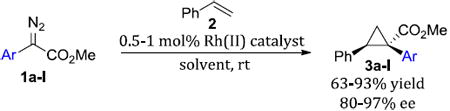
Abstract
Catalytic enantioselective methods for the generation of cyclopropanes has been of longstanding pharmaceutical interest. Chiral dirhodium(II) catalysts prove to be an effective means for the generation of diverse cyclopropane libraries. Rh2(R-DOSP)4 is generaally the most effective catalyst for asymmetric intermolecular cyclopropanation of methyl aryldiazoacetates with styrene. Rh2(S-PTAD)4 provides high levels of enantioinduction with ortho-substituted aryldiazoacetates. The less-established Rh2(R-BNP)4 plays a complementary role to Rh2(R-DOSP)4 and Rh2(S-PTAD)4 in catalyzing highly enantioselective cyclopropanation of 3- methoxy-substituted aryldiazoacetates. Substitution on the styrene has only moderate influence on the asymmetric induction of the cyclopropanation.
Keywords: Asymmetric cyclopropanation, Cyclopropanes, Donor/acceptor carbenoids, Dirhodium catalysis, Phenyldiazoacetate
1. Introduction
The metal-catalyzed decomposition of diazo compounds in the presence of alkenes is a general method for the stereoselective synthesis of cyclopropanes.1,2 We have previously shown the rhodium-catalyzed cyclopropanation of donor/acceptor carbenoids to be effective for the enantioselective synthesis of cyclopropanes with one or more quaternary stereogenic centers.3-6 As many cyclopropyl amines are known to have significant CNS activity,7,8 we have initiated a program to use our cyclopropanation methodology to access novel diarylcyclopropylamines as potential the rapeutic agents (Scheme 1). For the methodology to be broadly useful in drug discovery, access to a range of diarylcyclopropyl derivatives with high levels of enantioenrichment is required. Herein, we define the optimal catalysts for the cyclopropanation with various types of methyl aryldiazoacetates.
Scheme 1. Route to cyclopropyl amines from aryldiazoacetates.
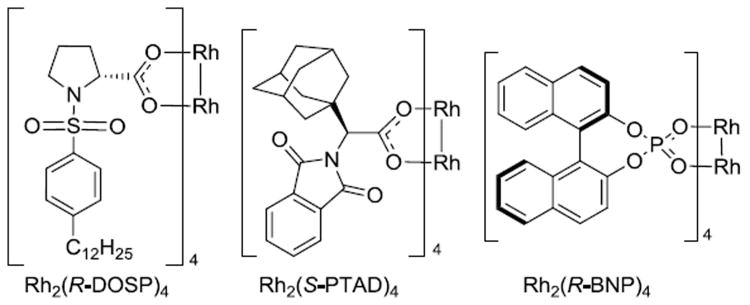
Three chiral dirhodium-(II) catalysts were selected for this study (Figure 1). The first catalyst, Rh2(R-DOSP)4, is considered to be the optimal catalyst for the reactions of donor/acceptor carbenoids when the acceptor group is a methyl ester.1,4 The substrate scope of Rh2(R-DOSP)4-catalyzed cyclopropanation is quite broad in terms of both the trapping agent and the donor group on the carbenoid.3,4,9-18 However, a detailed study on the influence of the aryl substituent of the aryldiazoacetate on Rh2(R- DOSP)4-catalyzed cyclopropanation has not been conducted. The second catalyst, Rh2(S-PTAD)4, is the optimal chiral catalyst when the acceptor group in the donor/acceptor carbenoid is a phosphonate,19 trifluoromethyl,20 cyano,21 or keto group.22 Due to the perceived superiority of Rh2(R-DOSP)4 in the reactions of aryldiazoacetates, Rh2(S-PTAD)4 has not been thoroughly evaluated in the reactions of this class of carbenoid precursor. The third catalyst, Rh2(R-BNP)4, is an interesting catalyst of D4-symmetry that has been applied to a number of carbenoid reactions,23-27 but has not been previously evaluated in the cyclopropanation of donor/acceptor carbenoids.
Figure 1. Dirhodium-(II) catalysts used in this study.
2. Results and Discussion
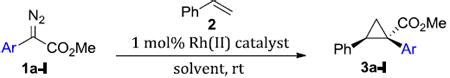
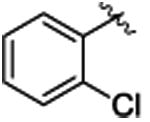
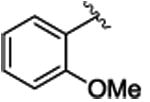
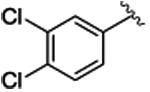
The study began by exploring the effect of aryl substitution on the enantioselectivity in the cyclopropanation of styrene by aryldiazoacetates. The results for a series of aryldiazoacetates (1a-l) using the three chiral dirhodium catalysts Rh2(R-DOSP)4, Rh2(S-PTAD)4 and Rh2(R-BNP)4 are summarized in Table 1. Excellent levels of diastereoselectivity were achieved in all cases (>95:5 dr) and the isolated yields of the cyclopropanes were generally high, ranging from 63-98%. The absolute configuration of the major isomer of the cyclopropanes is tentatively assigned as 1S,2R.28 Rh2(R-DOSP)4 provided high levels of enantioinduction when unsubstituted (1a) or 4-substituted (1b-e) methyl aryldiazoacetates were employed as substrates (Table 1, entries 1-5, 87-90% ee). In general, Rh2(S-PTAD)4 and Rh2(R-BNP)4 gave <60% ee in the cyclopropanation reactions of unsubstituted or 4-substituted aryldiazoacetates with these same substrates. The one exception, however, is the reaction of 4-methoxyphenyldiazoacetate 1e with Rh2(S-PTAD)4, which gave the cyclopropane 3e in exceptionally high enantioselectivity (entry 5, 96% ee). Rh2(R-DOSP)4 also performed well in the reactions of 2-substituted aryldiazoacetates 1f (entry 6, 92% ee) and 1g (entry 7, 86% ee), and moderately well with 3,4-dichlorophenyldiazoacate 1h (entry 8, 82% ee), confirming that Rh2(R-DOSP)4 is the optimal catalyst for a range of aryldiazoacetates. The enantioselection with Rh2(S-PTAD)4 is extremely variable. However, in certain cases, it provides exceptional levels of enantioselectivity, as seen in the reaction with 2-chlorophenyldiazoacetate 1f, which generates the cyclopropane 3f in 97% ee (entry 6).
Table 1. Examination of the influence of substitution on aryldiazoacetate.
| ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Rh2 (S-DOSP)4a | Rh2 (S-PTAD)4a | Rh2(R-BNP)4b | ||||||
|
| ||||||||
| entry | Ar | product | yield (%) | ee(%) | yield (%) | ee (%) | yield (%) | Ee(%) |
| 1 |
|
3a | 85 | 88 | 87b | 21b | 72 | 42 |
| 2 |
|
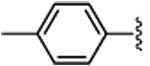
3b | 84 | 87 | 77c | 46c | 69 | 51 |
| 3 |
|
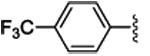
3c | 93 | 87 | 67c | 77c | 80 | 57 |
| 4 |
|
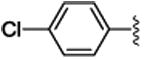
3d | 87 | 88 | 71c | 48c | 89 | 57 |
| 5 |
|
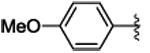
3e | 66 | 90 | 93c | 96c | 84 | 57 |
| 6 |
|
3f | 89 | 92 | 80c | 97c | 78 | 51 |
| 7 |
|
3g | 92 | 86 | 87c | 80c | 69 | 27 |
| 8 |
|
3h | 88b | 82b | 88b | 60b | 82 | 63 |
| 9 |
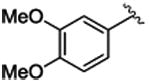
|
3i | 74 | 56 | 70c | 94c | 93 | 97 |
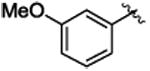
| 10 |
|
3j | 78b | 79b | 72b | 16 b | 82 | 88 |
| 11 |
|
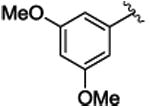
3k | 85b,c | 28b,c | 85b,c | 3b,c | 69c | 92c |
| 12 |
|
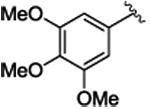
3l | 98b | 34b | 95b | 9b | 63 | 90 |
pentane,
toluene,
0.5 mol% catalyst loading
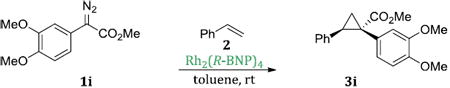
Interestingly, Rh2(R-DOSP)4 fails to provide high levels of enantioinduction when the aryldiazoacetate contains a 3-methoxysubstituent (1i-l) (entries 9-12), especially when the ring is polysubstituted (1i, k-l). In these cases, the cyclopropanes 3i, k-l are produced in 28-56% ee (entries 9, 11-12). Rh2(S-PTAD)4 is an effective catalyst only in the case of 3, 4-dimethoxyphenyldiazoacetate 1i, generating the cyclopropane 3i in 94% ee (entry 9). In contrast, Rh2(R-BNP)4 is an extremely effective catalyst with all of the 3-methoxy substituted aryldiazoacates 1i-l, generating the cyclopropanes 3i-l in 88-97% ee (entries 9-12).
One of the most attractive features of Rh2(R-DOSP)4 and Rh2(S-PTAD)4 as chiral catalysts is that they are capable of operating at low catalyst loadings.29 As Rh2(R-BNP)4 was found to be the optimal catalyst for 3-methoxyaryldiazoacetate derivatives, we investigated whether it could also operate at low catalyst loading. Reactions of aryldiazoacetate 1i, with successively lower loadings of Rh2(R-BNP)4 are summarized in Table 2. Rh2(R-BNP)4 was effective at catalyzing highly enantioselective cyclopropanation with catalyst loadings of 1.0 and 0.5 mol%, providing 3i with virtually the same level of enantioselectivity (entry 1 vs. 2, 97% ee vs. 96% ee). When the catalyst loading was decreased further to 0.1 mol% a more significant drop in the level of enantioselectivity was observed (entry 3, 91% ee). Further decreasing the catalyst loading to 0.01 mol%, however, caused a dramatic decrease in enantioinduction (entry 4, 40% ee). These results suggest that, although Rh2(R-BNP)4 is capable of moderately high turnover numbers (TONs), it may not be as robust a catalyst as Rh2(R-DOSP)4 or Rh2(S-PTAD)4, which are capable of TONs as high as 850,000 and 1,800,000 respectively in asymmetric intermolecular cyclopropanation.29.
Table 2. Examination of Rh2 (R-BNP)4 Loading.
| ||||
|---|---|---|---|---|
|
| ||||
| entry | catalyst loading (mol%) | reaction time(h) | yield (%) | ee (%) |
| 1 | 1 | 1 | 93 | 97 |
| 2a | 0.5 | 1 | 83 | 96 |
| 3 | 0.1 | 12 | 80 | 91 |
| 4 | 0.01 | 17 | 73 | 40 |
2.5 equiv. styrene used
At the onset of this study, the asymmetric induction of the cyclopropanation was not expected to be heavily influenced by the functionality on the aryldiazoacetate. Having observed considerable variation in enantioselectivity depending on the nature of the aryl group, we decided to explore the effect of modifying the substitution on the styrene. These studies were focused on Rh2(R-BNP)4-catalyzed cyclopropanation with the 3, 4-dimethoxyphenyldiazoacetate 1i (Table 3), because Rh2(R-BNP)4 had not been previously evaluated as a catalyst for this transformation. The levels of enantioselectivity in the cyclopropanation under Rh2(R-BNP)4-catalyzed conditions were found to be moderately influenced by the functionality on the styrene, as the cyclopropanes 5a-g were obtained with uniformly high levels of enantioinduction (80-90% ee). Previous studies have shown that Rh2(R-DOSP)4-catalyzed cyclopropanation is also not especially influenced by the nature of the styrene3,4,9 and the Rh2(R-DOSP)4-catalyzed cyclopropanation of a range of styrenes (4a-g) with 1i gave 5a-g with modest levels of enantioselectivity (48-67% ee).
Table 3. Substrate Scope of Styrene with Rh2(R-BNP)4.

| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Rh2 (R-BNP)4 | Rh2 (R-DOSP)4 | |||||
|
| ||||||
| entry | Ar | product | yield (%) | ee(%) | yield (%) | Ee(%) |
| 1 |
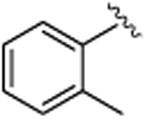
|
5a | 76 | 90 | 47 | 64 |
| 2 |
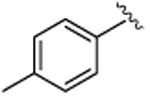
|
5b | 90 | 88 | 50 | 67 |
| 3 |
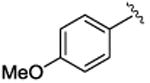
|
5c | 80 | 90 | 82 | 62 |
| 4 |
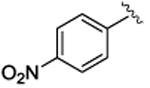
|
5d | 94 | 80 | 28 | 50 |
| 5 |
|
5e | 90 | 90 | 19 | 63 |
| 6 |
|
5f | 81 | 89 | 49 | 67 |
| 7 |
|
5g | 72 | 86 | 79 | 48 |
These studies reveal subtle differences in the ability of the chiral dirhodium tetracarboxylate catalysts to induce high enantioselectivity in the cyclopropanation of styrenes, as summarized in Table 4. The previous expectation that Rh2(R-DOSP)4 would give high asymmetric induction for all aryldiazoacetates was found not to be true, although it was found to be the most effective catalyst for the broadest range of substituted methyl aryldiazoacetates. In spite of the fact that Rh2(S-PTAD)4 is the most consistent catalyst when the acceptor group of the donor/acceptor carbenoid is modified,19-22 the level of enantioselectivity was found to be highly variable when aryl substitution of the aryldiazoacetate was modified. In particular, Rh2(S-PTAD)4 was very effective with 2-chlorophenyl aryldiazoacetate derivative. Rh2(R-BNP)4 becomes the best catalyst for 3-methoxyphenyl-substituted aryldiazoacetate derivatives. The cause for these subtle variations, at this stage, is not well understood. There is no clear steric or electronic trend to define which catalyst will perform best in a given system, which suggests the asymmetric induction may involve a combination of factors including π-stacking interactions. Computational studies are in progress to develop a rational model to explain these trends.
Table 4. A Guide to Chiral Dirhodium-(II) Catalyzed Cyclopropanation.
| |||
|---|---|---|---|
|
| |||
| Ar | catalyst | yield (%) | ee(%) |
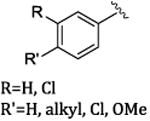
|
Rh2(R-DOSP)4 | 66-93 | 82-92 |
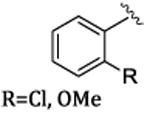
|
Rh2(S-PTAD)4 | 80-87 | 80-97 |
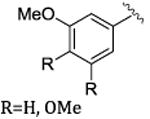
|
Rh2(R-BNP)4 | 63-93 | 88-97 |
3. Conclusion
This study provides guidelines for choosing the optimal chiral dirhodium-(II) catalyst for cyclopropanation of aryldiazoacetates. The nature of the aryl group on the aryldiazoacetate strongly affects the asymmetric induction imparted by the three catalysts studied. Depending on the aryl substituent, Rh2(R-DOSP)4, Rh2(S-PTAD)4, or Rh2(R-BNP)4 can be utilized to obtain the desired cyclopropane with a high level of enantioinduction. In contrast, the functionality on the styrene has only moderate influence on the level of asymmetric induction in cyclopropanation reactions. These studies set the chemical foundation for our ongoing studies to develop the rapeutically useful diarylcyclopropylamines, the results of which will be reported in due course.
