Abstract
Objectives
Our aim was to project the outcomes of using either efavirenz or nevirapine as part of initial antiretroviral therapy (ART) in women of childbearing age in Côte d’Ivoire.
Methods
We used an HIV computer simulation model to project both the mother’s survival and the birth defects at 10 years for a cohort of women who started ART with either efavirenz or nevirapine. The primary outcome was the ratio at 10 years of the difference in the number of women alive to the difference in the cumulative number of birth defects in women who started ART with efavirenz compared to nevirapine. In the base case analysis, the birth defect rate was 2.9% on efavirenz and 2.7% on nevirapine. In sensitivity analyses we varied all inputs across confidence intervals reported in the literature.
Results
In the base case analysis, for a cohort of 100,000 women, the additional number of women alive initiating ART with efavirenz at 10 years was 15 times the additional number of birth defects (women alive: nevirapine 67,969, efavirenz 68,880, difference = 911; birth defects: nevirapine 1,128, efavirenz 1,187, difference = 59). In sensitivity analysis, the teratogenicity rate with efavirenz had to be 6.3%, or 2.3 times higher than the rate with nevirapine, for the excess number of birth defects to outweigh the additional number of women alive at 10 years.
Conclusions
In Côte d’Ivoire, initiating ART with efavirenz instead of nevirapine is likely to substantially increase the number of women alive at 10 years with a smaller potential number of birth defects.
Keywords: efavirenz, women, sub-Saharan Africa, teratogenicity, survival, pregnancy, antiretroviral therapy
INTRODUCTION
World Health Organization (WHO) guidelines for antiretroviral treatment (ART) in resource- limited countries recommend that first-line ART regimens should consist of one non-nucleoside reverse transcriptase inhibitor (NNRTI) and two nucleoside reverse transcriptase inhibitors (NRTIs) (1). The rationale for using NNRTIs in first-line ART while sparing protease inhibitors for second line is due to consideration of cost, ease of use, and resistance (2–3).
Two NNRTIs are available for first-line therapy: efavirenz and nevirapine. Efavirenz has two advantages over nevirapine. First, although efavirenz and nevirapine have similar risks of mucocutaneous and liver toxicities, these side-effects are much less frequent and of lower severity with efavirenz than with nevirapine (4). The most frequent side-effects from efavirenz are transient central nervous system symptoms, which rarely lead to drug discontinuation (5–6). Second, efavirenz can be safely routinely prescribed with rifampicine, the first-line treatment for tuberculosis, while the interaction between nevirapine and rifampicine is still unclear (7). Subsequently, since HIV-associated tuberculosis is extremely common in sub-Saharan Africa, nevirapine often needs to be discontinued (8).
However, reports of teratogenicity gave efavirenz one major disadvantage over nevirapine. Based on animal studies and human case reports (9–11), the United States Food and Drug Administration classifies efavirenz as a category D drug, thereby strictly recommending against efavirenz use during pregnancy (12). The 2010 revised WHO guidelines for ART allows efavirenz to be prescribed during the second and third trimesters of pregnancy, but not during the first trimester (13). As a consequence, efavirenz should not be prescribed in women of child-bearing age who are not using effective contraception. Due to limited access and ineffective use of contraception in sub-Saharan Africa (14–16), many physicians prescribe nevirapine rather than efavirenz in women of child-bearing age (1).
The consequences of these recommendations are unclear. Neither the additional risk of teratogenicity from efavirenz compared to nevirapine nor the potential harmful long-term consequences for women of starting ART with nevirapine rather than with efavirenz have been accurately assessed. Because efavirenz is strictly not recommended in women of child-bearing age without effective contraception, clinical studies comparing the maternal survival and pregnancy outcomes with both drugs would not be ethical.
We therefore used a simulation model to assess the trade-off between potential excess birth defects and potential long-term clinical benefits for women starting ART with efavirenz, rather than with nevirapine.
METHODS
Analytic Overview
Using a previously-validated computer-based simulation model of HIV disease in resource-limited settings (17–19), we projected the clinical benefits for women and the risk of birth defects for their infants in two cohorts of women of child-bearing age in Côte d’Ivoire: one started ART with an efavirenz-based regimen (EFV), and the other started ART with a nevirapine-based regimen (NVP). Inputs were derived from cohort studies and clinical trials in sub-Saharan Africa. We used a mean pre-ART CD4 count of 154/μl, allowing the prescription of NVP. In the base case analysis, we assumed EFV and NVP had similar efficacy (20), but that NVP had a higher rate of toxicity-related drug discontinuation, as well as a higher rate of fatal toxicity than EFV (8). In sensitivity analysis, we varied major inputs widely. The main outputs were the number of women alive at 10 years and the cumulative number of birth defects at 10 years for a cohort of women starting EFV compared to one starting NVP. The cumulative number of birth defects was calculated in a separated decisions tree model using time of exposure to ART, the rate of pregnancy, the rate of live birth and the rate of birth defect. Using the main outputs, we calculated the ratio of the difference between both cohorts in the absolute number of women alive at 10 years to the difference in the cumulative absolute number of birth defects. Finally, we estimated the excess in birth defect rate with efavirenz compared to nevirapine that would equalize the additional number of women alive at 10 years and the additional number of birth defects with efavirenz compared to nevirapine.
Model description
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC)-International model is a state-transition, first-order Monte Carlo simulation model of HIV infection incorporating natural history, disease progression, and treatment (18–19, 21). The model divides HIV infection into “health states,” which include an acute state, a chronic state, and death. Simulated patients are randomly selected from an initial distribution of age, CD4 count, and HIV RNA level, and transition monthly to different health states. The probability of transition from one state to another depends on the CD4 stratum and the associated risk of opportunistic infections (OIs) or death (22) as well as ART. HIV RNA levels determine the rate of CD4 count decline in the absence of ART (23). Successful ART decreases HIV RNA and increases CD4 count. The model also takes into account ART toxicity, ART switching criteria, resistance, and number of ART lines available. Using the model, we simulated outcomes for two cohorts of 100,000 women, one starting ART with efavirenz and one with nevirapine.
