Abstract
Background
In sub-Saharan Africa, most HIV-infected patients receive antiretroviral therapy (ART) without virological monitoring. Longitudinal data on secondary resistance are rare.
Methods
We conducted a prospective cohort study of HIV-1 infected adults initiating ART in three clinics using computerized monitoring systems. Patients had plasma HIV-1 RNA viral load (VL) tests at month 12 (M12) and month 24 (M24) following ART initiation, and HIV-1 resistance genotype tests if VL was detectable (≥ 300 copies/ml).
Results
Overall, 1,573 patients initiated ART with stavudine/zidovudine plus lamivudine plus nevirapine/efavirenz. At M12 and M24, 944 and 844 patients, respectively, remained in active follow-up. Among them: 25% (M12) and 27% (M24) had detectable VLs, and 12% (M12) and 19% (M24) had virus resistant to at least one antiretroviral drug, accounting for 54% (M12) and 75% (M24) of patients with detectable VLs. Among the resistant strains, 95% (M12) and 97% (M24) were resistant to lamivudine/emtricitabine, efavirenz and/or nevirapine, the frequency of thymidine analog mutations (TAMs) increased from 8.1% (M12) to 14.7% (M24) and etravirine resistance increased from 13.5% (M12) to 24.5% (M24).
Conclusion
Of the patients with detectable VLs at M24, 25% still did not harbor resistant virus. Preventing mutations from emerging with adherence reinforcement in patients with detectable VLs remains important beyond M24. Switching therapy early in patients with resistance to 3TC/FTC and/or to NNRTIs to prevent extended resistance to NRTIs and etravirine resistance from occurring is also a major challenge.
INTRODUCTION
From 2004 through 2011, 5.5 million adults and children initiated antiretroviral treatment in sub-Saharan Africa 1. To reach this unprecedented level of success, physicians, scientists, patients, public-health authorities, and the entire community had to learn how to monitor ART under the routine conditions of large-scale programs in settings with limited facilities.
This success comes with the further need to address numerous major challenges. These challenges include: 1) how to achieve universal ART coverage when only approximately 50% of patients eligible for ART had accessed to it in 2011; 2) how to get patients who initiated ART to stay in care and take their drugs with maximal adherence 2; and 3) how to detect treatment failure and decide what to do, whether reinforcing adherence or switching to second-line therapies. 3,4,5
The facilities required to initiate ART are not the same as the facilities required to address the challenge of detecting early failure. It is quite easy to initiate ART in a setting with no access to viral load monitoring and even no access to CD4 counts 6–8. However, it is difficult to decide who needs to switch to an advanced level of therapy with no access to HIV-1 viral load or genotypic resistance testing. One aspect of the problem is how to balance the objective of switching “early enough” in patients harboring resistant viruses to prevent resistance mutations from accumulating against switching “too early” in patients who are failing treatment but whose viruses are still sensitive to the drugs they are taking.9–11
Describing the rate, pattern and order of appearance of resistance mutations and drug susceptibilities that arise during the first years of treatment may help inform these issues. Because access to resistance genotype testing is still very limited in sub-Saharan Africa, data on the emergence of resistance mutations over time are rare in program databases.
In 2006, we launched a prospective cohort study on HIV-infected adults who initiated ART at three HIV care centers equipped with computerized prescription databases in Abidjan, the economic capital of Côte d’Ivoire, West Africa.
We describe here the incidence of virological suppression and the incidence and pattern of resistance mutations and resistance to ARV drugs at 12 and 24 months of treatment in this routine-care cohort.
METHODS
The VOLTART cohort
We conducted a prospective cohort study of long-term virological outcomes on ART (VOLTART cohort).12,13 HIV-1 positive and HIV-1/2 positive adults who initiated ART between February 2006 and May 2007 at one of three HIV outpatient clinics in Abidjan and returned for their six-month visit were eligible for the study. HIV-2 positive patients were not eligible. Study subjects received the same standard care and treatment as other HIV-infected patients on ART at their respective clinics.
When we launched the cohort, plasma HIV-1 viral load testing and genotype tests were not available in routine in Côte d’Ivoire. We did as much as possible to make viral load measurement available in real-time every six months whenever this was feasible, and to get retrospective viral load measurements on frozen sample collected on a six-month basis otherwise.
Patients
We previously reported the evolution of plasma HIV-1 RNA and resistance tests between the 6-month (M6) and the 12-month (M12) visit for all patients enrolled in the VOLTART cohort, and showed that the medication possession ratio was strongly associated with virologic outcomes at both visits. Here, we report the evolution of virologic outcomes between the 12-month (M12) and the 24-month (M24) visits for all patients, with special attention being paid to resistance mutations. The study period was from February 2006 (first enrollment) to May 2009 (month-24 visit of the last patient).