4. Experimental Section
4.1 General
All reactions were conducted under anhydrous conditions in oven-dried glassware under an inert atmosphere of dry argon, unless otherwise stated. Hexanes, pentane, and toluene were dried by a solvent purification system (passed through activated alumina columns). All solvents were degassed by bubbling argon through the solvent for a minimum of 10 minutes prior to use. Unless otherwise noted, all other reagents were obtained from commercial sources and used as received. 1H Nuclear Magnetic Resonance (NMR) spectra were recorded at 400 MHz or 600MHz. Data presented as follows: chemical shift (in ppm on the δ scale relative to δH 7.27 for the residual protons in CDCl3), coupling constant (J/Hz), integration. Coupling constants were taken directly from the spectra and are uncorrected. 13C NMR spectra were recorded at 100 MHz and all chemical shift values are reported in ppm on the δ scale, with an internal reference of δC 77.23 for CDCl3. Mass spectral determinations were carried out by using APCI as ionization source. Melting points are uncorrected. Infrared spectral data are reported in units of cm−1. Analytical TLC was performed on silica gel plates using UV light. Flash column chromatography was performed on silica gel 60Å (230-400 mesh). Optical rotations were measured on Jasco polarimeters. Analytical enantioselective chromatographies were measured on Varian Prostar instrument and used isopropanol:hexane as gradient. Chiral HPLC conditions were determined by obtaining separation of the racemic product. Rh2(R/S-DOSP)4 was employed as the catalyst in the racemic reactions. Styrene (2) and substituted styrenes (4a-f) were commercially available and purified by pushing through a silica-filled pipette prior to use. Rh2(R- DOSP)4,3 Rh2(S-PTAD)4,19 Rh2(R-BNP)4,23 aryldiazoacetates 1a-1,2 and styrene 4g30 were all synthesized according published procedures.
4.2 General Procedure for the Synthesis of Methyl Phenyldiazoacetates31
The methyl arylacetate (1 equiv.) and p-ABSA (1.3 equiv.) were dissolved in acetonitrile and cooled to 0 °C using an ice bath under an argon atmosphere. 1, 8-Diazabicycloundec-7-ene (DBU, 1.3 equiv.) was then added to the stirring mixture over the course of 5 minutes. After the addition of the DBU, the reaction mixture continued to stir at 0 °C for an additional 15 minutes. Once this allotted time had passed, the ice bath was removed and the reaction mixture was stirred for 24 hours at room temperature. The resulting orange solution was quenched with saturated NH4Cl and the aqueous layer was extracted with diethyl ether (3×). The organic layer was then washed with deionized H2 O to remove any residual salts. The combined organic layers were dried over MgSO4 and filtered. The organic layer was then concentrated under reduced pressure. The residue was purified via flash chromatography on silica gel (10:1 Hexanes:EtOAc).
4.3 General Procedure for the Synthesis of Methyl Phenylcyclopropanecarboxylates with Rh2(R-DOSP)4
In a 25-mL round bottom flask (Flask A) equipped with a magnetic stir bar, styrene (5 equiv.) and Rh2(R-DOSP)4 (0.01 equiv.) were dissolved in dry, degassed pentane (3 mL). The reaction mixture was then degassed using vacuum/argon cycles (×3). In a separate 25-mL round bottom flask (Flask B), the methyl phenyldiazoacetate (0.5 mmol, 1 equiv.) was dissolved in dry, degassed pentane (5 mL) and degassed using vacuum/argon cycles (×3). The contents in Flask B were then added to Flask A using a syringe pump for the duration of 1 hour. After the addition, the reaction mixture continued to stir for 1 additional hour. Once the allotted time had passed, the reaction mixture was concentrated under reduced pressure and purified using silica gel column chromatography (increasing gradient starting at 10:1 Hexanes:EtOAc).
4.4 General Procedure for the Synthesis of Methyl Phenylcyclopropanecarboxylates with Rh2(S-PTAD)4
In a 25-mL round bottom flask (Flask A) equipped with a magnetic stir bar, styrene (5 equiv.) and Rh2(S-PTAD)4 (0.005 equiv.) were dissolved in dry, degassed pentane (3 mL). The reaction mixture was then degassed using vacuum/argon cycles (×3). In a separate 25-mL round bottom flask (Flask B), the methyl phenyldiazoacetate (0.5 mmol, 1 equiv.) was dissolved in dry, degassed pentane (5 mL) and degassed using vacuum/argon cycles (×3). The contents in Flask B were then added to Flask A using a syringe pump for the duration of 1 hour. After the addition, the reaction mixture continued to stir for 1 additional hour. Once the allotted time had passed, the reaction mixture was concentrated under reduced pressure and purified using silica gel column chromatography (increasing gradient starting at 10:1 Hexanes:EtOAc).
4.5 General Procedure for the Synthesis of Methyl Phenylcyclopropanecarboxylates with Rh2(R-BNP)4
In a 25-mL round bottom flask (Flask A) equipped with a magnetic stir bar, styrene (5 equiv.) and Rh2(R-BNP)4 (0.01 equiv.) were dissolved in dry, degassed toluene (3 mL). The reaction mixture was then degassed using vacuum/argon cycles (×3). In a separate 25-mL round bottom flask (Flask B), the methyl phenyldiazoacetate (0.5 mmol, 1 equiv.) was dissolved in dry, degassed pentane (5 mL) and degassed using vacuum/argon cycles (×3). The contents in Flask B were then added to Flask A using a syringe pump for the duration of 1 hour. After the addition, the reaction mixture continued to stir for 1 additional hour. Once the allotted time had passed, the reaction mixture was concentrated under reduced pressure and purified using silica gel column chromatography (increasing gradient starting at 10:1 Hexanes:EtOAc).
4.1.1. (1R,2S)-methyl 1, 2-diphenylcyclopropanecarboxylate (3a)
Title compound was prepared by general procedure and obtained as a white solid. 85% yield for Rh2(R-DOSP)4; 87% yield for Rh2(S-PTAD)4; 72% yield for Rh2(R-BNP)4; HPLC: (Chiralcel SS-WHELK, 1% i-PrOH in hexane, 1.0 mL/min, 1 mg/mL, 30min, λ = 254 nm) retention times of 8.62 min (major) and 10.22 min (minor), 88% ee for Rh2(R-DOSP)4; 21% ee for Rh2 (S- PTAD)4; 42% ee for Rh2(R-BNP)4; Rf = 0.25 (hexane: ethyl acetate 10:1); 1H NMR (600 MHz, CDCl3) δ 7.12-7.11 (m, 3H), 7.05-7.01 (m, 5H), 6.77-6.75 (m, 2H), 3.70 (s, 3H), 3.11 (dd, J = 7.2, 9.3, 1H), 2.13 (dd, J = 4.8, 9.3, 1H), 1.88 (dd, J = 4.8, 7.2, 1H); 13C NMR (100 MHz, CDCl3) δ 174.5, 136.5, 134.9, 132.1, 128.2, 127.9, 127.2, 126.5, 52.8, 37.6, 33.3, 20.7; Data are consistent with the literature5,6.