Base case inputs
Baseline cohort characteristics, morbidity, and mortality data were derived from four studies in Côte d’Ivoire (24–26) (Table 1 All women started ART upon entry into the model. Two lines of ART were available; the first consisted of two NRTIs (tenofovir and emtricitabine) and either efavirenz or nevirapine, and the second-line consisted of two NRTIs (didanosine and abacavir) and lopinavir/ritonavir (1). Patients had a CD4 count assessed every six months. HIV RNA testing was not routinely available. Switching from first-line to second-line was dictated by immunological criteria, as recommended by the WHO in the absence of HIV RNA monitoring (1).
Table 1.
Main model input parameters
| Base case value | Sensitivity analyses (4)
|
References | ||
|---|---|---|---|---|
| Range | Type | |||
|
|
|
|
||
| Baseline characteristics | ||||
| Mean age, years (SD) | 33.6 (7.7) | [26 – 41] | CI | (24) |
| Mean CD4, cells/μl (SD) | 154 (102) | [52 – 256] | CI | (20) |
| Sex, female, % | 100 | |||
| Plasma HIV-1 RNA, copies/ml, % | ||||
| >100,000 | 52.6 | (24) | ||
| 30,001–10,0000 | 21.8 | (24) | ||
| 10,001–30,000 | 13.1 | (24) | ||
| 3,001–10,000 | 5.5 | (24) | ||
| 501–3,000 | 3.1 | (24) | ||
| ≤ 500 | 3.9 | (24) | ||
| Monitoring | ||||
| Interval between clinic visits, months | 3 | 1; 6 months | Extr. values | ASMPT |
| Interval between CD4 tests, months | 6 | 3; 12 months | Extr. values | ASMPT |
| First-line ART efficacy and toxicity | ||||
| HIV-1 RNA suppression at 24 weeks, % (1) | 80.2 | [65 – 85] | Extr. Values | (2, 20) |
| CD4 cell/μl increase at 24 weeks (1) | 152 | (20)* | ||
| Toxicity, % | ||||
| EFV: | ||||
| Drug discontinuation | 0.1 | [0 – 6.3] | Extr. values | (8) |
| Fatal toxicity (2) | 0.1 | [0 – 1.0] | Extr. values | (27) |
| NVP: | ||||
| Drug discontinuation | 6.3 | [4.3 – 8.3] | CI | (8) |
| Fatal toxicity (2) | 1.0 | [0 – 3.0] | Extr. values | (28) |
| Pregnancy | ||||
| Pregnancy rate, per 100 woman-years (3) | 7.9 | [5.0 – 15.0] | Extr. values | (9) |
| Outcomes, % | ||||
| Abortion | 11.7 | (29) | ||
| Miscarriage | 5.2 | (29) | ||
| Stillborn | 6.7 | (29) | ||
| Live births (3) | 76.4 | [60.0 – 85.0] | Extr. values | (29) (9, 34) |
| Birth defects, % | ||||
| EFV | 2.9 | (30) | ||
| NVP | 2.7 | (30) | ||
EFV: ART regimen including efavirenz and 2 nucleoside reverse transcriptase inhibitors (tenofovir + emtricitabine); NVP: ART regimen including nevirapine and 2 nucleoside reverse transcriptase inhibitors (tenofovir + emtricitabine);
In the base case analysis we assumed that EFV and NVP had the same immunological and virological efficacy;
In the base case analysis we assumed that 0.1% of the 0.1% patients who had major toxicity on EFV died (0.001 × 0.001 = 0.00001), and 1% of the 6.3% patients who had major toxicity on NVP died (0.063 × 0.01 = 0.00063);
In the base case analysis we assumed that women on EFV or NVP had the same rate of pregnancy and live birth;
In one-way sensitivity analysis, we varied each parameter within the range of confidence interval (CI) or of highest or lowest values (Extreme values - Extr.values) as found in the literature. The column ‘references’ refers to the papers where CI and extreme values were found (ASMPT: assumption).
In the base case analysis, we used three assumptions, all of which were tested in sensitivity analyses. First, we assumed that EFV and NVP had similar virologic and immunologic efficacy (20, 26), and NVP had a higher rate of toxicity leading to drug discontinuation than EFV (8, 27–28). Second, we assumed that the rate of pregnancy was constant over time and disease stage, and did not vary from age 25 to 45 or according to ART regimen (9, 29). Finally, we assumed that the rate of birth defect was 0.2% higher with EFV than NVP, based on data from the US Antiretroviral Pregnancy Registry that had reported a birth defect rate of 2.7% for women on NVP and 2.9% for women on EFV (30). We used the same birth defect rate as NVP for the other ART regimens.
Model Outputs
The main model output was the number of women alive. Secondary outputs included time of exposure to each first-line and second-line ART regimen. We then incorporated the latter time of exposure into a decision tree, which included rates of pregnancy, live births, birth defects, abortions, miscarriages, and stillborns, to calculate the cumulative number of babies born with birth defects at 10 years using the following formula:
Where:
Time_expoi = total time of exposure to ART regimen i at 10 years;
Rate_Bdefi = the birth defect rate due to ART regimen i
i =1, 2, 3…n = the different lines of ART regimen
Study main outcomes
This study had two outcomes. The primary outcome was the difference in number of women alive per additional birth defect (“Δ NWA per additional birth defect”), defined as the ratio of the difference in the absolute number of women alive at 10 years to the difference between both populations in the cumulative absolute number of birth defects at 10 years. This outcome was calculated using the following formula:
Where:
NWA = number of women alive at 10 years
NBD = number of birth defects over 10 years
The secondary outcome was the “equivalence birth defect rate difference,” defined as the difference in birth defect rate per 100 live births that would be necessary to equalize the additional number of women alive at 10 years and the additional number of birth defects with EFV.
Sensitivity Analysis
Sensitivity analyses were done in three steps.