Standard care and treatment
The standard of HIV care for HIV-1 infected adults on ART in Côte d’Ivoire has been described elsewhere. 12 All patients followed from the three study clinics initiated ART according to the 2003–2006 recommendations of the World Health Organization 14. Chemistry exams (i.e., serum creatinine and transaminase levels) were performed prior to ART initiation. When patients were HIV-1 infected, first-line ART consisted of two nucleoside reverse transcriptase inhibitors (NRTIs zidovudine or stavudine plus lamivudine) and one non-nucleoside reverse transcriptase inhibitor (NNRTI; efavirenz or nevirapine). When patients were HIV-2 or HIV-1 and HIV-2 infected, first-line ART consisted of two NRTIs and one protease inhibitor (PI; lopinavir/ritonavir). CD4 counts, and complete blood counts were measured every six months. Patients paid a fixed rate of US$2 per month for antiretroviral drugs and laboratory tests until August 2008, when the national HIV program made them available free. All patients with CD4 counts ≤500/mm3 were also given cotrimoxazole prophylaxis. Isoniazid (INH) prophylaxis was not recommended, as it is not part of the national treatment guidelines. Support groups were organized to encourage patients to adhere to therapy, and a community-based team made telephone calls or home visits when patients did not show up for clinic visits or to pick up antiretroviral drugs. 8
Monitoring and tracking system
All three study centers used the same standardized forms to record the following variables during routine visits: (i) initial visit: date, sex, date of birth (or age), height, weight, HIV type (HIV-1, HIV-2, or both); (ii) follow-up visit: date, weight; (iii) ART initiation visit: date, WHO clinical stage, weight; (iv) drug prescription (antiretroviral or other): date, name and quantity of drugs delivered; (v) CD4 count and complete blood count measurement: date, CD4 count, CD4 percentage, hemoglobin level, and platelet, granulocyte and leukocyte counts; (vi) telephone call and home visit: dates on which patients were contacted and vital status on that date; (vii) patients known to have died: date of death.
Additional procedures
The care provided to patients who agreed to participate in the study differed from that provided to other patients at the same clinic and from the Côte d’Ivoire National AIDS Control Program guidelines in three ways.
First, real-time viral load measurement was obtained in one of the three participating centers thanks to the partnership we could establish with ESTHER, a French government program. The remaining two centers did not get direct funding for viral load testing and had to freeze plasma samples until further funding was found. In these two centers, M12 and M24 tests were then retrospectively funded by the ANRS, once all patients had already been followed more than 24 months. In all patients, plasma HIV-1 RNA tests were performed using ANRS real-time PCR (Biocentric, Bandol, France; detection threshold of 300 copies/ml).27
Second, if HIV-1 RNA exceeded 300 copies/ml, then genotypic resistance tests were performed using the consensus technique of the AC11 ANRS resistance group. 28 All genotype tests were made retrospectively, on frozen samples. The interpretation of drug resistance was made according to version 2.2 of the ANRS algorithm (http://www.hivfrenchresistance.org/2012/Algo-sep-2012.pdf). Genotypic resistance tests were performed in the virology laboratory of the Necker Hospital in Paris, France, which undergoes an annual external quality assurance evaluation. 29
Finally, a research coordinator was devoted to monitoring and managing the cohort data and to tracking patients by telephone and/or home visit.
Statistical analysis
Patients were defined as lost-to-follow-up if: (i) their last contact with the study team was <month 24; (ii) they were not known to be dead or transferred out before month 24; or (iii) no further information on their vital status could be obtained within the 6 months following the study endpoint (i.e., between months 24 and 30).
The medication possession ratio (MPR) was defined as the number of daily doses of antiretroviral drugs dispensed by the pharmacy to each patient divided by that patient’s total follow-up time in days since ART initiation.
Analyses were performed using the SAS® software, version 9.2 (SAS institute Inc., Cary, North Carolina, USA).
RESULTS
Patients
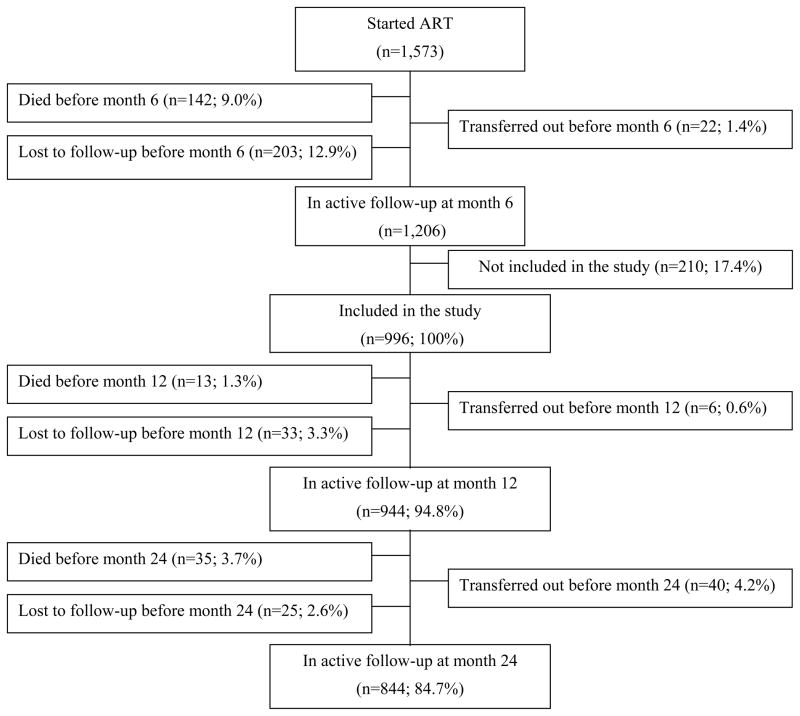
Overall, 1,573 adults initiated ART at the three study centers between February 2006 and May 2007. At M6, 1,206 patients were still alive and in care. Of these, 996 (HIV-1 positive n=944 [94.8%]; HIV-1/2 positive n=22 [2.2%]; HIV positive with no subtype discrimination n=30 [3.0%]) gave written informed consent to participate in the cohort. At M24, 844 (85%) were still alive and in active follow-up, 48 were dead, 58 were lost-to-follow-up, and 36 had been transferred out (Figure 1).
Figure 1.
Flow Chart
The median baseline age for the cohort was 36 years (interquartile range [IQR], 30–43), and 747 patients (75%) were women. Table 1 presents the main characteristics of the study participants at ART initiation, M12 and M24.
Table 1.
