Abstract
Amelogenin (AMELX) and matrix metalloproteinase-20 (MMP20) are essential for proper enamel development. Amelx and Mmp20 mutations cause amelogenesis imperfecta. MMP20, a protease secreted by ameloblasts, is responsible for processing enamel proteins, including AMELX, during the secretory stage of enamel formation. Of at least 16 different amelogenin splice products, the most abundant isoform found in murine ameloblasts and developing enamel is the M180 protein. To understand the role of MMP20 processing of M180 AMELX, we generated AmelxKO/Mmp20KO (DKO) mice with an amelogenin (M180Tg) transgene. We analyzed the enamel phenotype by SEM to determine enamel structure and thickness, µCT, and by nanoindentation to quantify enamel mechanical properties. M180Tg/DKO mouse enamel had 37% of the hardness of M180Tg/AmelxKO teeth and demonstrated a complete lack of normal prismatic architecture. Although molar enamel of M180Tg/AmelxKO mice was thinner than WT, it had similar mechanical properties and decussating enamel prisms, which were abolished by the loss of MMP20 in the M180Tg/DKO mice. Retention of the C-terminus or complete lack of this domain is unable to rescue amelogenin null enamel. We conclude that among amelogenins, M180 alone is sufficient for normal enamel mechanical properties and prism patterns, but that additional amelogenin splice products are required to restore enamel thickness.
Keywords: matrix metalloproteinase-20, knockout mouse, transgenic mouse, amelogenesis, imperfecta, ameloblasts, tooth calcification
Introduction
Development and mineralization of enamel, a highly organized, mostly inorganic structure, depend on numerous structural and proteolytic proteins. Amelogenins constitute 90% of the enamel organic matrix secreted by ameloblasts. The murine amelogenin gene encodes an abundant M180 amelogenin protein with distinct regions that are important for proper enamel mineralization (Wright et al., 2003). The amelogenin primary RNA transcript is extensively alternatively spliced (Simmer et al., 1994), and the splice variants may have different functions in developing enamel. Following secretion, amelogenins assemble into nanospheres, which occupy spaces between the enamel crystallites, to separate and support them (Fincham et al., 1995), and to bind mineral crystals and guide mineral growth (Iijima and Moradian-Oldak, 2004).
Ameloblasts also secrete proteases, which process amelogenins and other structural enamel proteins during mineralization (Smith, 1998; Lu et al., 2008). During crystal growth, the proteases matrix metalloproteinase-20 (MMP20) and kallikrein-4 (KLK4) process the enamel proteins (Bartlett and Simmer, 1999) until the overall protein content in the enamel is reduced from 30% to less than 3%. MMP20 is the predominant secretory-stage enzyme (Ryu et al., 1999) that cleaves the hydrophilic C-terminus from the hydrophobic region of the full-length amelogenin protein (Nagano et al., 2009) soon after secretion (Simmer and Hu, 2002), while the other enamel protease KLK4 is secreted by maturation-stage ameloblasts (Hu et al., 2002) to process the remaining enamel proteins.
Mice that do not make any amelogenin protein (AmelxKO) develop hypoplastic enamel lacking prismatic structure (Gibson et al., 2001). These defects can be partially rescued by mating the AmelxKO mice with mice expressing an amelogenin transgene (M180) (Li et al., 2008). M180 is the most abundant splice variant in mice, consisting of 180 amino acids, 13 of which are normally cleaved from the C-terminus by Mmp20 immediately after secretion. Amelogenin isoform processing by proteases during enamel formation has been characterized in recombinant porcine amelogenin (Nagano et al., 2009). Mice that contain M180 in an AmelxKO background do not carry the other alternatively spliced amelogenins. Transgenic mice that lack the C-terminus of M180 in an AmelxKO background display a phenotype similar to that of AmelxKO mice and have disorganized, aprismatic enamel (Pugach et al., 2010), indicating that the C-terminus is essential for the formation of prismatic enamel, which has been suggested by numerous studies (Moradian-Oldak et al., 2000, 2002; Paine et al., 2000; Beniash et al., 2005; Margolis et al., 2006; Fang et al., 2011; Wiedemann-Bidlack et al., 2011).