First, in one-way sensitivity analysis, we varied all major parameters in the model. These included patient and regimen characteristics (age, pre-ART CD4 count, virological efficacy of EFV and NVP-based regimens, pregnancy rate (31–32), birth defect rate, drug discontinuation and fatal toxicity rates of EFV and NVP-based regimens), as well as program characteristics (interval between clinic visits, interval between CD4 counts, third-line ART availability for patients failing second-line ART, rate of lost-to-follow-up, and availability of contraception). The latter included a mixed-scenario in which the women on effective contraception received EFV while the others received NVP. One-way sensitivity analyses were either confidence interval or extreme case range analyses, according to the confidence intervals or extreme values found in the literature.
Second, we classified the parameters into three groups according their impact on the outcomes. If the sensitivity analysis on a variable produced a variation in Δ number of women alive greater than ±10 per additional birth defect or a variation in equivalence birth defect rate difference greater than ±3%, that variable was classified as ‘highly-sensitive’. If the variation in Δ number of women alive per additional birth defect was between ±5 and ±10 or the variation in equivalence birth defect rate difference was between ±1.5% and ±3%, the variable was classified as ‘moderately sensitive’. Finally, if the variation was less than ±5 for the Δ number of women alive per additional birth defect and less than ±1.5% for the equivalence birth defect rate difference, the variable was classified as ‘minimally-sensitive’.
Third, we included all highly-sensitive variables in a multi-way sensitive analysis.
RESULTS
Base case analysis
During the first 10 years of ART, 92 of the 100,000 women who started ART with EFV experienced severe acute toxicity (of whom none died and all 92 switched to LPV/r), compared to 6,000 of the 100,000 women who started ART with NVP (of whom 62 died and 5937 switched to LPV/r). In women who started ART with EFV, the mean time per woman spent on EFV and on LPV/r was 7.03 years and 8.61 years. In women who started with NVP, the mean time spent on NVP and on LPV/r was 6.61 and 8.63 years. As shown in Table 2, 68,880 of the 100,000 women who started ART with EFV were alive at 10 years, compared to 67,969 women who started ART with NVP. The cumulative number of birth defects that occurred over the 10 years was 1,187 in women who started with EFV and 1,128 in women who started with NVP. Thus, the difference in absolute number of women alive at 10 years was 911, and the difference in absolute number of birth defects on EFV compared to NVP was 59. The “difference in number of women alive per additional birth defect,” was 15, and the “equivalence birth defect rate difference” was 3.4 per 100 live births. As the birth defect rate with EFV was 2.9 per 100, this means that the absolute birth defect rate with EFV had to be greater than 6.3 per 100 live births, and the birth defect rate with EFV had to be more than 2.3 times as higher as the birth defect rate with NVP for the additional number of birth defects to exceed the additional number of women alive at 10 years in women starting ART with EFV (Table 2).
Table 2.
10-year outcomes for a cohort of 100,000 women of child-bearing age starting on EFV or NVP-based ART regimens in Côte d’Ivoire: base case analysis
| EFV | NVP | Δ (EFV-NVP) | |
|---|---|---|---|
| Women | |||
| At 10 years, number of women: | |||
| Alive | 68,880 | 67,969 | 911 |
| Still on first-line regimen | 24,585 | 23,121 | 1,464 |
| With undetectable plasma HIV-1 RNA | 65,144 | 64,613 | 531 |
| Overall time on ART during child-bearing years, person-years | 694,341 | 692,413 | 1,928 |
| Including time on first-line ART (EFV or NVP) | 456,153 | 430,748 | 25,405 |
| Pregnancies and newborns | |||
| Pregnancies at 10 years, number | 54,853 | 54,701 | 152 |
| Abortions, miscarriages and stillbirths | 12,945 | 12,909 | 36 |
| Live births | 41,908 | 41,791 | 117 |
| Birth defects | 1,187 | 1,128 | 59 |
| Combined outcomes | |||
| Δ NWA per additional birth defect (1) | 15 | ||
| Equivalence birth defect rate difference (2) | 3.4% | ||
EFV: ART regimen including efavirenz and 2 nucleoside reverse transcriptase inhibitors (tenofovir + emtricitabine); NVP: ART regimen including nevirapine and 2 nucleoside reverse transcriptase inhibitors (tenofovir + emtricitabine);
Where:
- NWA = number of women alive at 10 years
- NBD = number of birth defects over 10 years
Equivalence birth defect rate difference: difference in birth defect rate per 100 live births between EFV and NVP that would be necessary to make the NWA at 10 years equivalent to the number of birth defects
Sensitivity analysis
In one-way confidence interval and extreme case sensitivity analysis, both the Δ NWA per additional birth defect and/or the equivalence birth defect rate difference were highly sensitive to the pregnancy rate, the difference in virologic efficacy between EFV and NVP and EFV toxicity-induced drug discontinuation (Table 3).
Table 3.