Summary of key characteristics at ART initiation, month 12 and month 24
| ART initiation (M0) | Month 12 (M12) | Month 24 (M24) | ||||
|---|---|---|---|---|---|---|
| n=996
|
n=944
|
n=844
|
||||
| Regimen, n (%) | ||||||
| d4T 3TC NVP | 582 | (58%) | 440 | (47%) | 361 | (43%) |
| d4T 3TC EFV | 96 | (10%) | 132 | (14%) | 110 | (13%) |
| ZDV 3TC EFV | 254 | (26%) | 281 | (30%) | 260 | (31%) |
| Others | 84 | (6%) | 91 | (9%) | 113 | (13%) |
| Treatment modification, n (%) | ||||||
| One drug only | - | - | 224 | (24%) | 274 | (33%) |
| Entire regimen | - | - | 19 | (2%) | 28 | (3.3%) |
| CD4 count*, cells/mm3 | 148 | (68;229) | 292 | (195;427) | 382 | (246; 522) |
| CD4 change since M0* | - | - | +152 | (+80;+241) | +221 | (+112;+348) |
| CD4 change since M12* | - | - | - | - | +58 | (−28;+153) |
| Body Mass Index*, kg/m2 | 19.8 | (17.8;22.1) | 22.8 | (20.4;25.2) | 22.8 | (20.4;25.8) |
| BMI change since M0* | - | - | +2.6 | (+0.8;+4.6) | +2.6 | (+0.8;+4.8) |
| BMI change since M12* | - | - | - | - | +0.0 | (−1.1;+1.2) |
| Medication Possession Ratio* | - | - | 0.90 | (0.77–0.98) | 0.88 | (0.74;0.96) |
| Plasma HIV-1 RNA, n (%) | ||||||
| Unavailable | 19 | (2%) | 81 | (10%) | ||
| Available | 925 | (98%) | 763 | (90%) | ||
| < 300 copies/ml | - | - | 693 | (75%) | 559 | (73%) |
| ≥ 300 copies/ml | - | - | 232 | (25%) | 204 | (27%) |
| Genotype tests | ||||||
| Non available ** | 25 | (11%) | 13 | (6%) | ||
| Available | 207 | (89%) | 191 | (94%) | ||
| No mutation | 96 | (46%) | 48 | (25%) | ||
| At least one mutation | 111 | (54%) | 143 | (75%) | ||
ART: antiretroviral therapy; NRTI: nucleoside reverse transcriptase inhibitor; d4T: stavudine; 3TC: lamivudine; EFV: efavirenz; NVP: nevirapine; ZDV: zidovudine.
BMI: body mass index.
Median (interquartile range [IQR]).
Including: at M12, 25 with test performed but amplification impossible; at M24, 8 with test performed but amplification impossible, and 4 with test not performed.
Other missing values:
- At M0 (among 996 included patients): weight, n=3 (0.3%).
- At M12 (944 patients who were neither dead nor lost-to-follow-up before M12): CD4, n=26 (2.8%); weight, n=23 (2.4%).
- At M24 (among 844 patients who were neither dead nor lost-to-follow-up before M24): CD4, n=64 (8%); weight, n=75 (9%).
Plasma HIV-1 RNA
We obtained HIV-1 RNA results for 925 patients at M12 and 763 patients at M24. At M12, 25% of the patients with available tests had HIV-1 RNA ≥300 copies/ml; among them, 85% had HIV-1 RNA ≥1,000 copies/ml. At M24, 27% of the patients with available tests had HIV-1 RNA ≥300 copies/ml; among them, 87% had HIV-1 RNA ≥1,000 copies/ml (Table 1).
Of the 169 patients who had HIV-1 RNA ≥300 copies/ml at M12 and HIV-1 RNA test results at M24, 35% had undetectable viral loads at M24. Of the 585 patients who had undetectable viral loads at M12 and HIV-1 RNA test results at M24, 15% had HIV-1 RNA ≥300 copies/ml at M24. Of the 204 patients who had HIV-1 RNA ≥300 copies/ml at M24, 110 (54%) already had HIV-1 RNA ≥300 copies/ml at M12 and thus remained on a failed ART regimen for more than 6 months (Table 2).
Table 2.
Virological status at months 12 and 24 (n= 944)
| Month 12 | Month 24
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (Col%) | Viral load
|
Genotype
|
|||||||||
| NA | Undetectable | Detectable | NA | No resistance | Any resistance* | |||||||
| N | N | (Row%) | N | (Row%) | N | N | (Row%) | N | (Row%) | |||
|
|
|
|
|
|
|
|||||||
| Overall | 944 | 181 | 559 | (73%) | 204 | (27%) | 13 | 48 | (25%) | 143 | (75%) | |
| VL unavailable | 19 | - | 10 | 5 | (56%) | 4 | (44%) | 0 | 2 | (50%) | 2 | (50%) |
| VL undetectable | 693 | (75%) | 108 | 495 | (85%) | 90 | (15%) | 8 | 27 | (33%) | 54 | (67%) |
| VL detectable | 232 | (25%) | 63 | 59 | (35%) | 110 | (65%) | 4 | 19 | (18%) | 87 | (82%) |
| Genotype | ||||||||||||
| NA | 25 | - | 3 | 15 | (68%) | 7 | (32%) | 1 | 1 | (17%) | 5 | (83%) |
| No resistance | 96 | (46%) | 26 | 36 | (51%) | 34 | (49%) | 3 | 15 | (48%) | 16 | (52%) |
| Any resistance* | 111 | (54%) | 34 | 8 | (10%) | 69 | (90%) | 0 | 3 | (4%) | 66 | (96%) |
N: number of patients.
NA: unavailable (patients dead, lost-to-follow-up or transferred out, or in active follow-up with test not performed).