Mice lacking MMP20 develop hypoplastic, hypomature enamel that separates from the dentin (Caterina et al., 2002) and has decreased mineral content and hardness (Bartlett et al., 2004), presumably due to failure to cleave the enamel matrix proteins properly. Like AmelxKO mice, Mmp20KO mice produce a thin layer of disorganized enamel (Caterina et al., 2002). Mmp20KO murine enamel was approximately 37% softer than wild-type enamel, contained around 53% less mineral, and had 7% to 16% higher water and protein content (Bartlett et al., 2004). While amelogenin-null enamel mineral is plate-like, Mmp20KO enamel has a disrupted prism pattern (Bartlett et al., 2006), demonstrating that both genes are essential for production of full-thickness enamel with decussating prisms.
Here we determined whether the presence of the C-terminus, in the absence of the protease that generates it, would be sufficient to rescue the enamel defects in AKO mice. The M180/DKO mice do not make any alternatively spliced amelogenins or Mmp20, so the M180Tg would be unable to be initially cleaved at the C-terminus. By overexpressing M180 in Amelx and Mmp20 double-knock-out mice, we sought to elucidate the relative contribution of this most abundant amelogenin splice variant to the enamel phenotype, particularly in terms of thickness, mechanical properties, and prism pattern.
Materials & Methods
Transgenic and Knockout Mice
All procedures were performed after approval by the University of Pennsylvania Institutional Animal Care and Use Committee. Mice were generated at the UPenn Transgenic Core Facility and maintained in an AAALAC-accredited facility. AmelxKO and Mmp20KO mice were generated by introduction of a deletion into the coding region as described previously (Gibson et al., 2001; Caterina et al., 2002). The C-terminus truncated (CTRNCTg) and M180 transgenic mice were generated as previously described (Pugach et al., 2010; Li et al., 2008). To determine the genotype of AmelxKO, Mmp20KO, and transgene-positive offspring, we isolated high-molecular-weight genomic DNA from mouse tails (Chen et al., 2003). The amelogenin, Mmp20, CTRNCTg, and M180Tg PCR primers and conditions used have been described (Caterina et al., 2002; Li et al., 2008; Pugach et al., 2010).
Protein Analysis
For protein analysis, first molar mandibular teeth were dissected from transgene-positive or knockout offspring at post-natal day 4. To identify transgenic and endogenous protein in teeth from M180Tg/AmelxKO/Mmp20KO and CTRNCTg/AmelxKO matings, we prepared protein extracts and SDS-PAGE gels as previously described (Chen et al., 2003) and stained them with Coomassie blue (Bio-Rad, Hercules, CA, USA).
µCT Analysis of Teeth
Scans for volume and density of enamel in molars (n = 6) were performed as described (Pugach et al., 2010), with a microtomograph imaging system (μCT 40, Scanco Medical AG, Brüttisellen, Switzerland) with 16-µm resolution at 70 kVp. The images were processed by three-dimensional reconstruction software (μCT Evaluation Program v6.0, Scanco Medical) and analyzed for determination of enamel density and volume. Hydroxyapatite standards were used for instrument calibration.
Scanning Electron Microscopy Analysis of Enamel
Molars dissected from adult (6 wks and 3 mos old) mouse mandibles (n = 6) were dehydrated through graded ethyl alcohols and embedded in eponate 12 resin (Ted Pella, Inc., Redding, CA, USA). Embedded teeth were sectioned with a Buehler Isomet low-speed saw (Buehler, Lake Bluff, IL, USA) to produce 200-µm-thick sections, which were polished with 600-grit SiC paper, 15-µm polishing paper, and with 1-µm and 0.25-µm diamond suspensions (Buehler). Sections were etched with 20% phosphoric acid for 10 sec prior to being mounted for scanning electron microscopic (SEM) analysis. SEM analysis of enamel mesiodistal cross-sections from molars was completed at 15 kV (FEI Quanta 200 FEG, Hillsboro, OR, USA). Enamel structure was analyzed and measurements of molar enamel thickness were calculated with Image J. Molar enamel thickness was measured on the thickest part (center) of distal and mesial enamel, adjacent to the respective cusps.
