Abstract
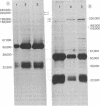
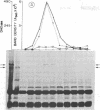
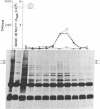
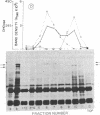
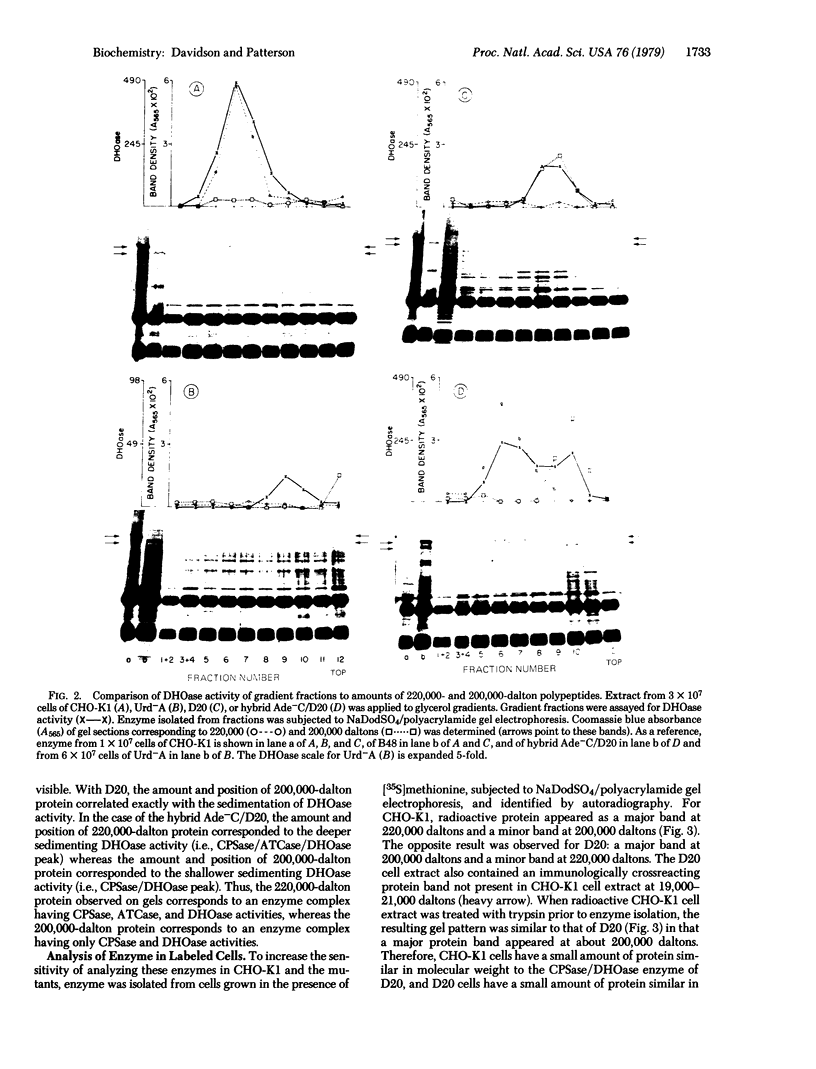
A combined genetic, biochemical, and immunological approach has clarified structural relationships involving the first three enzymes of de novo pyrimidine biosynthesis. A procedure involving antibody and protein A-Sepharose was used to isolate the enzymes carbamoyl-phosphate synthase [ATP:carbamate phosphotransferase (dephosphorylating, amido-transferring), EC 2.7.2.9], aspartate transcarbamoyltransferase (carbamoylphosphate:L-aspartate carbamoyltransferase, EC 2.1.3.2), and dihydro-orotase (L-5,6-dihydroorotate amidohydrolase, EC 3.5.2.3) from Chinese hamster ovary cell CHO-K1, the uridine-requiring auxotroph Urd-A, and selected Urd-A revertants. The enzymes of Urd-A and the Urd-A revertants were significantly altered in activity, native structure, and molecular weight from those of CHO-K1. The results presented permit the conclusion that (i) these three enzymes reside in a single multifunctional 220,000-dalton polypeptide; (ii) the aspartate transcarbamoyltransferase activity is located on a portion (≈20,000 daltons) at one end of the polypeptide; (iii) this portion may also be required for monomers to aggregate into the multimeric from present in mammalian cells; (iv) the mutations in Urd-A and the Urd-A revertants lie in the structural gene for this multifunctional protein; and (v) increased sensitivity to proteases could account for the alterations in the structure of these enzymes in the mutants.