Col%: the percentage of patients with detectable and undetectable viral load at M12 are among those with available viral load measurements (N=925); the percentage of patients with wild-type strains and resistant strains at M12 are among those with available genotype results (N=207).
Row%: the percentage of patients with detectable and undetectable viral load at M24 are among those with available viral load measurements (N=763); the percentage of patients with no resistance and resistance at M24 are among those with available genotype results (N=191).
Resistance to antiretroviral drugs
At M12, 111 patients were infected with HIV-1 virus, which was resistant to at least one antiretroviral drug. These patients represented 12% of the 925 patients with available HIV-1 RNA tests and 54% of the 207 patients with HIV-1 RNA ≥300 copies/ml (Table 3).
Table 3.
Resistance to drugs
| Month 12 | Month 24 | |||||
|---|---|---|---|---|---|---|
| N | % detect (N=207) | % pop (N =925) | N | % detect (N =191) | % pop (N =763) | |
|
|
|
|||||
| Resistance to at least 1 drug | 111 | 54% | 12% | 143 | 75% | 19% |
| By groups | ||||||
| Resistance to one class | 44 | 21% | 5% | 38 | 20% | 5% |
| 3TC/FTC | 7 | 3% | 1% | 6 | 3% | <1% |
| NVP | 1 | <1% | <1% | 1 | 1% | <1% |
| EFV/NVP | 29 | 14% | 3% | 26 | 14% | 4% |
| EFV/NVP/ETR | 1 | <1% | <1% | 2 | 1% | <1% |
| ETR | 5 | 2% | <1% | 3 | 1.6% | <1% |
| ZDV/d4T | 1 | <1% | <1% | 0 | 0% | 0% |
| Resistance to 3TC/FTC + NNRTIs | 53 | 26% | 6% | 84 | 44% | 11% |
| 3TC/FTC + NVP | 2 | <1% | <1% | 4 | 2% | <1% |
| 3TC/FTC + EFV/NVP | 45 | 22% | 5% | 58 | 31% | 8% |
| 3TC/FTC + NVP/ETR | 0 | 0% | 0% | 1 | 1% | <1% |
| 3TC/FTC + EFV/NVP/ETR | 6 | 3% | <1% | 21 | 11% | 3% |
| Others | 14 | 7% | 1.5% | 21 | 11% | 3% |
| 3TC/FTC + ZDV/d4t + EFV/NVP | 4 | 2% | <1% | 9 | 5% | 1% |
| 3TC/FTC + ZDV/d4t + EFV/NVP/ETR | 1 | <1% | <1% | 2 | 2% | <1% |
| 3TC/FTC +d4t+NVP | 0 | 0% | 0% | 1 | 1% | <1% |
| 3TC/FTC +d4t+EFV/NVP/ETR | 1 | <1% | <1% | 1 | 1% | <1% |
| 3TC/FTC +ZDV/d4T+ETR | 0 | 0% | 0% | 1 | 1% | <1% |
| 3TC/FTC + ddI + EFV/NVP/ETR | 0 | 0% | 0% | 1 | 1% | <1% |
| 3TC/FTC + ZDV/d4t + TDF + ABC + EFV/NVP | 1 | <1% | <1% | 3 | 2% | <1% |
| 3TC/FTC + TDF + ddI + ABC + EFV/NVP | 1 | <1% | <1% | 0 | 0% | 0% |
| XTC + ZDV/d4t + TDF + EFV/NVP/ETR | 0 | 0% | 0% | 1 | 1% | <1% |
| XTC + ddI + ABC + EFV/NVP | 2 | 1% | <1% | 0 | 0% | 0% |
| XTC + ddI + ABC + EFV/NVP/ETR | 1 | <1% | <1% | 0 | 0% | 0% |
| XTC + ZDV/d4t + ddI + ABC + EFV/NVP/ETR | 0 | 0% | 0% | 1 | 1% | <1% |
| XTC + d4t + ddI + ABC + TDF + EFV/NVP/ETR | 0 | 0% | 0% | 1 | 1% | <1% |
| Miscellaneous * | 3 | 1.5% | <1% | 0 | 0% | 0% |
| By drugs | ||||||
| NVP | 97 | 47% | 10% | 133 | 70% | 17% |
| EFV | 94 | 45% | 10% | 126 | 66% | 17% |
| XTC | 72 | 35% | 8% | 111 | 58% | 15% |
| ETR | 15 | 7% | 2% | 35 | 18% | 5% |
| d4T | 9 | 4% | 1% | 20 | 9% | 2% |
| ZDV | 8 | 4% | 1% | 17 | 9% | 2% |
| TDF | 2 | 1% | 0% | 5 | 3% | 1% |
| ABC | 5 | 2% | 1% | 5 | 3% | 1% |
| ddI | 5 | 2% | <1% | 3 | 1% | <1% |
| IDV | 1 | 0% | 0% | 0 | 0% | 0% |
| NFV | 1 | 0% | 0% | 0 | 0% | 0% |
% detect: percentage of patients with resistant virus among those who have a viral load 300 copies/ml and who have a genotype test available.
% pop: percentage of patients with resistant virus among those who have a viral load test available.
NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; ZDV/d4T: zidovudine and/or stavudine; 3TC/FTC: lamivudine or emtricitabine; EFV: efavirenz; NVP: nevirapine; ETR: etravirine; ddi: didanosine; ABC: abacavir; TDF: tenofovir; IDV: indinavir; NFV: nelfinavir.
Miscellaneous at M12: ddI, NVP/EFV, n=1; 3TC/FTC, IDV, NFV, n=1; ZDV/d4T, NVP/EFV, n=1.