4.1.2.(1S,2R)-methyl 2-phenyl-1-(p-tolyl)cyclopropanecarboxylate (3b)
Title compound was prepared by general procedure and obtained as a white solid. 84% yield for Rh2(R-DOSP)4; 77% yield for Rh2(S-PTAD)4; 69% yield for Rh2(R-BNP)4; HPLC: (Chiralcel SS-WHELK, 1% i- PrOH in hexane, 1.0 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 10.57 min (major) and 14.13 min (minor), 87% ee for Rh2(R-DOSP)4; 46% ee for Rh2(S-PTAD)4; 51% ee for Rh2(R-BNP)4; Rf = 0.44 (4:1 Hexanes:EtOAc); [α] 20D: + 17.2° (c. 1, chloroform); 1H NMR (400 MHz; CDCl3) δ 7.11-7.07 (m, 3H), 6.98-6.92 (m, 4H), 6.82-6.80 (m, 2H), 3.69 (s, 3H), 3.12 (dd, J = 9.2 and 7.2 Hz, 1H), 2.27 (s, 3H), 2.16 (dd, J = 9.6 and 4.8 Hz, 1H), 1.88 (dd, J = 7.2 and 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.8, 136.8, 136.7, 131.9, 131.8, 128.7, 128.3, 127.9, 126.5, 52.9, 37.3, 33.4, 21.4, 20.9; IR (film): 3028, 2950, 1716, 1255; m/z (ESI) 267.1 (100%, M+H), 268.1 (20%); HRMS-ESI m/z 167.138 (C18 H19 O2 requires 267.138).
4.1.3.(1S,2R)-methyl 2-phenyl-1-(4-(trifluoromethyl)phenyl)cyclopropanecarboxylate (3c)
Title compound was prepared by general procedure and obtained as a transparent oil. 93% yield for Rh2(R-DOSP)4; 67% yield for Rh2(S-PTAD)4; 80% yield for Rh2(R-BNP)4; HPLC: (Chiralcel OD-H, 0.5% i-PrOH in hexanes, 1.0 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 8.27 min (minor) and 10.34 min (major), 87% ee for Rh2(R-DOSP)4; 77% ee for Rh2(S-PTAD)4; 57% ee for Rh2(R-BNP)4; Rf = 0.44 (4:1 Hexanes:EtOAc); [α] 20D: 19.1 (c. 1, chloroform); 1H NMR (400 MHz; CDCl3) δ 7.36 (d, J = 8 Hz, 2H), 7.12 (d, J = 8 Hz, 2H), 7.06 (m, 3H), 6.74 (m, 2H), 3.66 (s, 3H), 3.14 (dd, J = 9.6 and 7.6 Hz, 1H), 2.17 (dd, J = 9.2 and 5.2 Hz, 1H), 1.88 (dd, J = 7.2 and 5.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 173.9, 139.2, 135.8, 132.4, 128.1, 126.9, 125.0, 124.9, 124.8, 124.7, 53.0, 37.2, 33.5, 20.4; IR (film): 3032, 2954, 1719, 1501, 1322, 1257; m/z (ESI) 321.1 (100%, M+H), 322.1 (21%); HRMS- m/z 321.1097 (C18 H16 O2 F3 requires 321.1097).
4.1.4. (1S,2R)-methyl 1-(4-chlorophenyl)-2-phenylcyclopropanecarboxylate (3d)
Title compound was prepared by general procedure and obtained as a transparent oil. 87% yield for Rh2(R-DOSP)4; 71% yield for Rh2(S-PTAD)4; 89% yield for Rh2(R-BNP)4; HPLC: (Chiralcel OJ-H, 1% i-PrOH in hexanes, 1.0 mL/min, 1mg/mL, 30 min, λ = 254 nm) retention times of 8.62 min (minor) and 12.58 min (major), 88% ee for Rh2(R-DOSP)4; 48% ee for Rh2(S-PTAD)4; 57% ee for Rh2(R-BNP)4; Rf = 0.56 (4:1 Hexanes:EtOAc); [α] 20D: 4.8 (c. 1, chloroform); 1H NMR (300 MHz; CDCl3) δ 7.12-7.06 (m, 5H), 6.95 (d, J = 8.7 Hz, 2H), 6.79-6.76 (m, 2H), 3.67 (s, 3H), 3.12(dd, J = 9.3 and 7.5 Hz, 1H), 2.15 (dd, J = 9.3 and 4.8 Hz, 1H), 1.85 (dd, J = 7.2 and 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.1, 136.1, 133.7, 133.4, 133.2, 128.3, 128.2, 128.1, 126.8, 52.9, 36.9, 33.4, 20.6; IR (film): 3031, 2951, 1717, 1493, 1255; m/z (ESI) 287.1 (100%, M+H), 288.1 (16.42%), 289.1 (29.3%); HRMS-ESI m/z 287.0833 (C17 H16 O2 Cl requires 287.0833).
4.1.5. (1S,2R)-methyl 1-(4-methoxyphenyl)-2-phenylcyclopropanecarboxylate (3e)
Title compound was prepared by general procedure and obtained as a white solid. 66% yield for Rh2(R-DOSP)4; 93% yield for Rh2(S-PTAD)4; 84% yield for Rh2(R-BNP)4; HPLC: (Chiralcel OD-H, 0.7% i-PrOH in hexanes, 1.0 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 11.97 min (major) and 17.71 min (minor), 90% ee for Rh2(R-DOSP)4; 96% ee for Rh2(S-PTAD)4; 57% ee for Rh2(R-BNP)4; Rf = 0.39 (4:1 Hexanes:EtOAc); [α] 20D = + 5.1° (c = 1, chloroform); 1H NMR (400 MHz; CDCl3) δ 7.10-7.07 (m, 3H), 6.95 (d, J = 8.8 Hz, 2H), 6.80-6.77 (m, 2H), 6.68 (d, J = 8.8 Hz, 2H), 3.74 (s, 3H), 3.68 (s, 3H), 3.09 (dd, J = 9.2 and 7.6 Hz, 1H), 2.14 (dd, J = 9.2 and 4.8 Hz, 1H), 1.84 (dd, J = 7.2 and 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.9, 158.6, 136.7, 133.1, 128.3, 127.9, 127.0, 126.5, 113.4, 55.3, 52.8, 36.9, 33.4, 21.0; IR(film): 3031, 2951, 2836, 1715, 1515, 1245; m/z (ESI) 283.1(100%, M+H), 284.1 (18.7%); HRMS-ESI m/z 283.1328(C18 H19 O3 requires 283.1329).
4.1.6. (1S,2R)-methyl 1-(2-chlorophenyl)-2-phenylcyclopropanecarboxylate (3f)
Title compound was prepared by general procedure and obtained as a transparent oil.89% yield for Rh2(R-DOSP)4; 80% yield for Rh2(S-PTAD)4; 78% yield for Rh2(R-BNP)4; HPLC: (Chiralcel OJ-H, 0.5% i-PrOH in hexanes, 1.0 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 11.47 min (major) and 15.27 min (major), 92% ee for Rh2(R-DOSP)4; 97% ee for Rh2(S-PTAD)4; 51% ee for Rh2(R-BNP)4; Rf = 0.43 (4:1 Hexanes:EtOAc); [α] 20D: 61.8 (c. 1, chloroform); 1H NMR (300 MHz; CDCl3) δ 7.18-7.02 (m, 7H), 6.84-6.76 (m, 2H), 3.68 (s, 3H), 3.13 (t, J = 8.7 Hz, 1H), 2.10 (m, 1H), 1.91 (dd, J = 7.2 and 5.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 173.6, 137.5, 133.5, 129.6, 128.9, 128.2, 127.6, 126.6, 126.4, 52.9, 33.5, 29.9, 21.7; IR (film): 3031, 2950, 1718, 1251, 694; m/z (ESI) 287.1 (100%, M+H), 288.1 (17%), 289.1 (32%); HRMS- m/z 287.0833 (C17 H16 O2 Cl requires 287.0833).
