestinal Cancer; Barcelona, Spain. 22–25 june 2011. [Google Scholar]; ▪▪ Randomized Phase III trial demonstrates a survival benefit with aflibercept in the second-line setting, and will likely lead to FDA approval.
- 21.Hecht JR, Trarbach T, Jaeger E, et al. A randomized, double-blind, placebocontrolled, Phase III study in patients with metastatic adenocarcinoma of the colon or rectum receiving first-line chemotherapy with oxaliplatin/5-fluorouracil/leucovorin and PTK787/ZK 222584 or placebo (CONFIRM-1) J Clin Oncol. 2005;23(Suppl. 16):3. [Google Scholar]
- 22.Koehne C, Bajetta E, Lin E, et al. Results of an interim analysis of a multinational randomized, double-blind, Phase III study in patients with previously treated metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK787/ZK 222584 (PTK/ZK) or placebo (CONFIRM 2) J Clin Oncol. 2006;24(Suppl. 18):3508. [Google Scholar]
- 23.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saltz LB, Rosen LS, Marshall JL, et al. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25(30):4793–4799. doi: 10.1200/JCO.2007.12.8637. [DOI] [PubMed] [Google Scholar]
- 25.Kim T, Saunders M, Salazar R, et al. A randomized, double-blind, placebo-controlled Phase II study of vandetanib plus FOLFIRI in patients with advanced colorectal cancer (CRC). 2009 Gastrointestinal Cancers Symposium; San Fransisco, CA, USA. 15–17 January 2009; Abstract 188. [Google Scholar]
- 26.Robertson JD, Botwood NA, Rothenberg ML, Schmoll HJ. Phase III trial of FOLFOX plus bevacizumab or cediranib (AZD2171) as first-line treatment of patients with metastatic colorectal cancer: HORIZON III. Clin Colorectal Cancer. 2009;8(1):59–60. doi: 10.3816/CCC.2009.n.010. [DOI] [PubMed] [Google Scholar]
- 27.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 28.Strumberg D, Scheulen ME, Frost A, et al. Phase I study of BAY 73-4506, an inhibitor of oncogenic and angiogenic kinases, in patients with advanced refractory colorectal carcinoma (CRC) J Clin Oncol. 2009;27(Suppl. 15) Abstract 3560. [Google Scholar]
- 29.Grothey A, Sobrero AF, Siena S, et al. Results of a Phase III randomized, double-blind, placebo-controlled, multicenter trial (CORRECT) of regorafenib plus best supportive care (BSC) versus placebo plus BSC in patients (pts) with metastatic colorectal cancer (mCRC) who have progressed after standard therapies. J Clin Oncol. 2012;30(Suppl. 4) Abstract LBA385. [Google Scholar]; ▪▪ Randomized Phase III trial demonstrates a survival benefit of regorafenib for refractory mCRC, and will likely lead to FDA approval, and the incorporation of regorafenib into the treatment algorithm for all patients with mCRC.
- 30.Saif MW. Colorectal cancer in review: the role of the EGFR pathway. Expert Opin Investig Drugs. 2010;19(3):357–369. doi: 10.1517/13543781003593962. [DOI] [PubMed] [Google Scholar]
- 31.Marshall J. Clinical implications of the mechanism of epidermal growth factor receptor inhibitors. Cancer. 2006;107(6):1207–1218. doi: 10.1002/cncr.22133. [DOI] [PubMed] [Google Scholar]; ▪ Comprehensive review of the EGF receptor pathway in cancer with emphasis on the clinical implications.
- 32.Vincenzi B, Zoccoli A, Pantano F, et al. Cetuximab: from bench to bedside. Curr Cancer Drug Targets. 2010;10(1):80–95. doi: 10.2174/156800910790980241. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]; ▪▪ First landmark Phase III trial study that demonstrates an increase in overall survival with an anti-EGF receptor monoclonal antibody in mCRC.
- 34.Saltz LB, Meropol NJ, Loehrer PJ, Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 35.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]; ▪▪ Based on this Phase III trial, the evaluation of KRAS mutational status began to be used as a predictive biomarker of response to cetuximab and panitumumab.
- 36.Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised Phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (nordic flox) versus flox alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30(15):1755–1762. doi: 10.1200/JCO.2011.38.0915. [DOI] [PubMed] [Google Scholar]
- 38.Gibson TB, Ranganathan A, Grothey A. Randomized Phase III trial results of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6(1):29–31. doi: 10.3816/CCC.2006.n.01. [DOI] [PubMed] [Google Scholar]
- 39.Douillard JY, Siena S, Cassidy J, et al. Randomized, Phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 40.Peeters M, Price TJ, Cervantes A, et al. Randomized Phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706–4713. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 41.De Roock W, De Vriendt V, Normanno N, et al. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011;12(6):594–603. doi: 10.1016/S1470-2045(10)70209-6. [DOI] [PubMed] [Google Scholar]
- 42.Lievre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 43.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 44.Grothey A, Lenz HJ. Explaining the unexplainable: EGFR antibodies in colorectal cancer. J Clin Oncol. 2012;30(15):1735–1737. doi: 10.1200/JCO.2011.40.4194. [DOI] [PubMed] [Google Scholar]; ▪ Outstanding analysis of the state of using KRAS mutational status and the use of anti-EGF receptor monoclonal antibodies in mCRC.