Of the 111 resistant viruses, 105 (95%) were resistant to lamivudine/emtricitabine and/or nevirapine/efavirenz (lamivudine/emtricitabine only, n=7; nevirapine/efavirenz only, n=31; both n=67). Nine patients harbored a virus with thymidine analog mutations (TAMs), accounting for 0.97% of the 925 patients with available HIV-1 RNA tests, 4.3% of the 207 patients who had detectable HIV-1 RNA and for 8.1% of the 111 patients harboring a virus resistant to at least one antiretroviral drug. Fifteen patients harbored a virus resistant to etravirine (Y181V n=1; E138K, n=2; Y181C + H221Y, n=4) or possibly resistant to etravirine (E138A/G/Q/R, n=8), accounting for 1.6% of the 925 patients with available HIV-1 RNA tests, 7.2% of the 207 patients with HIV-1 RNA ≥300 copies/ml and 13.5% of the 111 patients harboring a virus resistant to at least one antiretroviral drug (Tables 3 and 4).
Table 4.
Resistance mutations
| Month 12
|
Month 24
|
|||||
|---|---|---|---|---|---|---|
| N | % detect (n=207) | % pop (n=925) | N | % detect (n=191) | % pop (n=763) | |
|
|
|
|||||
| At least one mutation | 111 | 54% | 12% | 143 | 75% | 19% |
| NRTI | 86 | 123 | ||||
| M184V/I | 70 | 34% | 8% | 99 | 52% | 13% |
| T215Y/F | 5 | 2% | 1% | 6 | 3% | 1% |
| K70R, M184V/I | 1 | <1% | <1% | 3 | 2% | 0% |
| V75A/M/S/T | 2 | 1% | <1% | 3 | 2% | <1% |
| M41L, M184V/I, L210W, T215Y/F | 1 | <1% | <1% | 2 | 1% | <1% |
| D67N, K70R, T215A/C/D/E/G/H/I/L/N/S/V, K219Q/E, M184V/I | 0 | 0% | 0% | 2 | 1% | <1% |
| Q151M | 0 | 0% | 0% | 1 | 1% | 0% |
| L74V/I | 4 | 2% | 0% | 1 | 1% | <1% |
| M41L, E44D, M184V/I, L210W, T215Y/F | 0 | 0% | 0% | 1 | 1% | <1% |
| K65R | 1 | <1% | <1% | 1 | 1% | <1% |
| Y115F | 0 | 0% | 0% | 1 | 1% | <1% |
| T215A/C/D/E/G/H/I/L/N/S/V | 2 | 1% | <1% | 0 | 0% | <1% |
| D67N, M184V/I, T215Y/F | 0 | 0% | 0% | 1 | 1% | <1% |
| M41L, M184V/I, T215Y/F | 0 | 0% | 0% | 1 | 1% | <1% |
| D67N, T69D/N/S, K70R, M184V/I, T215Y/F | ||||||
| K219Q/E | 0 | 0% | 0% | 1 | 1% | <1% |
| NNRTI | 146 | 190 | ||||
| K103H/N/S/T | 57 | 28% | 6% | 75 | 39% | 10% |
| G190A/C/E/Q/S/T/V | 14 | 7% | 2% | 23 | 12% | 3% |
| L100I | 2 | 1% | <1% | 0 | 0% | 0% |
| Y181C/I | 17 | 8% | 2% | 16 | 8% | 2% |
| K101E | 8 | 4% | 1% | 13 | 7% | 2% |
| Y181C+H221Y | 4 | 2% | <1% | 11 | 6% | 1% |
| E138A/G/Q/R | 8 | 4% | <1% | 11 | 6% | 1% |
| V106A/M | 8 | 4% | 1% | 8 | 4% | 1% |
| P225H | 9 | 4% | 1% | 6 | 3% | 1% |
| Y188C/L | 9 | 4% | 1% | 5 | 3% | 1% |
| M230L | 6 | 3% | 1% | 5 | 3% | 1% |
| Y188H | 1 | 0% | 0% | 2 | 1% | 0% |
| A98S | 0 | 0% | 0% | 2 | 1% | <1% |
| E138K | 2 | 1% | <1% | 1 | 1% | <1% |
| A98G, K101E/H/I/P/R, V179D/F/I/L/M/T, G190A/S | 0 | 0% | 0% | 1 | 1% | <1% |
| V90I, A98G, Y181C/I, G190A/S, H221Y | 0 | 0% | 0% | 1 | 1% | <1% |
| Y181V | 1 | <1% | <1% | 1 | 1% | <1% |
| V90I, V179D/F/I/L/M/T, Y181C/I | 0 | 0% | 0% | 1 | 1% | <1% |
| V90I, V179D/F/I/L/M/T, Y181C/I, H221Y | 0 | 0% | 0% | 1 | 1% | <1% |
| A98G, K101E/H/I/P/R, Y181C/I | 0 | 0% | 0% | 1 | 1% | <1% |
| A98G, V106I, G190A/S | 0 | 0% | 0% | 1 | 1% | <1% |
| K101E/H/I/P/R, V179D/F/I/L/M/T, G190A/S | 0 | 0% | 0% | 1 | 1% | <1% |
| V179D/F/I/L/M/T, G190A/S, M230L | 0 | 0% | 0% | 1 | 1% | <1% |
| A98G, K101E/H/I/P/R, G190A/S | 0 | 0% | 0% | 1 | 1% | <1% |
| K101E/H/I/P/R, V179D/F/I/L/M/T, Y181C/I | 0 | 0% | 0% | 1 | 1% | <1% |
| A98G, V179D/F/I/L/M/T, Y181C/I | 0 | 0% | 0% | 1 | 1% | <1% |
| Protease Inhibitors | 1 | 0 | ||||
| V82A/F/S/T, M36I, V77I | 1 | <1% | <1% | 0 | 0% | 0% |
% detect: percentage of patients with resistant virus among those who have a viral load >300 copies/ml and who have a genotype test available.