4.1.7. (1S,2R)-methyl 1-(2-methoxyphenyl)-2-phenylcyclopropanecarboxylate (3g)
Title compound was prepared by general procedure and obtained as a white solid. 92% yield for Rh2(R-DOSP)4; 87% yield for Rh2(S-PTAD)4; 69% yield for Rh2(R-BNP)4; HPLC: (SS-WHELK column, 1% i-PrOHin hexanes, 1.0 mL/min, 1mg/mL, 30 min, λ = 254 nm) retention times of 11.65 min (minor) and 14.00 min (major), 86% ee for Rh2(R-DOSP)4; 80% ee for Rh2(S-PTAD)4; 27% ee for Rh2(R-BNP)4; Rf = 0.45 (4:1 Hexanes:EtOAc); [α] 20D: + 133.3° (c. 1, chloroform); 1H NMR (400 MHz; CDCl3) δ 7.19-7.10 (m, 2H), 7.04-6.98 (m, 3H), 6.86 (td, J = 7.6, 1.1 Hz, 1H), 6.81-6.74 (m, 2H), 6.53 (d, J = 8 Hz, 1H), 3.65 (s, 3H), 3.36 (s, 3H), 3.24 (dd, J = 9.6 and 7.6 Hz, 1H), 1.98 (dd, J = 9.2 and 4.8 Hz, 1H), 1.85(dd, J = 7.2 and 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.7, 159.2, 137.0, 131.8, 128.9, 127.9, 127.3, 126.1, 124.1, 120.1, 110.5, 55.2, 52.7, 34.3, 32.6, 20.8; IR (film): 3030, 2950, 2835, 1716, 1496, 1242; m/z (ESI) 283.1 (100%, M+H), 284.1(19%); HRMS-ESI m/z 283.1329 (C18 H19 O3 requires 283.1329).
4.1.8. (1S,2R)-methyl 1-(3, 4-dichlorophenyl)-2-phenylcyclopropanecarboxylate (3h)
Title compound was prepared by general procedure and obtained as a clear oil; 88% yield for Rh2(R-DOSP)4; 88% yield for Rh2(S-PTAD)4; 82% yield for Rh2(R-BNP)4; HPLC: (Chiralcel OD-H, 1% i-PrOH inhexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 7.54 min (minor) and 9.78 min (major), 82% ee for Rh2(R-DOSP)4; 60% ee for Rh2(S-PTAD)4; 63% ee for Rh2(R-BNP)4; Rf = 0.23 (Hexane:EtOAc = 7:1); [α] 20D: _8.1o (c = 1.60, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.18-7.16 (m, 2H), 7.14-7.10 (m, 3H), 6.82-6.79 (m, 3H), 3.68 (s, 3H), 3.14 (dd, J = 9.2, 7.6 Hz, 1H), 2.15 (dd, J = 9.2, 4.8 Hz, 1H), 1.84 (dd, J = 7.6, 5.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 173.3, 135.3, 135.2, 133.7, 131.6, 131.4, 131.2, 129.6, 128.0, 127.9, 126.8, 52.8, 36.3, 33.3, 20.2; IR (film): 2925, 1719, 1257, 1163, 695; HRMS (ESI) calcd for C17 H13 O2 Cl2 (M-H) + 319.02927 found 319.02985.
4.1.9. (1S,2R)-methyl 1-(3, 4-dimethoxyphenyl)-2-phenylcyclopropanecarboxylate (3i)
Title compound was prepared by general procedure and obtained as a white solid. 74% yield for Rh2(R-DOSP)4; 70% yield for Rh2(S-PTAD)4; 93% yield for 1 mol% Rh2(R-BNP)4; 83% yield for 0.5 mol% Rh2(R-BNP)4; 80% yield for 0.1 mol% Rh2(R-BNP)4; 73% yield for 0.01 mol% Rh2(R-BNP)4; HPLC: (Chiralcel OD-H, 6% i-PrOHin hexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 13.99 min (minor) and 17.97 min (major), 56% ee for Rh2(R-DOSP)4; 94% ee for Rh2(S-PTAD)4; 97% ee for 1 mol% Rh2(R-BNP)4; 96% ee for 0.5 mol% Rh2(R-BNP)4; 91% ee for0.1 mol% Rh2(R-BNP)4; 40% ee for 0.01 mol% Rh2(R-BNP)4; Rf = 0.20 (hexane: ethyl acetate 4:1); mp 88-89°C; [α] 20D +28.4° (c= 1.51, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.09-7.07 (m, 3H), 6.81-6.79 (m, 2H), 6.69 (m, 2H), 6.36 (s, 1H), 3.81 (s, 3H), 3.68 (s, 3H), 3.56 (s, 3H), 3.08 (dd, J = 9.2, 7.6 Hz, 1H), 2.14 (dd, J = 9.2, 4.4 Hz, 1H), 1.84 (dd, J = 7.6, 4.8 Hz, 1H); 13CNMR (100 MHz, CDCl3) δ 174.5, 147.8, 136.4, 127.9, 127.7, 127.2, 126.3, 123.9, 115.3, 110.2, 55.5, 52.6, 36.9, 33.1, 20.8; IR (film): 2924, 1716, 1518, 1455, 1434, 1413, 1341, 1229, 1208, 1177; HRMS (ESI) calcd for C19H21O4 (M+H) + 313.14398 found 313.14332.
4.1.10. (1S,2R)-methyl 1-(3-methoxyphenyl)-2-phenylcyclopropanecarboxylate (3j)
Title compound was prepared by general procedure and obtained as a yellow oil. 78% yield for Rh2(R-DOSP)4; 72% yield for Rh2(S-PTAD)4; 82% yield for Rh2(R-BNP)4; HPLC: (Chiralcel OD-H, 0.7% i-PrOH inhexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm), retention times of 9.50 min (major) and 11.38 min (minor), 79% ee for Rh2(R-DOSP)4; 16% ee for Rh2(S-PTAD)4; 88% ee for Rh2(R-BNP)4; Rf = 0.25 (hexane: ethyl acetate 9:1); [α] 20D +21.1° (c =1.25, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.09-7.03 (m, 4H), 6.81-6.79 (m, 2H), 6.69-6.63 (m, 2H), 6.53-6.52, (m, 1H), 3.67(s, 3H), 3.59 (s, 3H), 3.12 (dd, J = 9.6, 7.2 Hz, 1H), 2.12 (dd, J = 9.2, 4.8 Hz, 1H), 1.87 (dd, J = 7.6, 5.2 Hz, 1H); 13C NMR (100MHz, CDCl3) δ 174.2, 158.8, 136.4, 136.2, 128.5, 127.9, 127.7, 126.3, 124.4, 117.5, 112.9, 55.0, 52.6, 37.3, 33.1, 20.6; IR (film): 2925, 1717, 1602, 1454, 1239, 697; HRMS (ESI) calcd for C18 H19 O3 (M+H) + 283.13344 found 283.13288.