Keywords: carbamoyl-phosphate synthase (glutamine), aspartate carbamoyltransferase, dihydro-orotase, intracellular proteolysis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken D. M., Bhatti A. R., Kaplan J. G. Characterization of the aspartate carbamoyltransferase subunit obtained from a multienzyme aggregate in the pyrimidine pathway of yeast. Activity and physical properties. Biochim Biophys Acta. 1973 May 5;309(1):50–57. doi: 10.1016/0005-2744(73)90316-1. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Davidson J. N., Carnright D. V., Patterson D. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: III. Association of carbamyl phosphate synthetase, aspartate transcarbamylase, and dihydroorotase in mutants of cultured Chinese hamster cells. Somatic Cell Genet. 1979 Mar;5(2):175–191. doi: 10.1007/BF01539159. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry B. Isolation of a multifunctional complex containing the first three enzymes of pyrimidine biosynthesis in drosophila melanogaster. FEBS Lett. 1976 Nov;70(1):71–75. doi: 10.1016/0014-5793(76)80728-4. [DOI] [PubMed] [Google Scholar]
- Kent R. J., Lin R. L., Sallach H. J., Cohen P. P. Reversible dissociation of a carbamoyl phosphate synthase-aspartate transcarbamoylase-dihydroorotase complex from ovarian eggs of Rana catesbeiana: effect of uridine triphosphate and other modifiers. Proc Natl Acad Sci U S A. 1975 May;72(5):1712–1716. doi: 10.1073/pnas.72.5.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Buxton F. P., Radford A. A possible model for the structure of the Neurospora carbamoyl phosphate synthase-aspartate carbamoyl transferase complex enzyme. Mol Gen Genet. 1978 May 31;161(3):297–304. doi: 10.1007/BF00331004. [DOI] [PubMed] [Google Scholar]
- Mori M., Ishida H., Tatibana M. Aggregation states and catalytic properties of the multienzyme complex catalyzing the initial steps of pyrimidine biosynthesis in rat liver. Biochemistry. 1975 Jun 17;14(12):2622–2630. doi: 10.1021/bi00683a010. [DOI] [PubMed] [Google Scholar]
- Mori M., Tatibana M. Multi-enzyme complex of glutamine-dependent carbamoyl-phosphate synthetase with aspartate carbamoyltransferase and dihydroorotase from rat ascites-hepatoma cells. Purification, molecular properties and limited proteolysis. Eur J Biochem. 1978 May 16;86(2):381–388. doi: 10.1111/j.1432-1033.1978.tb12320.x. [DOI] [PubMed] [Google Scholar]
- Mori M., Tatibana M. Purification of homogeneous glutamine-dependent carbamyl phosphate synthetase from ascites hepatoma cells as a complex with aspartate transcarbamylase and dihydroorotase. J Biochem. 1975 Jul;78(1):239–242. [PubMed] [Google Scholar]
- Patterson D. Biochemical genetics of Chinese hamster cell mutants with deviant purine metabolism: biochemical analysis of eight mutants. Somatic Cell Genet. 1975 Jan;1(1):91–110. doi: 10.1007/BF01538734. [DOI] [PubMed] [Google Scholar]
- Patterson D., Carnright D. V. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: I. Isolation of a mutant defective in the early steps of de novo pyrimidine synthesis. Somatic Cell Genet. 1977 Sep;3(5):483–495. doi: 10.1007/BF01539120. [DOI] [PubMed] [Google Scholar]