% pop: percentage of patients with resistant virus among those who have a viral load test available.
NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor.
At M24, 143 patients were infected with a virus resistant to at least one antiretroviral drug. These patients represented 19% of the 763 patients with available HIV-1 RNA tests and 75% of the 191 patients with HIV-1 RNA ≥300 copies/ml.
Of the 143 resistant viruses, 139 (97%) were resistant to lamivudine/emtricitabine and/or nevirapine/efavirenz (lamivudine/emtricitabine only, n=6; nevirapine/efavirenz only, n=29; both n=104). Twenty one patients harbored a virus with TAMs, accounting for 2.8% of the 763 patients with available HIV-1 RNA tests, 11.0% of the 191 patients who had detectable HIV-1 RNA and 14.7% of the 143 patients harboring a virus resistant to at least one antiretroviral drug. Thirty-five patients harbored a virus resistant to etravirine (Y181V, n=1; E138K, n=1; Y181C + H221Y, n=13; 4 mutations, n=1) or possibly resistant to etravirine (E138A/G/Q/R, n=11; 3 mutations, n=8)(Table 4), accounting for 4.6% of the 763 patients with available HIV-1 RNA tests, 18.3% of the 191 patients with HIV-1 RNA ≥300 copies/ml and 24.5% of the 143 patients harboring a virus resistant to at least one antiretroviral drug.
Of the 143 patients who harbored a resistant virus to at least one antiretroviral drug at M24, 66 (46%) already had a resistant strain at M12, 16 (11%) had detectable HIV-1 RNA but no mutation at month 12, 54 (38%) had undetectable HIV-1 RNA at month 12, and 7 had unavailable HIV-1 and/or genotype tests at month 12 (Table 2). Among the 35 patients who had strains with resistance to etravirine at M24, 10 (29%) had undetectable HIV-1 RNA levels at M12, 25 (71%) had detectable HIV-1 RNA levels at M12 with no resistance, 18 (51%) had resistance to EFV/NVP but no resistance to etravirine at M12, and 12 (34%) had resistance to etravirine at M12 (including 4 to etravirine only and 8 to EFV/NVP/etravirine).
Finally, 110 patients had a HIV-1 RNA ≥300 copies/ml both at M12 and at M24. Among these 110 patients, four (2.6%) and 17 (15.4%) harbored a virus with TAMs at M12 and M24 (p=0.001); and 12 (10.9%) and 25 (22.7%) harbored a virus resistant to etravirine at M12 and M24 (p=0.0002);
Discussion
We conducted a prospective cohort study nested in three large HIV care programs in Abidjan, Côte d’Ivoire, a country where the CRF02_AG subtype of HIV is predominant and the rate of primary HIV-1 resistance to antiretroviral drugs remains below five percent. 15
The study participants received the same care and treatment as non-participants at the same HIV care centers. In addition to providing regular follow-up, they also had plasma collection for HIV-1 RNA testing every six months and genotypic resistance testing if their HIV RNA levels were >300 copies/ml. For most patients, tests were done retrospectively on frozen samples, when funds were obtained. Therefore, most virological tests results were not available to patients and physicians on a real-time basis and could not be used to adapt the treatment between M12 and M 24,.
Because this was a cohort study, our data provide clear estimates of virological outcomes in the entire population of patients who initiated ART within the study period. Data are missing for patients lost-to-follow-up (16.2% prior to M12, 2.6% between M12 and M24) and for 2% and 10% of the patients who were still in the active file at M12 and M24, respectively.
We determined four main findings.
First, 25% and 27% of the patients who initiated ART and were still in active follow-up had a plasma HIV-1 RNA level ≥300 copies at month 12 and month 24, respectively. The percentage of patients infected with resistant HIV-1 strains was 12% at month 12 and 19% at month 24, accounting for 54% and 75% of patients with plasma HIV-1 RNA ≥300 copies at month 12 and month 24, respectively.
Second, among patients who had reached undetectable viral loads at month 12, 15% had detectable viral loads at month 24, of whom two out of three harbored resistant viruses. These results illustrate that maintenance of adherence is a permanent battle and that in regard to monitoring adherence, it is necessary to repeat viral load testing regularly. Reaching undetectable viral loads early does not preclude failing treatment within the following months.
Third, 46% of patients who had detectable HIV-1 RNA and available genotype test results at month 12 did not yet have resistance mutations. This percentage was half as high (25%) but still significant at month 24. 16 Thus, preventing resistance from emerging with interventions aiming at reinforcing adherence in patients with detectable viral loads is still worthwhile beyond the first two years of treatment. This suggests that systematic viral load measurements during the first two years of ART may help identify those patients who could still benefit from adherence reinforcement. 17
Fourth, most of the resistance mutations that developed in the first year of NNRTI-based first-line ART were to lamivudine/emtricitabine, nevirapine and/or efavirenz. 16,18–22 During the study period, most patients initiated ART with ZDV or d4T plus 3TC plus NVP or EFV. As expected, the most frequent NRTI mutations were M184V and TAMs, while V75A/M/S/T, K65R and Q151M were very rare. 16,19 The frequency of TAMs increased from 8.1% to 12.6% in all resistant strains from month 12 to month 24. This trend illustrates the challenge of targeting patients who harbor viruses resistant to lamivudine/emtricitabine and/or nevirapine/efavirenz as early as possible to switch patients to a second-line regimen before they select resistance to other NRTIs. 11,20,23 This is all the more important because the reported rates of virological failure to second-line therapy in resource-limited settings are high and are associated with the duration of exposure to previous drug regimens. 5 Because patients on second-line therapies who are treated with LPV/r monotherapy have worse outcomes than those treated with LPV/r and Truvada 24, it is important that patients who switch from a first-line NNRTI-based ART to a second-line PI-based one can receive effective NRTI in association with their PI.