10-year outcomes for a cohort of 100,000 women on EFV or NVP-based ART regimens in Côte d’Ivoire: One-way sensitivity analysis (patients and treatment characteristics)
| Δ Women alive, at 10 years (1) | Δ Birth defects, at 10 years (1) | Δ NWA per additional birth defect (2) | Equivalence birth defect rate difference (3) | |
|---|---|---|---|---|
| Base case | 911 | 59 | 15 | 3.4% |
| Mean Age, years | ||||
| 26 | 913 | 68 | 13 | 3.0% |
| 41 | 836 | 38 | 22 | 4.8% |
| Mean CD4, cells/μl | ||||
| 52 | 795 | 49 | 16 | 3.7% |
| 256 | 949 | 66 | 14 | 3.2% |
| HIV-1 RNA suppression at 6 months, % | ||||
| Varying both regimens, with EFV=NVP | ||||
| EFV : 65 ; NVP : 65 | 922 | 53 | 17 | 4.0% |
| EFV: 85 ; NVP: 85 | 1023 | 61 | 17 | 3.7% |
| Varying EFV only | ||||
| EFV : 78 ; NVP : 80.2 | 323 | 53 | 6 | 1.3% |
| EFV: 82 ; NVP: 80.2 | 1474 | 63 | 23 | 5.4% |
| EFV-based regimen toxicity, % | ||||
| Drug discontinuation | ||||
| 0 (EFV < NVP: −6.3%) | 961 | 58 | 17 | 3.6% |
| 6.3 (EFV = NVP) | 190 | 54 | 4 | 0.9% |
| Fatal toxicity (2) | ||||
| 0 (EFV < NVP: −1%) | 1040 | 59 | 18 | 3.9% |
| 1.0 (EFV = NVP) | 915 | 59 | 16 | 3.5% |
| NVP-based regimen toxicity, % | ||||
| Drug discontinuation | ||||
| 4.3 (NVP > EFV: +4.2) | 937 | 59 | 16 | 3.5% |
| 8.3 (NVP > EFV: +8.2) | 930 | 60 | 16 | 3.5% |
| Fatal toxicity | ||||
| 0.1 (NVP = EFV) | 973 | 58 | 17 | 3.7% |
| 3.0 (NVP > EFV: +2.9) | 979 | 59 | 17 | 3.7% |
| Pregnancy rate/100 person-years | ||||
| 5.0 | 911 | 35 | 26 | 5.3% |
| 15.0 | 911 | 105 | 9 | 1.8% |
| Live births, % | ||||
| 60.0 | 911 | 43 | 21 | 4.3% |
| 85.0 | 911 | 61 | 15 | 3.0% |
EFV: ART regimen including efavirenz and 2 nucleoside reverse transcriptase inhibitors (tenofovir + emtricitabine); NVP: ART regimen including nevirapine and 2 nucleoside reverse transcriptase inhibitors (tenofovir + emtricitabine);
Δ: Difference between EFV and NVP
Where:
- NWA = number of women alive at 10 years
- NBD = number of birth defects over 10 years
Equivalence birth defect rate difference: difference in birth defect rate per 100 live births between EFV and NVP that would be necessary to make the number of women alive at 10 years equivalent to the number of birth defects
Decreasing the pregnancy rate increased the ratio of the benefits for women to the risks for children on EFV. When the pregnancy rate decreased from 15 per 100 woman-years (32) to 5 per 100 woman-years (31), the “Δ NWA per additional birth defect” increased from 9 to 26 and the “equivalence birth defect rate difference” increased from 1.8 per 100 live births (i.e. absolute birth defect rate with EFV was 4.7 per 100 live births, 1.7 times as high as the birth defect rate with NVP) to 5.3 per 100 live births (i.e. absolute birth defect rate with EFV was 8.2 per 100 live births, 3 times as high as the birth defect rate with NVP) (Table 3). To make the “Δ NWA per additional birth defect” lower than 1.0, the pregnancy rate had to be over 50 per 100 woman-years.
When assuming that EFV efficacy at 6 months was 2% higher than that of NVP, the “Δ NWA per additional birth defect” increased to 23, and the “equivalence birth defect rate difference” increased to 5.4 per 100 live births (i.e. absolute birth defect rate with EFV was 8.3 per 100 live births, 3 times as high as the birth defect rate with NVP). To make the “ Δ NWA per additional birth defect” lower than 1.0, the efficacy of EFV at 6 months had to be 3% lower than the efficacy of NVP.
When assuming that EFV had the same rate of severe toxicity leading to drug discontinuation as NVP, the “Δ NWA per additional birth defect” was 4.0 and the “equivalence birth defect rate difference” was 0.9 per 100 live births (i.e. absolute birth defect rate with EFV was 3.8 per 100 live births, or 1.4 times as high as the birth defect rate with NVP) (Table 3). To make the “Δ NWA per additional birth defect” lower than 1.0, the absolute rate of EFV severe toxicity leading to drug discontinuation had to be 7.0%, i.e. 0.7% higher than NVP’s base case rate of severe toxicity.
If a third-line ART regimen was available in the country, the “Δ NWA per additional birth defect” ranged from 5 to 12, and the “equivalence birth defect rate difference” ranged from 1.2 to 2.5 per 100 live births, depending on third-line efficacy (Table 4). When assuming a scenario under which EFV is given only to women who take contraception while other women receive NVP, both the “Δ NWA per additional birth defect” and the “equivalence birth defect rate difference” were at least as high as in the base case analysis, and tended to be even higher with decreasing rates of pregnancy in women receiving contraception. If loss-to-follow-up rates were considered, the women in both strategies did worse in terms of survival, and there were fewer birth defects (Table 4). As a result, there was no substantial change in the main results
Table 4.
10-year outcomes for a cohort of 100,000 women on EFV or NVP-based ART regimens in Côte d’Ivoire: One-way sensitivity analysis (program characteristics)
| Δ Women alive, at 10 years (1) | Δ Birth defects, at 10 years (1) | Δ NWA per additional birth defect (2) | Equivalence birth defect rate difference (3) | |
|---|---|---|---|---|
| Interval between scheduled visits | ||||
| Every months | 1031 | 59 | 17 | 3.9% |
| Every 6 months | 994 | 59 | 17 | 3.8% |
| Routine CD4 tests | ||||
| Every 3 months | 1025 | 58 | 18 | 4.0% |
| Every 12 months | 863 | 62 | 14 | 3.1% |
| EFV only for women on contraception (4) | ||||
| Pregnancy rate = 90% × base case | 546 | 31 | 17 | 3.8% |
| Pregnancy rate = 50% × base case | 546 | 17 | 31 | 6.8% |
| Pregnancy rate = 30% × base case | 546 | 10 | 52 | >10.0% |
| Third-line ART available | ||||
| 6-month HIV-1 RNA suppression: 30 % | 304 | 58 | 5 | 1.2% |
| 6-month HIV-1 RNA suppression: 50 % | 401 | 57 | 7 | 1.6% |
| 6-month HIV-1 RNA suppression: 80 % | 658 | 56 | 12 | 2.5% |
| Lost to follow-up (LTFU) on ART | ||||
| 12-month LTFU percentage: 8 % | 696 | 43 | 16 | 3.5% |
| 12-month LTFU percentage: 16% | 482 | 41 | 12 | 2.6% |
EFV: ART regimen including efavirenz and 2 nucleoside reverse transcriptase inhibitors (tenofovir + emtricitabine); NVP: ART regimen including nevirapine and 2 nucleoside reverse transcriptase inhibitors (tenofovir + emtricitabine);
Δ : Difference between EFV and NVP
Where:
- NWA = number of women alive at 10 years
- NBD = number of birth defects over 10 years
Equivalence birth defect rate difference: difference in birth defect rate per 100 live births between EFV and NVP that would be necessary to make the number of women alive at 10 years equivalent to the number of birth defects
In this analysis, we assumed that 60% of HIV-infected women actually take effective contraception, and we compared two strategies: (i) all women start ART with NVP, irrespective of whether they are on contraception or not; (ii) women on contraception start ART with EFV, and other women start ART with NVP.