Nanomechanical Property Measurements of Teeth
Mandibles were dissected from mouse heads as described (Li et al., 2008). Elastic modulus and hardness of molar enamel were determined in adult (6 wks and 3 mos old) mice (n = 6) by a Nanoindenter XP (MTS Systems, Oak Ridge, TN, USA). Half-mandibles were embedded in Acrymount embedding resin (Electron Microscopy Sciences, Hatfield, PA, USA) and ground from the mesial side with 400-grit SiC paper until the interior of the first molar was exposed to reveal longitudinal cross-sections of molars. The exposed interior of first molars was further polished by 800- and 1200-grit SiC papers, and 1-µm and 0.25-μm diamond suspensions. Nanoindentations were performed with a Berkovich diamond tip, with a trapezoidal force profile with peak loads at 300 µN. Twenty indentations were made in the enamel of each tooth, in mesial cusps of 1st molars, in mature enamel. Each indentation yielded a load-deformation curve, from which the elastic modulus, and hardness, were determined according to the method of Oliver and Pharr (1992; Doerner and Nix, 1986).
Statistical Analysis
We used analysis of variance (ANOVA) with the Tukey post hoc test to detect differences (p < .05) between groups of teeth analyzed for enamel density and volume, as measured by µCT, enamel thickness as measured by SEM, and nanomechanical properties (GraphPad Software, San Diego, CA, USA).
Results
Protein Analyses of Transgenic/KO Mice
SDS-PAGE analysis was used to detect transgenic and endogenous protein in developing molars (Fig. 1). Wild-type (WT) and Mmp20KO (MKO) four-day-old mouse molars expressed endogenous amelogenins, at least 16 of which were alternative splice products in WT mice (Simmer et al., 1994). M180Tg/Mmp20KO (M180/MKO) molars in the secretory stage expressed both transgenic and endogenous amelogenins, while M180Tg/ AmelxKO (M180/AKO) and M180Tg/doubleKO (M180/DKO) molars expressed only transgenic amelogenin of approximately 23 kDa with no alternative splice variants. Without MMP20, the endogenous amelogenin splice variants and transgenic M180 in M180/MKO mouse molars could not be normally cleaved, as indicated by the darker stained band approximately 23 kDa compared with that of WT mice (Fig. 1). In the M180/DKO molars, only M180Tg remained uncleaved, and no other amelogenin splice variants were present. In only the MKO and M180/MKO mouse enamel was an additional band visible at approximately 24 kDa, which has been previously reported (Caterina et al., 2002) and is presumably an uncleaved amelogenin alternative splice variant that is rapidly cleaved by Mmp20 during the secretory stage in WT enamel (Fig. 1).
Figure 1.
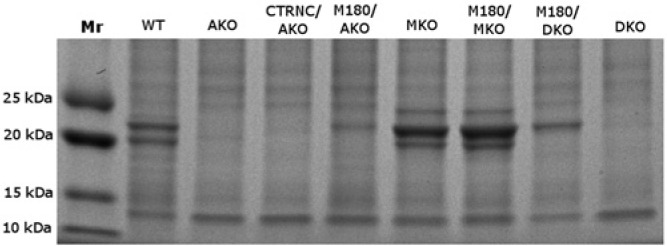
SDS-PAGE of four-day-old molar extracts stained with Coomassie blue. Non-amelogenin enamel proteins are evident by comparing AKO and DKO with the other lanes. An additonal band at approximately 24 kDa (either alternative splice variant or uncleaved protein) is noticeable in the MKO and M180/MKO lanes. The absence of several lower-Mr bands in MKO, M180/MKO, and M180/DKO that appear in WT and M180/AKO illustrates the differences in amelogenin cleavage. CTRNC/AKO mice expressed only the cleaved form of M180Tg and no other splice variants, and CTRNCTg is visible as a faint band slightly below 23 kDa.