While 15 strains were resistant to etravirine at month 12, etravirine resistance developed rapidly over the second year of treatment. 20 At month 24, 18% of patients with detectable HIV-1 RNA at month 24 harbored strains resistant to etravirine, accounting for 4% of the entire cohort. The most frequent mutations causing etravirine resistance were Y181C + H221Y and E138A/G/Q/R. This again highlights the importance of making viral load testing routinely available as a tool for detecting incomplete viral suppression and prompting appropriate interventions before mutations accumulate. 11 Consistent data regarding resistance to etravirine were recently reported by researchers from Mali. 25
To our knowledge, this is one the largest cohort studies on medium term virological outcomes in sub-Saharan Africa. This study has several limitations. First, we did not have genotype test results prior to ART initiation. Virologic resistance at 6 months may in part be due to primary resistance. 26 However, the prevalence of resistance mutations in adults with recent HIV infection in Côte d’Ivoire was estimated to be lower than 5%. 15 Second, some data are missing because 13% of the patients who were in active follow-up at month 6 refused to enroll in the study, 2% of the patients who enrolled at month 6 did not receive HIV RNA measurements at month 12, and 11% of patients who had detectable HIV RNA did not have available genotype test results. Third, the threshold of HIV RNA detectability was ≥300 copies/ml, which may have led us to underestimate the proportion of patients who were not completely virologically suppressed and thus the proportion of patients with resistance. Fourth, nearly one-third of the patients initiating ART in these programs either died, transferred out or were lost-to-follow-up before month 6. However, their outcomes were likely worse than those of patients who enrolled in the study, and the association we found between MPR and virological outcomes in patients who enrolled is likely to also exist in patients who did not.
In conclusion, preventing the emergence of resistance mutations by implementing adherence reinforcement in patients with detectable VLs is important beyond M24. Switching therapy early in patients with early resistance to 3TC/FTC and/or to first generation NNRTIs to prevent TAMs and etravirine resistance is a challenge. Three options could be pursued to address this challenge: begin patients on more forgiving first-line regimens 27,28, make genotype testing widely available, or validate alternative methods (e.g., repeating viral load measurements after an intensive phase of adherence reinforcement) for distinguishing patients who harbor resistant viruses in settings where genotype tests are available. 29,30
Acknowledgments
Source of Funding: EM and MLC are currently receiving a grant (ANRS 12186) from the French National Agency for or Research on AIDS and viral hepatitis (ANRS, Paris, France).
Funding: The Agence Nationale de Recherches sur le SIDA et les hepatites virales (Grant ANRS 12136 and ANRS 12212), the programme “Ensemble pour une Solidarité Thérapeutique Hospitalière En Réseau” – ESTHER (Network for Therapeutic Solidarity in Hospitals), the National Institute of Allergy and Infectious Diseases (Grant R01 AI058736, K24 AI062476) and the National Institutes of Health (IeDEA West Africa Collaboration, Grant 12273).
We are indebted to all patients who participate in this cohort.
We gratefully acknowledge the valuable contributions of the CeDReS, CEPREF, CIRBA, CMSDS, CNTS, Hopital Général de Yopougon-Attié, Programme PACCI and INSERM U897 teams.
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
References
- 1.WHO. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. 2010 http://www.who.int/hiv/pub/2010progressreport/en/index.html.
- 2.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eholie SP, Aoussi FE, Ouattara IS, Bissagnene E, Anglaret X. HIV treatment and care in resource-constrained environments: challenges for the next decade. J Int AIDS Soc. 15:17334. doi: 10.7448/IAS.15.2.17334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binford MC, Kahana SY, Altice FL. A Systematic Review of Antiretroviral Adherence Interventions for HIV-Infected People Who Use Drugs. Curr HIV/AIDS Rep. 2012 doi: 10.1007/s11904-012-0134-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajose O, Mookerjee S, Mills E, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. AIDS. 2012;26:929–38. doi: 10.1097/QAD.0b013e328351f5b2. [DOI] [PubMed] [Google Scholar]
- 6.Severe P, Leger P, Charles M, Noel F, Bonhomme G, Bois G, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 7.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 8.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts T, Bygrave H, Fajardo E, Ford N. Challenges and opportunities for the implementation of virological testing in resource-limited settings. J Int AIDS Soc. 2012;15:17324. doi: 10.7448/IAS.15.2.17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen ML, van der Laan MJ, Napravnik S, Eron JJ, Moore RD, Deeks SG. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS. 2008;22:2097–106. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigaloff KC, Hamers RL, Wallis CL, Kityo C, Siwale M, Ive P, et al. Unnecessary Antiretroviral Treatment Switches and Accumulation of HIV Resistance Mutations; Two Arguments for Viral Load Monitoring in Africa. J Acquir Immune Defic Syndr. 2011;58:23–31. doi: 10.1097/QAI.0b013e318227fc34. [DOI] [PubMed] [Google Scholar]
