Abstract
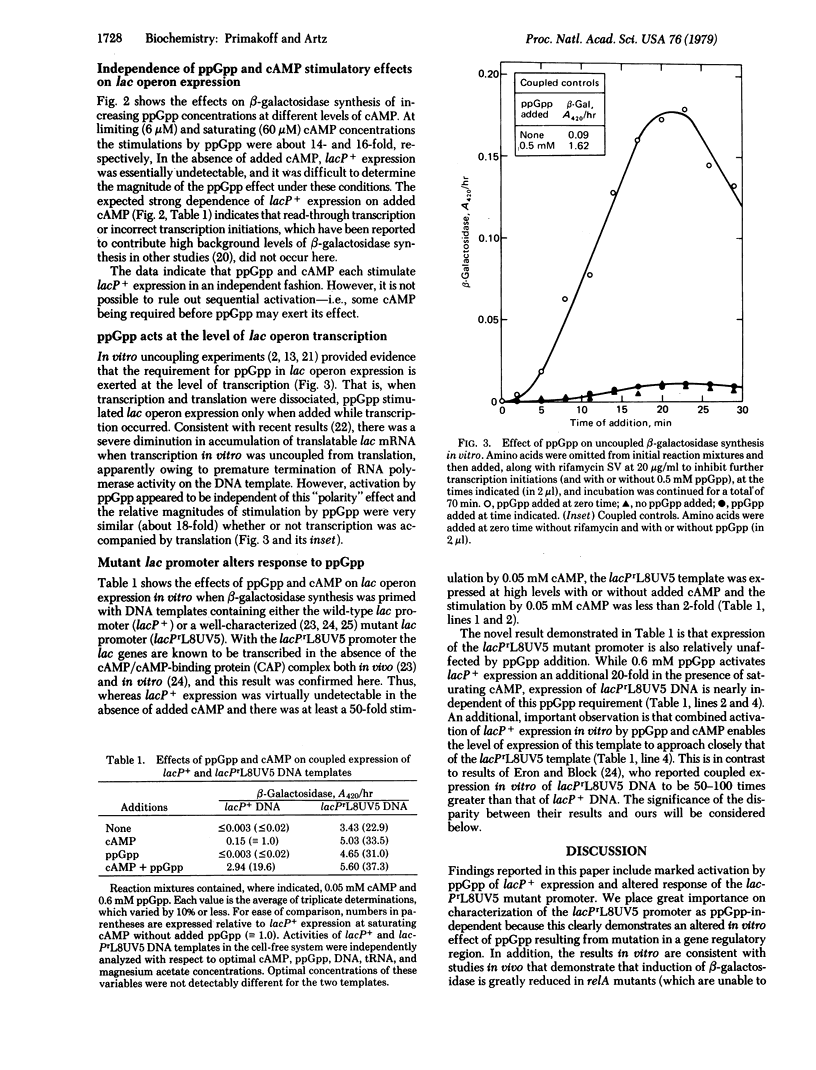
Maximal expression of the Escherichia coli lactose operon in a coupled in vitro transcription-translation system from a Salmonella typhimurium relA mutant was strongly dependent upon addition of guanosine 5′-diphosphate 3′-diphosphate (ppGpp). Without added ppGpp, at saturating 3′,5′-cyclic AMP (cAMP) concentrations, synthesis of β-galactosidase (β-D-galactoside galactohydrolase, EC 3.2.1.23) was reproducibly only 5-7% of that which can be obtained with 0.5-0.8 mM ppGpp. Experiments in which transcription was uncoupled from translation indicated that this 14- to 20-fold stimulation by ppGpp occurred at the level of transcription. When coupled β-galactosidase synthesis was primed with a template containing a well-characterized mutant lac promoter (lacPrL8UV5), the dependence on ppGpp was greatly reduced. This result provides an important experimental control previously unavailable for verifying the significance of ppGpp effects on gene regulation in vitro; it indicates that activation of lacP+ expression by ppGpp is specifically an effect of increased transcription initiations. Furthermore, the large ppGpp stimulation of lacP+ DNA enabled the level of expression of this template to approach that of lacPrL8UV5 DNA, an observation expected from results in vivo but not obtained with other transcription-translation systems in vitro. The importance of these results is considered with respect to previous ideas on the physiological role of ppGpp as a supercontrol molecule in bacterial regulation.