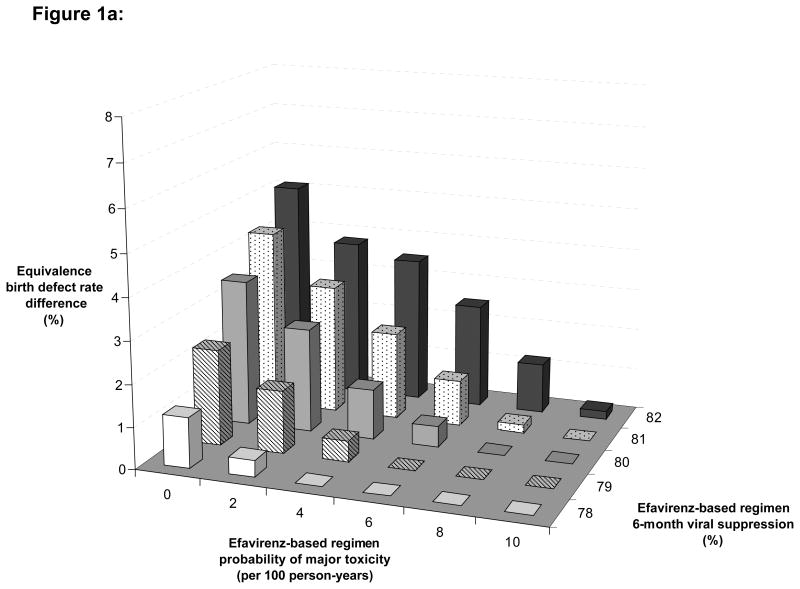
Finally, we varied EFV efficacy compared to NVP and EFV toxicity-induced drug discontinuation in different scenarios of pregnancy rates, to estimate the impact of three-way variations on the “equivalence birth defect rate difference” (Figure 1). In settings with medium rates of pregnancy (Figure 1a), an equivalence birth defect rate difference lower than 1% was found in two situations : First, when the rate of EFV toxicity-inducing drug discontinuation was higher than 1% and the EFV 6-month virological suppression was 2% lower than the NVP 6-month virological suppression; second, when the rate of EFV toxicity-inducing drug discontinuation was higher than 2% and the EFV 6-month virological suppression was 1% lower than the NVP 6-month virological suppression.
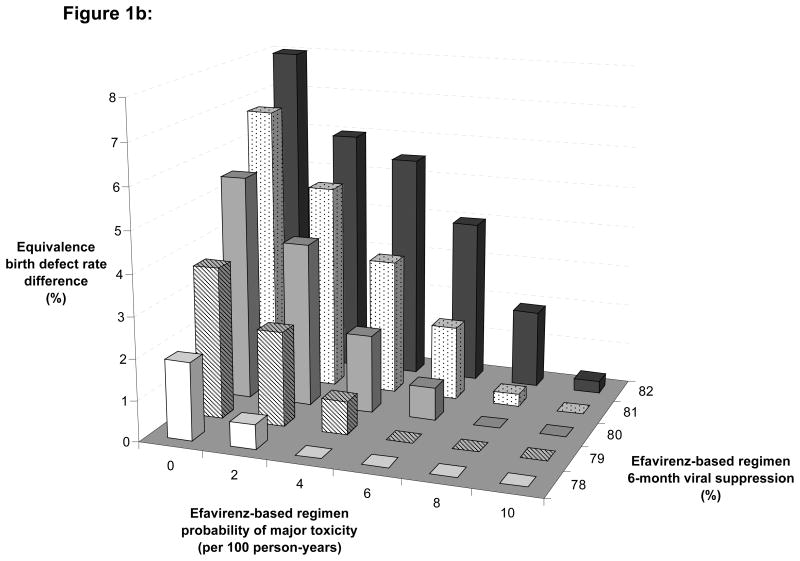
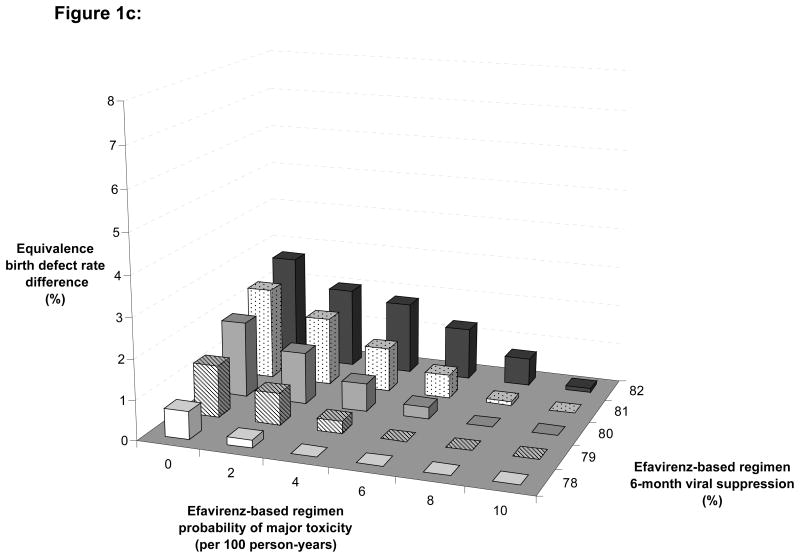
Figure 1. Multi-way sensitivity analysis.