- 12.Messou E, Chaix ML, Gabillard D, Minga A, Losina E, Yapo V, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Cote d’Ivoire. J Acquir Immune Defic Syndr. 2011;56:356–64. doi: 10.1097/QAI.0b013e3182084b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messou E, Kouakou M, Gabillard D, Gouesse P, Kone M, Tchehy A, et al. Medication Possession Ratio: Predicting and Decreasing Loss to Follow-Up in Antiretroviral Treatment Programs in Cote d’Ivoire. J Acquir Immune Defic Syndr. 2011;57 (Suppl 1):S34–9. doi: 10.1097/QAI.0b013e3182208003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Antiretroviral Therapy For HIV Infection in adults and adolescents: Recommendations for a public health approach (2006 revision) 2006 Available from: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 15.Ayouba A, Lien TT, Nouhin J, Vergne L, Aghokeng AF, Ngo-Giang-Huong N, et al. Low prevalence of HIV type 1 drug resistance mutations in untreated, recently infected patients from Burkina Faso, Cote d’Ivoire, Senegal, Thailand, and Vietnam: the ANRS 12134 study. AIDS Res Hum Retroviruses. 2009;25:1193–6. doi: 10.1089/aid.2009.0142. [DOI] [PubMed] [Google Scholar]
- 16.El-Khatib Z, Ekstrom AM, Ledwaba J, Mohapi L, Laher F, Karstaedt A, et al. Viremia and drug resistance among HIV-1 patients on antiretroviral treatment: a cross-sectional study in Soweto, South Africa. AIDS. 2010;24:1679–87. doi: 10.1097/QAD.0b013e32833a097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith DM, Schooley RT. Running with scissors: using antiretroviral therapy without monitoring viral load. Clin Infect Dis. 2008;46:1598–600. doi: 10.1086/587110. [DOI] [PubMed] [Google Scholar]
- 18.Barth RE, Wensing AM, Tempelman HA, Moraba R, Schuurman R, Hoepelman AI. Rapid accumulation of nonnucleoside reverse transcriptase inhibitor-associated resistance: evidence of transmitted resistance in rural South Africa. AIDS. 2008;22:2210–2. doi: 10.1097/QAD.0b013e328313bf87. [DOI] [PubMed] [Google Scholar]
- 19.Kouanfack C, Montavon C, Laurent C, Aghokeng A, Kenfack A, Bourgeois A, et al. Low levels of antiretroviral-resistant HIV infection in a routine clinic in Cameroon that uses the World Health Organization (WHO) public health approach to monitor antiretroviral treatment and adequacy with the WHO recommendation for second-line treatment. Clin Infect Dis. 2009;48:1318–22. doi: 10.1086/597779. [DOI] [PubMed] [Google Scholar]
- 20.Hamers RL, Sigaloff KC, Wensing AM, Wallis CL, Kityo C, Siwale M, et al. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis. 2012;54:1660–9. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 21.Wadonda-Kabondo N, Hedt BL, van Oosterhout JJ, Moyo K, Limbambala E, Bello G, et al. A retrospective survey of HIV drug resistance among patients 1 year after initiation of antiretroviral therapy at 4 clinics in Malawi. Clin Infect Dis. 2012;54 (Suppl 4):S355–61. doi: 10.1093/cid/cis004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ugbena R, Aberle-Grasse J, Diallo K, Bassey O, Jelpe T, Rottinghaus E, et al. Virological response and HIV drug resistance 12 months after antiretroviral therapy initiation at 2 clinics in Nigeria. Clin Infect Dis. 2012;54 (Suppl 4):S375–80. doi: 10.1093/cid/cir1064. [DOI] [PubMed] [Google Scholar]
- 23.Bennett DE, Jordan MR, Bertagnolio S, Hong SY, Ravasi G, McMahon JH, et al. HIV drug resistance early warning indicators in cohorts of individuals starting antiretroviral therapy between 2004 and 2009: World Health Organization global report from 50 countries. Clin Infect Dis. 2012;54 (Suppl 4):S280–9. doi: 10.1093/cid/cis207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunupuradah T, Chetchotisakd P, Ananworanich J, Munsakul W, Jirajariyavej S, Kantipong P, et al. A randomized comparison of second-line lopinavir/ritonavir monotherapy vs. tenofovir/lamivudine/lopinavir/ritonavir in patients failing NNRTI-regimens: the HIV STAR study. Antivir Ther. 2012 doi: 10.3851/IMP2222. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Maiga AI, Fofana DB, Cisse M, Diallo F, Maiga MY, Traore HA, et al. Characterization of HIV-1 antiretroviral drug resistance after second-line treatment failure in Mali, a limited-resources setting. J Antimicrob Chemother. 2012 doi: 10.1093/jac/dks310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghokeng AF, Kouanfack C, Laurent C, Ebong E, Atem-Tambe A, Butel C, et al. Scale-up of antiretroviral treatment in Cameroon is accompanied by increasing HIV-1 drug resistance mutations in drug-naive patients. AIDS. 2011 doi: 10.1097/QAD.0b013e32834bbbe9. [DOI] [PubMed] [Google Scholar]
- 27.Stadeli KM, Richman DD. Rates of emergence of HIV drug resistance in resource-limited settings: a systematic review. Antivir Ther. 2012 doi: 10.3851/IMP2437. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Wyl V, Cambiano V, Jordan MR, Bertagnolio S, Miners A, Pillay D, et al. Cost-effectiveness of tenofovir instead of zidovudine for use in first-line antiretroviral therapy in settings without virological monitoring. PLoS One. 2012;7:e42834. doi: 10.1371/journal.pone.0042834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy RA, Sunpath H, Lu Z, Chelin N, Losina E, Gordon M, et al. Outcomes after virologic failure of first-line ART in South Africa. AIDS. 24:1007–12. doi: 10.1097/QAD.0b013e3283333639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levison JH, Wood R, Scott CA, Ciaranello AL, Martinson NA, Rusu C, et al. The Clinical and Economic Impact of Genotype Testing at First-line Antiretroviral Therapy Failure for HIV-infected Patients in South Africa. Clin Infect Dis. 2012 doi: 10.1093/cid/cis887. [DOI] [PMC free article] [PubMed] [Google Scholar]