We recently described a motion-related artifact in resting state functional connectivity MRI (rs-fcMRI) data that arises from subject head motion (Power et al. (2012), see also Satterthwaite et al. (2012); Van Dijk et al. (2012)). Head motion produces well-known disruptions in BOLD signal (Friston et al., 1996), and these artifactual modulations of BOLD signal, which are similar at nearby voxels, create spurious patterns in correlations in rs-fcMRI. Specifically, head motion augments short-distance correlations and weakens long-distance correlations. Thus, for instance, a higher-motion dataset would typically display weakened correlations between (distantly spaced) default mode regions but enhanced correlations between (closely spaced) visual regions in comparison to a low-motion data-set. We described this artifact in cohorts of children, adolescents, and adults, and its severity (magnitude) was related to the prevalence of motion within a cohort. Motion-related functional connectivity artifact is thus a substantial confound in the examination of single rs-fcMRI datasets and in comparisons of multiple rs-fcMRI datasets.
In our report we proposed a volume censoring technique (‘scrubbing’) to identify volumes where signal was likely compromised by motion. As proof of principle, we identified volumes where both bulk head position and brain-wide BOLD signal changed substantially from volume to volume and withheld these volumes from correlation calculations to establish the presence and nature of a motion-related functional connectivity artifact. As we stated in our report, this approach was not designed to remove all motion-contaminated data, and we called for efforts to test and improve strategies for removing this artifact.
In a Comment on our article (Carp, 2011), Carp proposes a modification of our data processing steps with the goal of improving artifact removal. In our article, following our standard functional connectivity processing procedures, the data were first band-pass filtered (0.009 Hz<f<0.08 Hz), then spatially blurred (6 mm FWHM), and, finally, nuisance variables (e.g., white matter signal, ventricular signal, etc.) were regressed from the data. For reasons explained below, volume censoring only occurred after these steps. In his Comment, Carp suggests a modified order of operations that censors time-points (replaced by interpolation) before functional connectivity processing. Using synthetic timeseries in which 5% of the data are contaminated with artifactual spikes, Carp shows that this modified order of operations eliminates a greater proportion of artifact-induced correlation while removing less data than the ‘scrubbing’ procedure we initially described. While we welcome these simulation-based findings, we suspect that they may have limited applicability in real datasets.
As we noted in Power et al. (2012), there are at least 3 ways in which the ‘scrubbing’ procedure as implemented in our report did not remove all motion-related signal: 1) the thresholds used to identify and censor volumes were lenient and were not meant to identify all motion-contaminated data, 2) band-pass filtering temporally spreads compromised signal into non-compromised volumes, and 3) the multiple regression of nuisance variables was fit to all data and was not tailored to ignore compromised data. We address briefly each of these issues below.
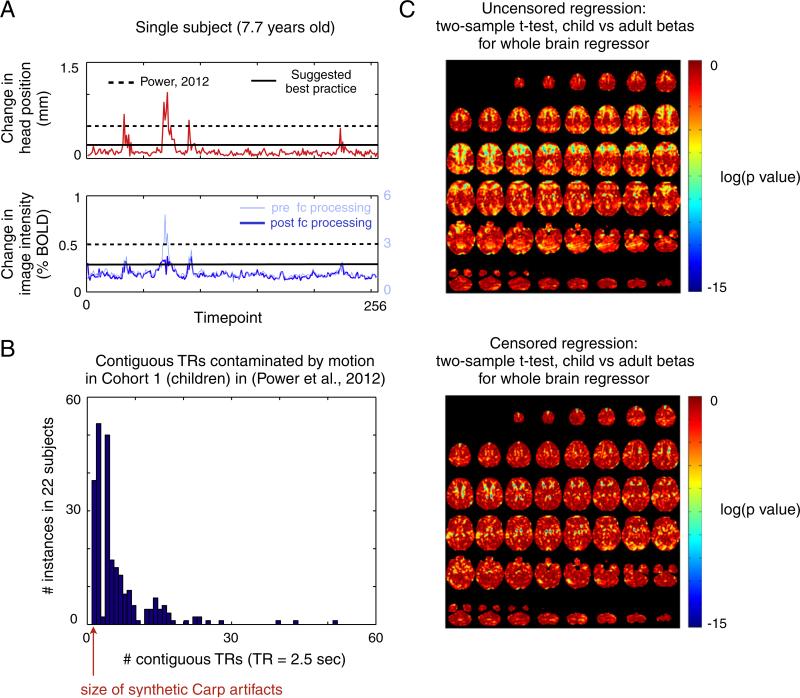
First, since our analyses were proof of principle in nature, we chose lenient thresholds for identifying compromised volumes and only removed the most egregious instances of motion contamination, not all contaminated data. Fig. 1A shows, in a representative subject, the 2 indices (in red and blue) that we used to identify motion-contaminated volumes (see also Figs. 3 and S5 of Power et al. (2012)). Framewise displacement (FD), shown in red, is calculated from derivatives of the rigid body realignment estimates that are used to realign BOLD data during fMRI preprocessing. The DVARS measure, shown in blue, is the RMS (root mean squared) change in BOLD signal from volume to volume calculated over all voxels within the brain. DVARS can be calculated before (light blue) or after (blue) functional connectivity processing, and in our hands it makes little practical difference which version is used (note the similarity between the blue and light blue traces). We use DVARS values calculated after functional connectivity processing (blue) since these are the data most directly related to rs-fcMRI analyses. In our initial report we only removed volumes with head displacements >0.5 mm and BOLD signal displacements >0.5% (the dotted lines in Fig. 1A).