These figures show the variation of the equivalence birth defect rate difference in different scenarios of pregnancy rate, according to different rates of drug discontinuation in women on EFV (X-axis) and to different rates of 6-month HIV-1 RNA suppression on EFV (Z-axis).
Figure 1a: Base case scenario: pregnancy rate = 7.9 per 100 person-years
Figure 1b: Low pregnancy rate scenario: pregnancy rate = 5.0 per 100 person-years
Figure 1c: High pregnancy rate scenario: pregnancy rate = 15.0 per 100 person-years
DISCUSSION
While EFV is better tolerated than NVP, many women start ART with NVP in sub-Saharan Africa. This is mainly due to the fear of efavirenz teratogenicity, which may be higher than that of nevirapine. This is a particular concern in countries where pregnancy rates are high and where contraception is not widely available.
Many physicians, however, are concerned with guidelines that recommend the more toxic NVP for women on the basis of a suspected, but not clearly known, higher rate of teratogenicity with EFV. Starting ART with a more toxic drug may lead to a switch in ART regimen earlier, thus shortening the time on effective ART for women in countries where the number of available ART lines is limited. This, in turn, may decrease long-term survival. Because EFV is strictly contraindicated in women without effective contraception, randomized trials could not be conducted to measure the rate of teratogenicity in women starting ART with EFV, and compare it to women starting ART with NVP. Furthermore, even if such trials were feasible, they would be unable to estimate outcomes over a 10-year period.
Models can explore long-term clinical outcomes in situations where trials are not feasible or ethical. In this study we used a simulation model of HIV infection to compare survival in women and pregnancy outcomes at 10 years in cohorts of women starting ART with EFV or with NVP in Côte d’Ivoire. Using a conservative approach, we first assumed that the two drugs had similar virological efficacy, and that the only differences between drugs were a higher rate of acute toxicity in women with NVP and a higher rate of birth defects in infants with EFV. We used published data for the difference in toxicity, for which there is extensive evidence (6, 8, 33). For birth defect rates, we first assumed that the difference between drugs was reasonably small (29–30, 34). We then did extensive sensitivity analyses on all of these parameters.
We found that starting ART with the drug that leads to the lowest rate of switching due to acute toxicity provides a benefit in survival at 10 years, even when assuming that both drugs have the same efficacy. In a cohort of 100,000 women, there were 911 more women alive at 10 years with EFV compared to NVP, approximately a 0.9% benefit. Albeit small, the additional number of women alive was higher than the additional number of birth defects occurring with EFV compared to NVP over 10 years (59 birth defects). Consequently, the ratio of additional number of women alive per additional number of birth defect was very high (15 NWA per addition birth defects). To make the additional number of women alive equivalent to the additional number of birth defects, the rate of birth defects with EFV needed to be at least 2.3 times the rate with NVP, a very unlikely figure. These outcomes were found under conservative assumptions regarding differences in toxicity and efficacy between EFV and NVP. When assuming that EFV may have a slightly higher rate of virologic efficacy than NVP (33, 35–39), or that NVP may have a higher rate of fatal toxicity than that we used in the base case (28, 33), we found results even more favourable to EFV.
There are several limitations to this study. First, we used birth defect rates from the Antiretroviral Pregnancy Report (APR), which pools data from different sources (30). Other studies reported different birth defect rates (9, 29, 34). Furthermore, other model inputs were also from different African countries. However, we varied birth defect rates and other inputs widely in sensitivity analysis, and found that the outcomes were robust. Second, our primary outcome is an atypical one, as it posed survival in women against birth defects in children. One may argue that birth defects and women’s deaths are altogether different. Birth defects lead to a higher risk of death in children (40–41), but also to consequences in terms of quality of life (42–43) that we did not take into account in this study. Finally, both birth defects and women’s death may also affect other family members both in terms of mortality and of quality of life (44–45) that we also did not take into account. For a direct comparison of outcomes in women and in infants related to the use of NVP or EFV, or for a cost-effectiveness analysis, QALYs and DALYs would be more appropriate outcomes than the ones we used. However, our aim was not to compare adult deaths to birth defect, but to evaluate in parallel the severe clinical outcomes related to the use of NVP or EFV in both he infants and their mothers. For the infants, the most severe outcome associated with EFV is birth defect. For the mothers, the most severe outcome associated with starting ART either with EFV or with NVP is death. The first outcome can be measured early. The second one requires a long-term analysis, because it’s most important determinant is not virological efficacy but the rate of toxicity-induced drug discontinuation. Showing these two outcomes in parallel could allow people to take the long-term survival of mothers into consideration when debating the merits of EFV versus NVP. Currently birth defects are considered the most important part of this debate, and survival in mothers is rarely taken into consideration.
Our aim was not to suggest that birth defects are preferable to women’s deaths. Our aim was to estimate the extent to which recommending NVP over EFV might affect women’s long-term survival. Our analysis suggests that women’s survival could be substantially improved if EFV was recommended first in all women of childbearing age, and that the additional number of birth defects would be likely to be lower than the additional number of women alive over a 10-year period. This trade off should be carefully considered when recommending first-line ART regimens in resource-limited countries.
Acknowledgments
This research was supported by:
The Agence Nationale de recherche sur le Sida et les hépatites virales (ANRS 12212), France The National Institute of Allergy and Infectious Diseases (R01 085276), USA
We extend our gratitude to the entire CEPAC-International team and investigators including: Xavier Anglaret, Ingrid Bassett, Linda-Gail Bekker, Andrea Ciaranello, Christine Danel, Timothy Flanigan, Kenneth A. Freedberg, Sue J. Goldie, Nagalingeswaran Kumarasamy, Marc Lipsitch, Elena Losina, Neil A. Martinson, Kenneth Mayer, Eugene Messou, Eric Ouattara, A. David Paltiel, Stephen Resch, George R. Seage III, Soumya Swaminathan, Rochelle P. Walensky, Milton C. Weinstein, Robin Wood, Yazdan Yazdanpana.