Keywords: coupled protein synthesis in vitro, transcription initiation, supercontrol systems, overlapping metabolic domains, stringent phenomenon
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Pastan I. Activation of transcription by guanosine 5'-diphosphate,3'-diphosphate, transfer ribonucleic acid, and novel protein from Escherichia coli. J Biol Chem. 1975 Mar 25;250(6):2189–2195. [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arditti R. R., Scaife J. G., Beckwith J. R. The nature of mutants in the lac promoter region. J Mol Biol. 1968 Dec;38(3):421–426. doi: 10.1016/0022-2836(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Artz S. W., Broach J. R. Histidine regulation in Salmonella typhimurium: an activator attenuator model of gene regulation. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3453–3457. doi: 10.1073/pnas.72.9.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J., Grodzicker T., Arditti R. Evidence for two sites in the lac promoter region. J Mol Biol. 1972 Aug 14;69(1):155–160. doi: 10.1016/0022-2836(72)90031-9. [DOI] [PubMed] [Google Scholar]
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- Cashel M. Preparation of guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) from Escherichia coli ribosomes. Anal Biochem. 1974 Jan;57(1):100–107. doi: 10.1016/0003-2697(74)90056-6. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Cieslà Z., Salvatore F., Broach J. R., Artz S. W., Ames B. N. Histidine regulation in Salmonella typhimurium. XVI. A sensitive radiochemical assay for histidinol dehydrogenase. Anal Biochem. 1975 Jan;63(1):44–55. doi: 10.1016/0003-2697(75)90187-6. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Gottesman M., Pastan I., Varmus H. E., Emmer M., Perlman R. L. Regulation of lac mRNA synthesis in a soluble cell-free system. Nat New Biol. 1971 Mar 10;230(10):37–40. doi: 10.1038/newbio230037a0. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Block R. Mechanism of initiation and repression of in vitro transcription of the lac operon of Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1828–1832. doi: 10.1073/pnas.68.8.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Inouye H., Pratt C., Beckwith J., Torriani A. Alkaline phosphatase synthesis in a cell-free system using DNA and RNA templates. J Mol Biol. 1977 Feb 15;110(1):75–87. doi: 10.1016/s0022-2836(77)80099-5. [DOI] [PubMed] [Google Scholar]
- Jacobs K. A., Shen V., Schlessinger D. Coupling of lac mRNA transcription to translation in Escherichia coli cell extracts. Proc Natl Acad Sci U S A. 1978 Jan;75(1):158–161. doi: 10.1073/pnas.75.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Simmons C. Synthesis and decay of messenger ribonucleic acid from the lactose operon of Escherichia coli during amino-acid starvation. J Mol Biol. 1972 Oct 14;70(3):451–464. doi: 10.1016/0022-2836(72)90552-9. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Brot N., Spears C., Chen B., Weissbach H. Studies on the in vitro transcription and translation of the lac operon. Arch Biochem Biophys. 1974 Jan;160(1):168–174. doi: 10.1016/s0003-9861(74)80023-8. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Fox J. E., Spears C., Brot N., Weissbach H. Studies on the role of ribosomal proteins L 7 and L 12 in the in vitro synthesis of -galactosidase. J Biol Chem. 1973 Jul 25;248(14):5012–5015. [PubMed] [Google Scholar]
- Maher D. L., Dennis P. P. In vivo transcription of E. coli genes coding for rRNA, ribosomal proteins and subunits of RNA polymerase: influence of the stringent control system. Mol Gen Genet. 1977 Oct 20;155(2):203–211. doi: 10.1007/BF00393161. [DOI] [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. Specific binding of CAP factor to lac promoter DNA. Nature. 1975 Aug 21;256(5519):672–674. doi: 10.1038/256672a0. [DOI] [PubMed] [Google Scholar]
- Martin R. G. Polarity in relaxed strains of Salmonella typhimurium. J Mol Biol. 1968 Jan 14;31(1):127–134. doi: 10.1016/0022-2836(68)90060-0. [DOI] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Ramey W. D., Ishiguro E. E. Site of inhibition of peptidoglycan biosynthesis during the stringent response in Escherichia coli. J Bacteriol. 1978 Jul;135(1):71–77. doi: 10.1128/jb.135.1.71-77.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher G., Ehring R. RNA-directed cell-free synthesis of the galactose enzymes of Escherichia coli. Mol Gen Genet. 1973 Aug 28;124(4):329–344. doi: 10.1007/BF00267662. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Tong B. Construction of phi80 dhis carrying Salmonella typhimurium histidine operon mutations. J Bacteriol. 1974 Dec;120(3):1223–1226. doi: 10.1128/jb.120.3.1223-1226.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman A. J., Gilvarg C. Protein turnover in amino acid-starved strains of Escherichia coli K-12 differing in their ribonucleic acid control. J Biol Chem. 1969 Nov 25;244(22):6304–6306. [PubMed] [Google Scholar]
- Tomkins G. M. The metabolic code. Science. 1975 Sep 5;189(4205):760–763. doi: 10.1126/science.169570. [DOI] [PubMed] [Google Scholar]
- Voll M. J. Derivation of an F-merogenote and a phi-80 high-frequency transducing phage carrying the histidine operon os Salmonella. J Bacteriol. 1972 Feb;109(2):741–750. doi: 10.1128/jb.109.2.741-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Hermoni E. Repression of alkaline phosphatase in Salmonella typhimurium carrying a phoA+ phoR- episome from Escherichia coli. J Bacteriol. 1976 Nov;128(2):661–664. doi: 10.1128/jb.128.2.661-664.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Urm E., Heiness G., Cashel M. Effects of guanosine tetraphosphate, guanosine pentaphosphate, and beta-gamma methylenyl-guanosine pentaphosphate on gene expression of Escherichia coli in vitro. Proc Natl Acad Sci U S A. 1974 Jan;71(1):63–67. doi: 10.1073/pnas.71.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]



