Abstract
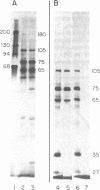
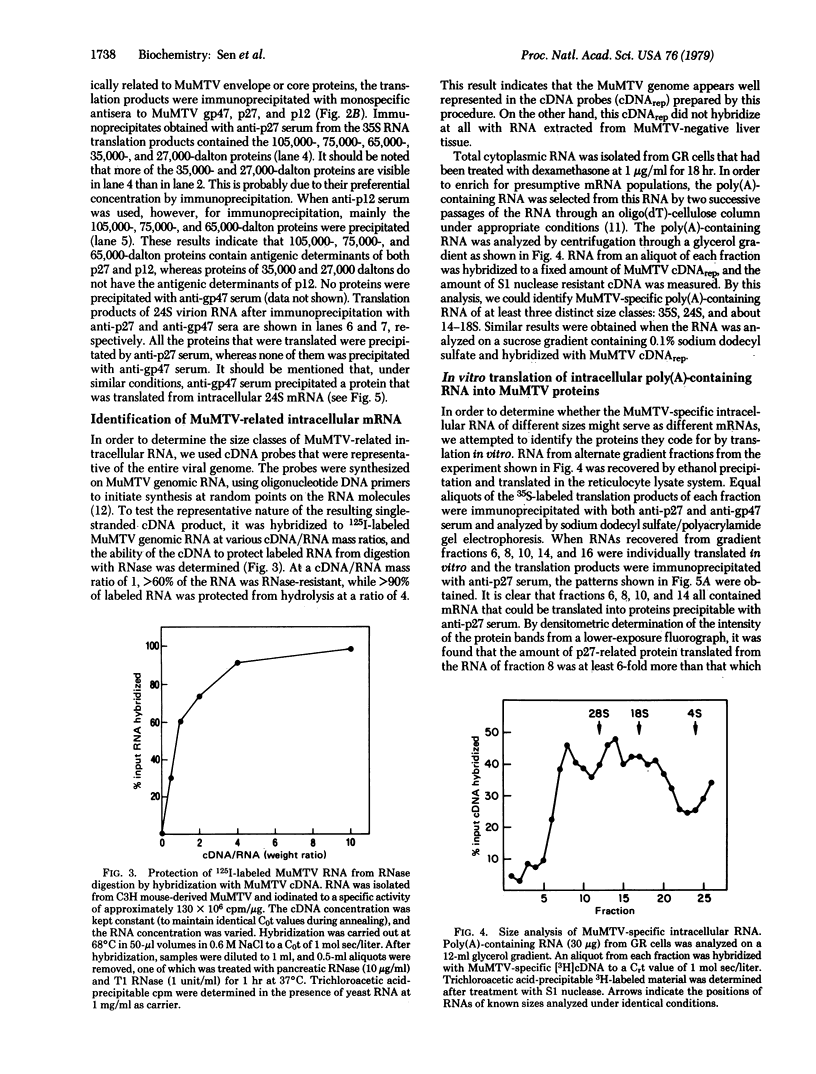
Full-length (35S) genomic RNA from murine mammary tumor virus (MuMTV) was translated in vitro, using a reticulocyte lysate system, into proteins of 105,000, 75,000, 65,000, 35,000, and 27,000 daltons. These proteins were all immunoprecipitable with a monospecific antiserum to the major viral core protein, p27, but not with antiserum to the major viral envelope glycoprotein, gp47. Translation in vitro of RNA of about 24S size extracted from MuMTV yielded proteins similar in size and immunoreactivity to the products of the 35S RNA translation. Polyadenylylated RNA isolated from an MuMTV-producing cell line was fractionated according to size by velocity sedimentation and subsequently hybridized to MuMTV complementary DNA probes. These studies identified at least three size classes (35S, 24S, and 14-18S) of intracellular MuMTV-specific RNA. The 35S intracellular RNA was translated into MuMTV-specific proteins identical in size and immunoreactivity to the products of the virion-derived 35S RNA. On the other hand, translation of the intracellular 24S RNA fraction resulted in the synthesis of proteins, of which two (of about 70,000 daltons) could be immunoprecipitated with anti-gp47 serum, but not with anti-p27 serum. From these data we conclude that MuMTV core and envelope proteins are synthesized from two different mRNAs with approximate sizes of 35S and 24S, respectively. Our results also imply that the intracellular 24S mRNA is synthesized by a process more complex than simple cleavage of the 35S RNA.
Keywords: translation in vitro, molecular hybridization, immunoprecipitation, type B oncornavirus
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Weiss S. R., Varmus H. E., Bishop J. M. At least 104 nucleotides are transposed from the 5' terminus of the avian sarcoma virus genome to the 5' termini of smaller viral mRNAs. Cell. 1978 Sep;15(1):79–91. doi: 10.1016/0092-8674(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Polyproteins related to the major core protein of mouse mammary tumor virus. J Virol. 1978 Jun;26(3):660–672. doi: 10.1128/jvi.26.3.660-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Blair P. B. Isolation of the nucleic acid of mouse mammary tumor virus (MTV). Proc Natl Acad Sci U S A. 1966 Jun;55(6):1490–1497. doi: 10.1073/pnas.55.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamine J., Buchanan J. M. Cell-free synthesis of two proteins unique to RNA of transforming virions of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1977 May;74(5):2011–2015. doi: 10.1073/pnas.74.5.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Synthesis and processing of precursor polypeptides to murine mammary tumor virus structural proteins. J Virol. 1978 Jan;25(1):374–383. doi: 10.1128/jvi.25.1.374-383.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Long C. W., Oroszlan S., Arthur L., Fine D. L. Isolation of separate precursor polypeptides for the mouse mammary tumor virus glycoproteins and nonglycoproteins. Virology. 1978 Mar;85(1):168–174. doi: 10.1016/0042-6822(78)90421-x. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Paucha E. Extraction and fingerprint analysis of simian virus 40 large and small T-antigens. J Virol. 1978 Oct;28(1):140–153. doi: 10.1128/jvi.28.1.140-153.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Bishop J. M. Synthesis and isolation of DNA complementary to nucleotide sequences encoding the variable region of immunoglobulin kappa chain. Biochemistry. 1977 Sep 20;16(19):4225–4232. doi: 10.1021/bi00638a015. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Devare S. G., Reynolds F. H., Jr Translational products of type-C RNA tumor viruses. Adv Cancer Res. 1978;27:1–53. doi: 10.1016/s0065-230x(08)60929-x. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]