4.1.11. (1S,2R)-methyl 1-(3, 5-dimethoxyphenyl)-2-phenylcyclopropanecarboxylate (3k)
Title compound was prepared by general procedure and obtained as a yellow oil. 85% yield for Rh2(R-DOSP)4; 85% yield for Rh2(S-PTAD)4; 69% yield for Rh2(R-BNP)4; HPLC: (Chiralcel OD-H, 6% i-PrOH inhexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 7.33 min (major) and 9.07 min (minor), 28% ee for Rh2(R-DOSP)4; 3% ee for Rh2(S-PTAD)4; 92% ee for Rh2(R-BNP)4; Rf = 0.31 (hexane: ethyl acetate 5:1); [α] 20D +33.7° (c =1.1, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.10-7.08 (m, 3H), 6.82 (dd, J = 7.2, 2.4 Hz, 2H), 6.24 (t, J =2.4 Hz, 1H), 6.15 (d, J =2.4 Hz, 2H), 3.68 (s, 3H), 3.56 (s, 6H), 3.08 (dd, J = 9.2, 7.2 Hz, 1H), 2.10 (dd, J = 9.2, 4.8 Hz, 1H), 1.84 (dd, J = 7.2, 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.4, 160.1, 137.2, 136.6, 128.2, 128.0, 126.6, 110.4, 99.8, 55.4, 52.9, 37.7, 33.3, 20.9; IR(film): 2951, 2840, 1715, 1594, 1456, 1425, 1260, 1203, 1151, 1046, 715, 692; HRMS (APCI) calcd for C19H21O4 (M+H) + 313.14344 found 313.14348.
4.1.12. (1S,2R)-methyl 2-phenyl-1-(3, 4, 5-trimethoxyphenyl)cyclopropanecarboxylate (3l)
Title compound was prepared by general procedure and obtained as a yellow oil. 98% yield for Rh2(R-DOSP)4; 95% yield for Rh2(S-PTAD)4; 63% yield for Rh2(R-BNP)4; HPLC: (Chiralcel OD-H, 6% i-PrOH inhexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm), retention times of 10.78 min (minor) and 11.79 min (minor), 34% ee for Rh2(R-DOSP)4; 9% ee for Rh2(S-PTAD)4; 90% ee for Rh2(R-BNP)4; Rf = 0.20 (hexane: ethyl acetate 5:1); [α] 20D +17.5° (c =2.02, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.09-7.07 (m, 3H), 6.81-6.78 (m, 2H), 6.16 (s, 2H), 3.76 (s, 3H), 3.69 (s, 3H), 3.59(s, 6H), 3.07 (dd, J = 9.2, 7.2 Hz, 1H); 2.14 (dd, J = 9.6, 4.8 Hz, 1H), 1.82 (dd, J = 7.6, 5.2 Hz, 1H); 13C NMR (100 MHz, CDCl3)δ 174.2, 152.3, 136.5, 130.3, 128.0, 127.7, 126.4, 109.4, 60.7, 55.9, 52.6, 37.4, 33.1 20.8; IR (film): 2925, 1716, 1587, 1413, 1258, 1235, 1153, 1124; HRMS (ESI) calcd for C20H23O4 (M+H) + 345.14852 found 345.14853.
4.1.13.(1S,2R)-methyl 1-(3,4-dimethoxyphenyl)-2-(m-tolyl)cyclopropanecarboxylate (5a)
Prepared by general procedure with methyl 2-diazo-2-(3,4-dimethoxyphenyl)acetate (118 mg, 0.5 mmol, 1 equiv), 2-methylstyrene (148 mg, 1.25 mmol, 2.5 equiv), and Rh2(R-BNP)4 (4 mg, 0.0025 mmol, 0.005 equiv). The remaining residue was purified on silica gel eluting with hexanes: ethyl acetate (5:1) to afford a yellow/green oil (114 mg, 76 % yield). Rf = 0.12 (hexane: ethyl acetate 5:1); [α] 20D +28.7° (c = 1.23, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.11 (d, J = 7.6 Hz, 1H), 7.00 (t, J = 7.6 Hz, 1H), 6.83 (t, J = 7.6 Hz, 1H), 6.63 (m, 2H), 6.42 (d, J = 7.6 Hz, 1H), 6.34 (s, 1H), 3.77 (s, 3H), 3.71 (s, 3H), 3.57 (s. 3H), 3.11 (dd, J = 9.2, 7.6 Hz, 1H), 2.51 (s, 3H), 2.06 (dd, J = 9.2, 5.0 Hz, 1H), 1.99 (dd, J = 7.6, 5.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.9, 147.9, 137.8, 134.7, 129.7, 127.8, 126.7, 126.0, 125.8, 123.7, 114.7, 110.3, 55.7, 55.6, 52.8, 36.0, 31.2, 20.2, 19.5; IR (film): 2951, 1713, 1516, 1463, 1435, 1251, 1140, 1026, 740; HRMS (APCI) calcd for C20H23O4 (M+H) + 327.15909 found 327.15909; HPLC: (Chiralcel OD-H, 3% i-PrOH in hexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 17.81 min (minor) and 19.31 min (major), 90% ee for Rh2(R-BNP)4, 64% ee for Rh2(R-DOSP)4.
4.1.14.(1S,2R)-methyl 1-(3,4-dimethoxyphenyl)-2-(p-tolyl)cyclopropanecarboxylate (5b)
Prepared by general procedure with methyl 2-diazo-2-(3,4-dimethoxyphenyl)acetate (118 mg, 0.5 mmol, 1 equiv), 4-methylstyrene (148 mg, 1.25 mmol, 2.5 equiv), and Rh2(R-BNP)4 (4 mg, 0.0025 mmol, 0.005 equiv). The remaining residue was purified on silica gel eluting with hexanes: ethyl acetate (5:1) to afford a yellow/green oil (147 mg, 90% yield). Rf = 0.12 (hexane: ethyl acetate 5:1); [α] 20D +27.7° (c = 1.24, CHCl3); 1H NMR (400 MHz, CDCl3) δ 6.88 (d, J = 8.0 Hz, 2H), 6.69-6.67 (m, 4H), 6.37 (s, 1H), 3.81 (s, 3H), 3.67 (s, 3H), 3.57 (s, 3H), 3.05 (dd, J = 9.2, 7.6 Hz, 1H), 2.22 (s, 3H), 2.11 (dd, J = 9.2, 4.8 Hz, 1H), 1.80 (dd, J = 7.6, 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.7, 147.9, 136.0, 133.5, 128.6, 128.0, 127.6, 124.1, 115.5, 110.3, 55.7, 55.6, 52.7, 36.9, 33.1, 21.0, 20.9; IR (film): 2951, 1714, 1589, 1516, 1435, 1413, 1341, 1315, 1250, 1228, 1155, 1027; HRMS (APCI) calcd for C20H23O4 (M+H) + 327.15909 found 327.15909; HPLC: (Chiralcel OD-H, 3% i-PrOH in hexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 17.3 min (minor) and 20.1 min (major), 88% ee for Rh2(R-BNP)4, 67% ee for Rh2(R-DOSP)4.