Fig. 1.
(A) The two indices used to create temporal masks in Power et al. (2012) are shown for a single subject. On top in red is the framewise displacement (FD) measure, which indexes volume-to-volume changes in head position. Below in blue is the DVARS measure, which indexes RMS BOLD signal change across the whole brain from volume to volume. The DVARS measure was calculated for this subject's data before (light blue) and after (blue) functional connectivity processing. Dashed lines represent the thresholds used in Power et al. (2012) and the solid lines indicate the thresholds presently used within the Petersen/Schlaggar laboratory to maximally remove motion-contaminated data. (B) A histogram of the sizes of the periods of movement detected in Cohort 1 using the standard thresholds of Power et al. (2012), modified from Figure S8 of Power et al. (2012). Most movements last several contiguous TRs and many movements are quite long. (C) Regressions for the child and adult cohorts were performed without (uncensored) and with (censored) temporal masks. Two-sample t-tests comparing the beta maps in children and adults were performed. The images show the resultant p-values for the whole brain signal regressor without and with volume censoring. Many differences in beta maps between children and adults are eliminated or lessened when high-motion data is excluded from the regression. This result is typical of the other beta maps produced by other regressors (e.g., ventricular signal, white matter signal, etc.)
More stringent thresholds, just above the values in volume-to-volume head displacement (0–0.2 mm) and BOLD signal intensity displacement (0–0.3% BOLD) seen in still subjects, are likely needed to most fully remove motion-contaminated data. In our original article, in each subject we studied, the change in BOLD signal at a given TR was related to the change in head position at that TR (Fig. 2 of Power et al. (2012)). These motion-induced changes are the source of functional connectivity artifact, and therefore one would expect that any and all motion would produce changes that will contribute to this artifact. Indeed, when we lowered thresholds below our proof-of-principle thresholds to more stringently identify suspect data, larger effects were produced and differences between children and adult functional connectivity matrices were further reduced (Figure S10 of Power et al. (2012)). In our laboratory, analyses are now done removing volumes with head displacements >~0.2 mm or BOLD signal displacements >~0.3% BOLD (solid lines in Fig. 1A). More stringent censoring removes more data; we prefer to examine smaller amounts of maximally cleaned data rather than larger amounts of semi-cleaned data. Even after these precautions, any motion that is either subthreshold or which was not captured by our quality measures will not be caught and eliminated from analysis.
Second, as Carp rightly notes, band-pass filtering spreads contaminated signal into non-contaminated volumes. It is desirable to avoid this spreading, but there are obstacles to avoiding this spreading in actual datasets. Carp replaces single bad timepoints of synthetic time-series by interpolating from adjacent timepoints. In real datasets, however, periods of motion are often not a single TR. As Fig. 1B shows (modified from Figure S8 of Power et al. (2012)), using the lenient thresholds in our report, most periods of motion span multiple TRs and dozens (38/249=15%) are longer than 10 TRs. The advantage of interpolation is that it can reduce the amplitude of signal that is spread during temporal filtering, but the disadvantage of interpolation is that it replaces data that may have characteristics of interest with a signal ramp that cannot preserve the characteristics of the replaced signal. The longer the interpolation, the more characteristics are lost. The essential question, then, becomes whether it is always advantageous to use interpolation to minimize artifactual signal amplitude, or whether this benefit, beyond some number of TRs, is balanced or outweighed by the loss of signal characteristics. The answer to this question may be complicated, depending on artifact sign, amplitude, duration, prevalence, and the temporal filter applied to the data.