Author’s contribution
| Eric N. Ouattara, MD, MPH | Conception and design |
| Doing runs and calculations | |
| Drafting and writing of the manuscript | |
| Tables and figures | |
| Xavier Anglaret, MD, PhD | Conception and design |
| Clinical expertise | |
| Modeling expertise | |
| Critical revision of the manuscript | |
| Angela Y. Wong, BS | Modeling expertise |
| Doing runs and calculations | |
| Critical revision of the manuscript | |
| Jennifer Chu, BS | Modeling expertise |
| Revision of the manuscript | |
| Heather E. Hsu, MPH | Modeling expertise |
| Revision of the manuscript | |
| Christine Danel, MD, PhD | Clinical expertise |
| Revision of the manuscript | |
| Serge Eholié, MD, MSc | Clinical expertise |
| Revision of the manuscript | |
| Raoul Moh, MD, MPH | Clinical expertise |
| Revision of the manuscript | |
| Delphine Gabillard, MSc | Providing input data |
| Revision of the manuscript | |
| Rochelle, P. Walensky, MD, MPH | Clinical expertise |
| Modeling expertise | |
| Revision of the manuscript | |
| Kenneth, A. Freedberg, MD, MSc | Conception and design |
| Clinical expertise | |
| Modeling expertise | |
| Critical revision of the manuscript |
Footnotes
Conflicts of Interest Statement
All authors declare no conflicts of interest.
References
- 1.World Health Organization. [Accessed May 12, 2011];Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010 revision. Available at http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [PubMed]
- 2.Maggiolo F. Efavirenz. Expert Opin Pharmacother. 2007;8:1137–45. doi: 10.1517/14656566.8.8.1137. [DOI] [PubMed] [Google Scholar]
- 3.Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, Klingman KL, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy RL. Defining the toxicity profile of nevirapine and other antiretroviral drugs. J Acquir Immune Defic Syndr. 2003;34 (Suppl 1):S15–20. doi: 10.1097/00126334-200309011-00004. [DOI] [PubMed] [Google Scholar]
- 5.Danel C, Moh R, Anzian A, Abo Y, Chenal H, Guehi C, et al. Tolerance and acceptability of an efavirenz-based regimen in 740 adults (predominantly women) in West Africa. J Acquir Immune Defic Syndr. 2006;42:29–35. doi: 10.1097/01.qai.0000219777.04927.50. [DOI] [PubMed] [Google Scholar]
- 6.Boulle A, Orrel C, Kaplan R, Van Cutsem G, McNally M, Hilderbrand K, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12:753–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 7.Maartens G, Decloedt E, Cohen K. Effectiveness and safety of antiretrovirals with rifampicin: crucial issues for high-burden countries. Antivir Ther. 2009;14:1039–43. doi: 10.3851/IMP1455. [DOI] [PubMed] [Google Scholar]
- 8.Messou E, Anglaret X, Duvignac J, Konan-N’dri E, Komena E, Gnokoro J, et al. Antiretroviral treatment changes in adults from Côte d’Ivoire: the roles of tuberculosis and pregnancy. AIDS. 2010;24:93–9. doi: 10.1097/QAD.0b013e32832ec1c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussmann H, Wester CW, Wester CN, Lekoko B, Okezie O, Thomas AM, et al. Pregnancy rates and birth outcomes among women on efavirenz-containing highly active antiretroviral therapy in Botswana. J Acquir Immune Defic Syndr. 2007;45:269–73. doi: 10.1097/QAI.0b013e318050d683. [DOI] [PubMed] [Google Scholar]
- 10.De Santis M, Carducci B, De Santis L, Cavaliere AF, Straface G. Periconceptional exposure to efavirenz and neural tube defects. Arch Intern Med. 2002;162:355. doi: 10.1001/archinte.162.3.355. [DOI] [PubMed] [Google Scholar]
- 11.Fundaro C, Genovese O, Rendeli C, Tamburrini E, Salvaggio E. Myelomeningocele in a child with intrauterine exposure to efavirenz. AIDS. 2002;16:299–300. doi: 10.1097/00002030-200201250-00025. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. [Accessed February 4, 2011];Supplement: Safety and Toxicity of Individual Antiretroviral Agents in Pregnancy - Update May 24, 2010. Available at http://www.aidsinfo.nih.gov/contentfiles/PerinatalGLSafetyTox_Sup.pdf.
- 13.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. [accessed the May 11, 2011];Recommendations for a public health approach. 2010 revision. Available at: http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. [PubMed]
- 14.Ouellet D, Hsu A, Qian J, Locke CS, Eason CJ, Cavanaugh JH, et al. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol. 1998;46:111–6. doi: 10.1046/j.1365-2125.1998.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kibuuka H, Guwatudde D, Kimutai R, Maganga L, Maboko L, Watyema C, et al. Contraceptive use in women enrolled into preventive HIV vaccine trials: experience from a phase I/II trial in East Africa. PLoS One. 2009;4:e5164. doi: 10.1371/journal.pone.0005164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Ibiary SY, Cocohoba JM. Effects of HIV antiretrovirals on the pharmacokinetics of hormonal contraceptives. Eur J Contracept Reprod Health Care. 2008;13:123–32. doi: 10.1080/13625180701829952. [DOI] [PubMed] [Google Scholar]
- 17.Freedberg KA, Scharfstein JA, Seage GR, 3rd, Losina E, Weinstein MC, Craven DE, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279:130–6. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 18.Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, Walensky RP, et al. Cost-effectiveness of HIV treatment in resource-poor settings--the case of Côte d’Ivoire. N Engl J Med. 2006;355:1141–53. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 19.Losina E, Toure H, Uhler LM, Anglaret X, Paltiel AD, Balestre E, et al. Cost-effectiveness of preventing loss to follow-up in HIV treatment programs: a Côte d’Ivoire appraisal. PLoS Med. 2009;6:e1000173. doi: 10.1371/journal.pmed.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messou E, Chaix ML, Gabillard D, Minga A, Losina E, Yapo V, et al. Association Between Medication Possession Ratio, Virologic Failure and Drug Resistance in HIV-1-Infected Adults on Antiretroviral Therapy in Côte d’Ivoire. J Acquir Immune Defic Syndr. 2011;56:356–64. doi: 10.1097/QAI.0b013e3182084b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 22.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Losina E, Anglaret X, Yazdanpanah Y, Wang B, Toure S, Seage GR, 3rd, et al. Impact of opportunistic diseases on chronic mortality in HIV-infected adults in Côte d’Ivoire. S Afr Med J. 2006;96:526–9. [PubMed] [Google Scholar]