4.1.15.(1S,2R)-methyl 1-(3,4-dimethoxyphenyl)-2-(4-methoxyphenyl)cyclopropanecarboxylate (5c)
Prepared by general procedure with methyl 2-diazo-2-(3,4-dimethoxyphenyl)acetate (118 mg, 0.5 mmol, 1 equiv), 4- methoxystyrene (168 mg, 1.25 mmol, 2.5 equiv), and Rh2(R-BNP)4 (4 mg, 0.0025 mmol, 0.005 equiv. The remaining residue was purified on silica gel eluting with hexanes: ethyl acetate (5:1) to afford an off-white solid (137 mg, 80% yield). Rf = 0.20 (hexane: ethyl acetate 3:1); mp 123-125°C; [α] 20D +33.1° (c = 1.4, CHCl3); 1H NMR (400 MHz, CDCl3) δ 6.73-6.61 (m, 6H), 6.41 (s, 1H), 3.81 (s, 3H), 3.70 (s, 3H), 3.67 (s, 3H), 3.60 (s, 3H), 3.03 (dd, J = 9.2, 7.6 Hz, 1H), 2.10 (dd, J = 9.2, 4.8 Hz, 1H), 1.76 (dd, J = 7.6, 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 175.1, 158.6, 148.3, 129.5, 128.9, 127.9, 124.6, 115.9, 113.7, 110.7, 56.1, 55.6, 53.1, 37.1, 33.2, 21.3; IR (film): 2952, 1714, 1611, 1589, 1515, 1463, 1437, 1413, 1341, 1248, 1228, 1177, 1153; HRMS (APCI) calcd for C20H22O5 (M+H) + 342.14618 found 342.14589; HPLC: (Chiralcel OD-H, 5% i-PrOH in hexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 19.2 min (minor) and 22.9 min (major), 90% ee for Rh2(R-BNP)4, 62% ee for Rh2(R-DOSP)4.
4.1.16.(1S,2R)-methyl 1-(3,4-dimethoxyphenyl)-2-(4-nitrophenyl)cyclopropanecarboxylate (5d)
Prepared by general procedure with methyl 2-diazo-2-(3,4-dimethoxyphenyl)acetate (118 mg, 0.5 mmol, 1 equiv), 4-nitrostyrene (186 mg, 1.25 mmol, 2.5 equiv), and Rh2(R-BNP)4 (4 mg, 0.0025 mmol, 0.005 equiv). The remaining residue was purified on silica gel eluting with hexanes: ethyl acetate (5:1) to afford yellow oil (168 mg, 94% yield). Rf = 0.28 (hexane: ethyl acetate 3:1); [α] 20D +15.5° (c = 1.07, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.94 (d, J = 8.8 Hz, 2H), 6.92 (d, J = 8.8 Hz, 2H), 6.67 (d, J = 8.2 Hz, 1H), 6.61 (dd, J = 8.2, 2.0 Hz, 1H), 6.43 (d, J = 2.0 Hz,, 1H), 3.81 (s, 3H), 3.69 (s, 3H), 3.62 (s, 3H), 3.16 (dd, J = 9.2, 7.6 Hz, 1H), 2.22 (dd, J = 9.2, 5.4 Hz, 1H), 1.91 (dd, J = 7.6, 5.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 173.9, 148.5, 145.1, 128.7, 126.2, 124.2, 123.0, 115.0, 110.6, 55.9, 55.8, 53.0, 38.4, 32.6, 29.8, 21.8; IR (film): 2924, 1717, 1597, 1515, 1463, 1415, 1342, 1229, 1206, 1156, 1141; HRMS (APCI) calcd for C19H20O6N1 (M+H) + 358.12851 found 358.12836; HPLC: (Chiralcel OD-H, 6% i-PrOH in hexane, 1 mL/min, 1 mg/mL, 60 min, λ = 254 nm) retention times of 30.4 min (minor) and 41.0 min (major), 80% ee for Rh2(R-BNP)4, 50% ee for Rh2(R-DOSP)4.
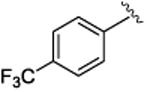
4.1.17.(1S,2R)-methyl 1-(3,4-dimethoxyphenyl)-2-(4-trifluoromethyl)phenyl)cyclopropanecarboxylate (5e)
Prepared by general procedure with methyl 2-diazo-2-(3,4-dimethoxyphenyl)acetate (118 mg, 0.5 mmol, 1 equiv), styrene (215 mg, 1.25 mmol, 2.5 equiv), and Rh2(R- BNP)4 (4 mg, 0.0025 mmol, 0.005 equiv). The remaining residue was purified on silica gel eluting with hexanes: ethyl acetate (6:1) to afford a yellow oil (171 mg, 90% yield); Rf = 0.24 (hexane: ethyl acetate 4:1); [α] 20D +16.5° (c = 1.6, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.33 (d, J = 8.2 Hz, 2H), 6.88 (d, J = 8.2 Hz, 2H), 6.70-6.65 (m, 2H), 6.35 (s, 1H), 3.81 (s, 3H), 3.68 (s, 3H), 3.56 (s, 3H), 3.11 (t, 1H), 3.12 (dd, J = 9.2, 7.2 Hz, 1H), 2.18 (dd, J = 9.2, 4.8 Hz, 1H), 1.85 (dd, J = 7.2, 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.5, 148.7, 148.6, 141.5, 128.7, 127.0, 124.8, 124.7, 124.6, 124.3, 115.6, 110.9, 56.1, 53.2, 38.0, 33.0, 21.7; IR (film): 2954, 1718, 1517, 1465, 1437, 1414, 1324, 1252, 1230, 1210, 1193; HRMS (APCI) calcd for C20H19F3O4 (M+H) + 380.12300 found 380.12251; HPLC: (Chiralcel OD-H, 6% i-PrOH in hexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 12.6 min (minor) and 15.6 min (major), 90% ee for Rh2(R-BNP)4, 63% ee for Rh2(R-DOSP)4.
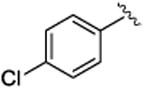
4.1.18. (1S,2R)-methyl 2-(4-chlorophenyl)-1-(3,4-dimethoxyphenyl)cyclopropanecarboxylate (5f)
Prepared bygeneral procedure with methyl 2-diazo-2-(3, 4-dimethoxyphenyl)acetate (118 mg, 0.5 mmol, 1 equiv), 4-chlorostyrene (173 mg, 1.25 mmol, 2.5 equiv), and Rh2(R-BNP)4 (4 mg, 0.0025 mmol, 0.005 equiv). The remaining residue was purified on silica gel eluting with hexanes: ethyl acetate (6:1) to afford clear oil (123 mg, 89% yield). Rf = 0.30 (hexane: ethyl acetate 3:1); [α] 20D +13.4° (c = 1.2, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.04 (d, J = 8.4 Hz, 2H), 6.72-6.63 (m, 4H), 6.38 (d, J = 1.6 Hz, 1H), 3.81 (s, 3H), 3.67 (s. 3H), 3.61 (s, 3H), 3.04 (dd, J = 9.2, 7.2 Hz, 1H), 2.13 (dd, J = 9.2, 4.8 Hz, 1H), 1.78 (dd, J = 7.2, 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.4, 148.2, 148.2, 135.4, 132.3, 129.4, 128.0, 127.0, 124.2, 115.4, 110.5, 55.9, 55.8, 52.8, 37.2, 32.6, 21.2; IR (film): 2951, 1715, 1589, 1517, 1496, 1435, 1413, 1371, 1341, 1307, 1228, 1177, 1155, 1139; HRMS (APCI) calcd for C19H19ClO4 (M+H) + 346.09664 found 346.09632; HPLC: (Chiralcel OD-H, 5% i-PrOH in hexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 14.9 min (minor) and 18.5 min (major), 89% ee for Rh2(R-BNP)4, 67% ee for Rh2(R-DOSP)4.