- 45.Hecht JR, Mitchell E, Chidiac T, et al. A randomized Phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 46.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 47.Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30(8):1426–1447. doi: 10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Fields AL, Rinadli DA, Henderson CA, et al. An open-label multicenter Phase II study of oral lapatinib (GW572016) as single agent, second-line therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2005;23(Suppl. 16):3583. [Google Scholar]
- 49.Weickhardt AJ, Price TJ, Chong G, et al. Dual targeting of the epidermal growth factor receptor using the combination of cetuximab and erlotinib: preclinical evaluation and results of the Phase II DUX study in chemotherapy-refractory, advanced colorectal cancer. J Clin Oncol. 2012;30(13):1505–12. doi: 10.1200/JCO.2011.38.6599. [DOI] [PubMed] [Google Scholar]
- 50.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11(1–2):32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11(2):102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rychahou PG, Kang J, Gulhati P, et al. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci USA. 2008;105(51):20315–20320. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bendell JC, Nemunaitis J, Vukelja SJ, et al. Randomized placebo-controlled Phase II trial of perifosine plus capecitabine as second- or third-line therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2011;29(33):4394–4400. doi: 10.1200/JCO.2011.36.1980. [DOI] [PubMed] [Google Scholar]
- 54.Bendell JC, Ervin TJ, Senzer NN, et al. Results of the X-PECT study: a Phase III randomized double-blind, placebo-controlled study of perifosine plus capecitabine (P-CAP) versus placebo plus capecitabine (CAP) in patients (pts) with refractory metastatic colorectal cancer (mCRC) J Clin Oncol. 2012;30(Suppl) Abstract LBA3501. [Google Scholar]; ▪ This trial was presented at the American Society of Clinical Oncology 2012 and is significant for the surprisingly negative results. Perifosine was highly anticipated to become part of the armamentarium for the treatment of mCRC.
- 55.Watson AJ. An overview of apoptosis and the prevention of colorectal cancer. Crit Rev Oncol Hematol. 2006;57(2):107–121. doi: 10.1016/j.critrevonc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Senzer N, Mani S, Rosemurgy A, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a Phase I study in patients with solid tumors. J Clin Oncol. 2004;22(4):592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- 57.Gregorc V, Citterio G, Vitali G, et al. Defining the optimal biological dose of NGR-hTNF, a selective vascular targeting agent, in advanced solid tumours. Eur J Cancer. 2010;46(1):198–206. doi: 10.1016/j.ejca.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Santoro A, Rimassa L, Sobrero AF, et al. Phase II study of NGR-hTNF, a selective vascular targeting agent, in patients with metastatic colorectal cancer after failure of standard therapy. Eur J Cancer. 2010;46(15):2746–2752. doi: 10.1016/j.ejca.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Mammoliti S, Andretta V, Bennicelli E, et al. Two doses of NGR-hTNF in combination with capecitabine plus oxaliplatin in colorectal cancer patients failing standard therapies. Ann Oncol. 2011;22(4):973–978. doi: 10.1093/annonc/mdq436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reidy DL, Vakiani E, Fakih MG, et al. Randomized, Phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28(27):4240–4246. doi: 10.1200/JCO.2010.30.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13(11):3109–3114. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- 62.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 63.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 64.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a Phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26(10):1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 65.Punt CJ, Boni J, Bruntsch U, et al. Phase I and pharmacokinetic study of CCI-779, a novel cytostatic cell-cycle inhibitor, in combination with 5-fluorouracil and leucovorin in patients with advanced solid tumors. Ann Oncol. 2003;14(6):931–937. doi: 10.1093/annonc/mdg248. [DOI] [PubMed] [Google Scholar]
- 66.Pishvaian MJ, Wang H, Hardesty RA, et al. A Phase I trial of the mTOR inhibitor temsirolimus (TEM) in combination with capecitabine (CAP) in patients with advanced malignancies. J Clin Oncol. 2012;30(Suppl) Abstract 3095. [Google Scholar]
- 67.Altomare I, Bendell JC, Bullock KE, et al. A Phase II trial of bevacizumab plus everolimus for patients with refractory metastatic colorectal cancer. Oncologist. 2011;16(8):1131–1137. doi: 10.1634/theoncologist.2011-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howard LA, Bullock KE, Bendell JC, et al. Bevacizumab (B) plus everolimus (E) and panitumumab (P) in refractory advanced solid tumors. J Clin Oncol. 2009;27(Suppl):15s. Abstract 3551. [Google Scholar]
- 69.El Zouhairi M, Charabaty A, Pishvaian MJ. Molecularly targeted therapy for metastatic colon cancer: proven treatments and promising new agents. Gastrointest Cancer Res. 2011;4(1):15–21. [PMC free article] [PubMed] [Google Scholar]
Website
- 101.AstraZeneca Announces Results of Recentin HORIZON II Phase III Trial in Metastatic Colorectal Cancer. www.astrazeneca.com/Media/Press-releases/Article/20100528--AstraZeneca-Announces-Results-of-Recentin-HORIZON-II-