- 24.Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, Kanga C, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–9. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 25.Minga A, Danel C, Abo Y, Dohoun L, Bonard D, Coulibaly A, et al. Progression to WHO criteria for antiretroviral therapy in a 7-year cohort of adult HIV-1 seroconverters in Abidjan, Côte d’Ivoire. Bull World Health Organ. 2007;85:116–23. doi: 10.2471/BLT.06.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Côte d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forna F, Liechty CA, Solberg P, Asiimwe F, Were W, Mermin J, et al. Clinical toxicity of highly active antiretroviral therapy in a home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 2007;44:456–62. doi: 10.1097/QAI.0b013e318033ffa1. [DOI] [PubMed] [Google Scholar]
- 28.Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191:825–9. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 29.Ekouevi DK, Coffie PA, Ouattara E, Moh R, Amani-Bosse C, Messou E, et al. Pregnancy outcomes in women exposed to efavirenz and nevirapine: an appraisal of the IeDEA West Africa and ANRS Databases, Abidjan, Côte d’Ivoire. J Acquir Immune Defic Syndr. 2011;56:183–7. doi: 10.1097/QAI.0b013e3181ff04e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 Jan 1989 – 31 July 2009. Wilmington, NC: Registry coordinating center; 2009. [accessed May 13, 2011]. Available at http://www.apregistry.com/forms/interim_report.pdf. [Google Scholar]
- 31.Brou H, Viho I, Djohan G, Ekouevi DK, Zanou B, Leroy V, et al. Contraceptive use and incidence of pregnancy among women after HIV testing in Abidjan, Ivory Coast. Rev Epidemiol Santé Publique. 2009;57:77–86. doi: 10.1016/j.respe.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Loko MA, Toure S, Dakoury-Dogbo N, Gabillard D, Leroy V, Anglaret X. Decreasing incidence of pregnancy by decreasing CD4 cell count in HIV-infected women in Côte d’Ivoire: a 7-year cohort study. AIDS. 2005;19:443–5. doi: 10.1097/01.aids.0000161776.30815.44. [DOI] [PubMed] [Google Scholar]
- 33.Van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–63. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 34.Bera E, McCausland K, Nonkwelo R, Mgudlwa B, Chacko S, Majeke B. Birth defects following exposure to efavirenz-based antiretroviral therapy during pregnancy: a study at a regional South African hospital. AIDS. 2010;24:283–9. doi: 10.1097/QAD.0b013e328333af32. [DOI] [PubMed] [Google Scholar]
- 35.Wester CW, Thomas AM, Bussmann H, Moyo S, Makhema JM, Gaolathe T, et al. Non-nucleoside reverse transcriptase inhibitor outcomes among combination antiretroviral therapy-treated adults in Botswana. AIDS. 2010;24 (Suppl 1):S27–36. doi: 10.1097/01.aids.0000366080.91192.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachega JB, Hislop M, Dowdy DW, Gallant JE, Chaisson RE, Regensberg L, et al. Efavirenz versus nevirapine-based initial treatment of HIV infection: clinical and virological outcomes in Southern African adults. AIDS. 2008;22:2117–25. doi: 10.1097/QAD.0b013e328310407e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keiser P, Nassar N, White C, Koen G, Moreno S. Comparison of nevirapine- and efavirenz-containing antiretroviral regimens in antiretroviral-naive patients: a cohort study. HIV Clin Trials. 2002;3:296–303. doi: 10.1310/M47B-R51C-X0MC-K3GW. [DOI] [PubMed] [Google Scholar]
- 38.Cozzi-Lepri A, Phillips AN, d’Arminio Monforte A, Piersantelli N, Orani A, Petrosillo N, et al. Virologic and immunologic response to regimens containing nevirapine or efavirenz in combination with 2 nucleoside analogues in the Italian Cohort Naive Antiretrovirals (I.Co.N.A) study. J Infect Dis. 2002;185:1062–9. doi: 10.1086/339821. [DOI] [PubMed] [Google Scholar]
- 39.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 40.Copeland GE, Kirby RS. Using birth defects registry data to evaluate infant and childhood mortality associated with birth defects: an alternative to traditional mortality assessment using underlying cause of death statistics. Birth Defects Res A Clin Mol Teratol. 2007;79:792–7. doi: 10.1002/bdra.20391. [DOI] [PubMed] [Google Scholar]
- 41.Duke CW, Alverson CJ, Correa A. Fetal death certificates as a source of surveillance data for stillbirths with birth defects. Public Health Rep. 2007;122:664–9. doi: 10.1177/003335490712200514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisman S, Wolf L. The handicapped child: psychological effects of parental, marital, and sibling relationships. Psychiatr Clin North Am. 1991;14:199–217. [PubMed] [Google Scholar]
- 43.Lawoko S, Soares JJ. Quality of life among parents of children with congenital heart disease, parents of children with other diseases and parents of healthy children. Qual Life Res. 2003;12:655–66. doi: 10.1023/a:1025114331419. [DOI] [PubMed] [Google Scholar]
- 44.Monasch R, Boerma JT. Orphanhood and childcare patterns in sub-Saharan Africa: an analysis of national surveys from 40 countries. AIDS. 2004;18 (Suppl 2):S55–65. doi: 10.1097/00002030-200406002-00007. [DOI] [PubMed] [Google Scholar]
- 45.Ng’weshemi J, Urassa M, Isingo R, Mwaluko G, Ngalula J, Boerma T, et al. HIV impact on mother and child mortality in rural Tanzania. J Acquir Immune Defic Syndr. 2003;33:393–404. doi: 10.1097/00126334-200307010-00015. [DOI] [PubMed] [Google Scholar]