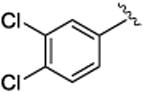
4.1.19. (1S,2R)-methyl 2-(3,4-dichlorophenyl)-1-(3,4-dimethoxyphenyl)cyclopropanecarboxylate (5g)
Methyl 2-diazo-2-(3, 4-dimethoxyphenyl)acetate (2.7g, 11.6 mmol, 1 equiv) in 153 mL dry and degassed toluene was added by syringe pump over 2 h to a solution of 1, 2-dichloro-4-vinylbenzene (2.4 g, 13.9 mmol, 1.2 equiv) and Rh2(R-BNP)4 (92.2 mg, 0.06 mmol, 0.005 equiv) in 77 mL toluene. After addition, the solution was allowed to stir overnight and toluene was removed in vacuo. The remaining residue was purified on silica gel (hexane:ethyl acetate 5:1) to afford a clear oil (3.2 g, 72% yield for Rh2(R-BNP)4 [enantiomer shown above]; 2.3g, 52% yield for Rh2(S-BNP)4) Rf = 0.17 (hexane: ethyl acetate 5:1); [α] 20D +4.1° (c = 1.03, CHCl3) for Rh2(R-BNP)4; [α] 20D -6.9° (c = 1.05, CHCl3) for Rh2(S-BNP)4; 1H NMR (600 MHz, CDCl3) δ 7.10 (d, J = 9.0 Hz, 1H), 7.01 (d, J = 2.4 Hz, 1H), 6.69 (d, J = 9.0 Hz, 1H), 6.63 (dd, J = 9.0, 2.4 Hz, 1H), 6.51 (dd, J = 9.0, 2.4 Hz, 1H), 6.44 (d, J = 1.8 Hz, 1H), 3.82 (s, 3H), 3.67 (s, 3H), 3.66 (s, 3H), 3.02 (dd, J = 9.0, 7.2 Hz, 1H), 2.13(dd, J = 9.0, 5.1 Hz, 1H), 1.78 (dd, J = 7.2, 5.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.1, 148.4, 148.3, 137.4, 131.9, 130.5, 130.4, 129.7, 127.0, 126.6, 124.2, 115.2, 110.6, 55.9, 55.8, 52.9, 37.4, 32.1, 21.1; IR (film): 2951, 2836, 1716, 1559, 1516, 1413, 1229, 1178, 1137, 1027, 764; HRMS awaiting results; HPLC: (Chiralcel OD-H, 5% i-PrOH in hexane, 1 mL/min, 1 mg/mL, 30 min, λ = 254 nm) retention times of 15.4 min (minor) and 17.9 min (major), 86% ee for Rh2(R-BNP)4; retention times of 15.3 min (major) and 19.7 min (minor) 85% ee for Rh2(S-BNP)4, 48% ee for Rh2(R-DOSP)4.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (DA023224).
Footnotes
Supplementary Material: Detailed experimental for the compounds, HPLC traces for all chiral compounds, 1H NMR and 13C NMR spectra for all new compounds are described in Supplementary data. Supplementary data associated with this article can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lebel H, Marcoux JF, Molinaro C, Charette AB. Chem Rev. 2003;103:977. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- 2.Davies HML, Antoulinakis EG. Org React. 2001;1:1–313. [Google Scholar]
- 3.Davies HML, Bruzinski PR, Lake DH, Kong N, Fall MJ. J Am Chem Soc. 1996;118:6897. [Google Scholar]
- 4.Davies HML, Rusiniak L. Tet Lett. 1998;39:8811. [Google Scholar]
- 5.Davies HML, Panaro SA. Tet Lett. 1999;40:5287. [Google Scholar]
- 6.Davies HML, Venkataramani C. Org Lett. 2003;5:1403. doi: 10.1021/ol034002a. [DOI] [PubMed] [Google Scholar]
- 7.Kozikowski A, Kurome T, Setola V, Roth B. US Patent. 2009 Aug 13;:203–750.
- 8.Cho SJ, Jensen NH, Kurome T, Kadari S, Manzano ML, Malberg JE, Caldarone B, Roth BL, Kozikowski AP. J Med Chem. 2009;52:1885. doi: 10.1021/jm801354e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies HML, Townsend RJ. J Org Chem. 2001;66:6595. doi: 10.1021/jo015617t. [DOI] [PubMed] [Google Scholar]
- 10.Davies HML, Nagashima T, Klino JL., III Org Lett. 2000;2:823. doi: 10.1021/ol005563u. [DOI] [PubMed] [Google Scholar]
- 11.Ventura DL, Li Z, Coleman MG, Davies HML. Tetrahedron. 2009;65:3052. [Google Scholar]
- 12.Davies HML, Coleman MG, Ventura DL. Org Lett. 2007;9:4971. doi: 10.1021/ol702218w. [DOI] [PubMed] [Google Scholar]
- 13.Davies HML, Boebel TA. Tet Lett. 2000;41:8189. [Google Scholar]
- 14.Hedley SJ, Ventura DL, Dominiak PM, Nygren CL, Davies HML. J Org Chem. 2006;71:5349. doi: 10.1021/jo060779g. [DOI] [PubMed] [Google Scholar]
- 15.Davies HML, Panaro SA. Tetrahedron. 2000;56:4871. [Google Scholar]
- 16.Bonge HT, Pintea B, Hansen T. Org Biomol Chem. 2008;6:3670. doi: 10.1039/b814374a. [DOI] [PubMed] [Google Scholar]
- 17.Bonge HT, Hansen T. J Org Chem. 2010;75:2309. doi: 10.1021/jo100113b. [DOI] [PubMed] [Google Scholar]
- 18.Bonge HT, Hansen T. Tet Lett. 2010;51:5298. [Google Scholar]
- 19.Reddy RP, Lee GH, Davies HML. Org Lett. 2006;8:3437. doi: 10.1021/ol060893l. [DOI] [PubMed] [Google Scholar]
- 20.Denton JR, Sukumaran D, Davies HML. Org Lett. 2007;9:2625. doi: 10.1021/ol070714f. [DOI] [PubMed] [Google Scholar]
- 21.Denton JR, Cheng K, Davies HML. Chem Commun. 2008:1238. doi: 10.1039/b719175h. [DOI] [PubMed] [Google Scholar]
- 22.Denton JR, Davies HML. Org Lett. 2009;11:787. doi: 10.1021/ol802614j. [DOI] [PubMed] [Google Scholar]
- 23.Pirrung MC, Zhang J. Tet Lett. 1992;33:5987. [Google Scholar]
- 24.McCarthy N, McKervey MA, Ye T, McCann M, Murphy E, Doyle MP. Tet Lett. 1992;33:5983. [Google Scholar]
- 25.Liao M, Wang J. Green Chem. 2007;9:184. [Google Scholar]
- 26.Hodgson DM, Stupple PA, Johnstone C. Chem Commun. 1999:2185. [Google Scholar]
- 27.Hodgson DM, Stupple PA, Pierard FYTM, Labande AH, Johnstone C. Chem Eur J. 2001;7:4465. doi: 10.1002/1521-3765(20011015)7:20<4465::aid-chem4465>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.The absolute configuration of the cyclopropanes is tenatively assigned assuming a similar assymmetric induction as was observed with styryldiazoacetates (see Davies HMLHNJS, Cantrell WR, Jr, Olive JL. J Am Chem Soc. 1993;115:9468..)
- 29.Pelphrey P, Hansen J, Davies HML. Chem Sci. 2010;1:254. [Google Scholar]
- 30.Brooks LA. J Am Chem Soc. 1944;66:1295. [Google Scholar]
- 31.Davies HML, Cantrell WR, Jr, Romines KR, Baum JS. Org Synth. 1992;70:93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.