We do not presently know the answer to this essential question. Comprehensive investigations using simulations and real datasets will likely be needed to provide convincing answers. For the moment we continue to censor volumes following band-pass filtering with the knowledge that some contaminated signal is spread into previously uncontaminated volumes. This spread is limited by using (as we always have) low-order Butterworth filters that do not display the overshoot or ringing warned against in Carp's article. It is worth noting that within our processing stream the volume “quality” rankings produced from DVARS calculated before and after functional connectivity processing (light blue vs. blue lines in Fig. 1A) are quite similar.
Third, the multiple regressions of nuisance signals from our data in our initial report fit regressors to all data, contaminated and uncontaminated. In general, if regressors fit a dataset in some useful way, adding noise to the dataset will corrupt the fit of the regressors. The strong signal outliers present during periods of motion thus likely corrupted the fits of nuisance regressors to data. Following this logic, to improve the efficacy of our regressions, we now tailor our regressions to use only data included within the temporal masks when calculating beta values. By definition, this maneuver should (and does) decrease the magnitude of the residuals of interest (the rs-fcMRI data within the temporal mask).
How might one establish whether this processing decision is a beneficial one? A thought experiment suggests a test: if two indistinguishable populations undergo regressions, the betas from each population should be indistinguishable. However, if noise is added to one population, the betas for that population will be corrupted, and differences in betas will be created between populations. Conversely, if the noise is removed from the noised population, the betas will again become indistinguishable. Our cohorts of children and adults represent high noise (high movement) and low noise (low movement) populations, respectively. These populations display many significant differences in betas when all data are included in regressions. To test the effect of noise removal on differences in betas, we computed the beta maps of children and adults after tailoring the regressions using the scrubbing settings in our report. Fig. 1C shows a representative result: the differences between the beta maps (here, for whole brain signal regressors) calculated for children and adults are dramatically decreased by tailored regressions. This result is echoed in the other beta maps (e.g., for ventricular signal, white matter signal, etc.) for these populations. Eliminating high-motion data from regressions therefore eliminates many differences that previously existed between the fits of regressions in children and adults, just as if one were removing noise from the data in the thought experiment.
We should also clarify two 2 minor points. First, Figure S4 in Power et al. (2012) shows that spatially adjacent brain regions share similar motion-induced signal changes. However, it also shows that regions at opposite ends of the brain often display opposite motion-induced signal changes. We raise this point since Carp adds identical spikes to pairs of timeseries (thus best modeling adjacent brain regions). This point does not modify Carp's conclusions but we want to ensure that our findings are clearly communicated. Second, our practice of also censoring volumes one back and two forward from any supra-threshold volume arose from considerations about the imprecise timing of head position estimates and the disruption of spin-history effects that occur during motion, not from considerations about the temporal spread of artifact due to band-pass filtering. Carp, in his synthetic timeseries, appears to limit inserted artifact to a single timepoint rather than to the multiple TRs that are typically impacted by motion in fMRI (Friston et al., 1996).
We welcome efforts to develop optimal methods to counter motion artifact in rs-fcMRI. The ‘impute-first’ methodology tested with simulations by Carp is theoretically useful, but it remains to be seen whether this approach is unambiguously helpful in real datasets. In this Comment, based on properties of our real dataset, we have pointed out several possible improvements to our procedure that can be explored in simulations. We agree with Carp that the most robust way forward is to combine modeling efforts with analysis of real datasets. To this end, we have released 3 of the datasets used in Power et al. (2012) to the neuroimaging community (data is currently being shared via the International Neuroimaging Data-sharing Initiative (INDI) based at NITRC; http://fcon_1000.projects.nitrc.org/indi/retro/Power2012.html). The datasets available are healthy children, adolescents, and adults scanned at 3 T, Cohorts 1–3 of our report. Now, investigators should be able to reproduce and extend our findings, develop methodology, and compare approaches using a common dataset. We thank Carp for his attention to this important issue and look forward to future progress in countering motion artifact.
Acknowledgments
This work was supported by NIH F30 MH940332 (JDP) and a McDonnell Foundation Collaborative Action Award (SEP).
This is a commentary on article Carp J. Optimizing the order of operations for movement scrubbing: Comment on Power et al. Neuroimage. 2013;76436-8.
References
- Carp J. Optimizing the order of operations for movement scrubbing: comment on Power et al. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.12.061. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]