Phenotype of Transgenic/Double-KO Enamel
Phenotype analysis of enamel by µCT in adult M180/DKO mice and controls revealed that molars from AKO, M180/DKO, and DKO mice had lower enamel density and volume than all other groups and were not different from each other (Fig. 2). While the µCT density and volume values of AKO, M180/DKO, and DKO appear to be zero in Fig. 2, they were below the detectable threshold and thus were recorded as a zero value, when in fact enamel was present on these mouse molars, as shown in Fig. 3.
Figure 2.
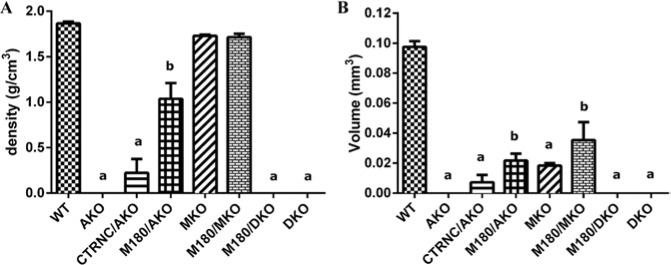
µCT of adult molar enamel from WT, AmelxKO (AKO), CTRNC/AmelxKO (CTRNC/AKO), M180Tg/AmelxKO (M180/AKO), Mmp20KO (MKO), M180Tg/Mmp20KO (M180/MKO), M180Tg/AmelxKO/Mmp20KO (M180/DKO), and AmelxKO/Mmp20KO (DKO) mouse models. (A) Density. (B) Volume. Although in some groups there is no column value for density or volume, there is actually enamel present; it was below the measurement threshold of the µCT system and thus was recorded as a 0 value. aSignificantly different from WT and b. bSignificantly different from WT and a (p < .05).
Figure 3.
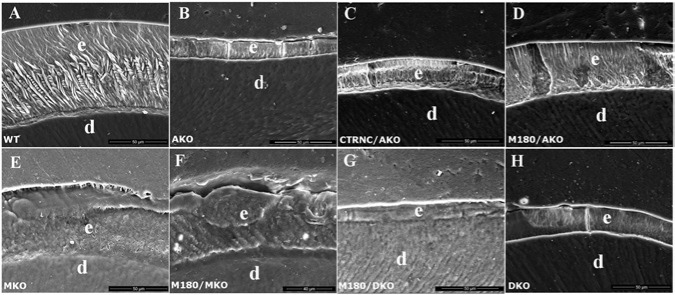
SEM analysis of polished, then etched, six-week-old adult molar enamel. SEM images of molar enamel, with enamel (e) and dentin (d) indicated, from (A) WT, (B) AmelxKO (AKO), (C) CTRNC/AmelxKO (CTRNC/AKO), (D) M180Tg/AmelxKO (M180/AKO), (E) Mmp20KO (MKO), (F) M180Tg/Mmp20KO (M180/MKO), (G) M180Tg/AmelxKO/Mmp20KO (M180/DKO), and (H) AmelxKO/Mmp20KO (DKO) mouse models. Note that the M180Tg/AKO (D) gained a prism pattern but did not attain full thickness.
To investigate the mature enamel structure of M180/DKO mice and controls, we analyzed cross-sections of etched molar enamel by SEM. WT enamel displayed the characteristic decussating prisms that extended through almost the full thickness of the enamel layer (Fig. 3A). In contrast, AKO molar enamel displayed no prisms but some flat crystals (Fig. 3B), while CTRNC/AKO enamel had a structure similar to that of AKO (Fig. 3C). M180/AKO molar enamel had an improved decussating prismatic structure, although this pattern did not extend through the entire thickness of the enamel layer (Fig. 3D). MKO molar enamel exhibited prisms with no inter-weaving that seemed to be obscured by organic matter (Fig. 3E), while M180/MKO molar enamel exhibited a prismatic enamel pattern that was improved over that of MKO enamel but still was obscured by organic material (Fig. 3F). Both M180/DKO and DKO molar enamel displayed neither prisms nor visible crystals (Figs. 3G, 3H).
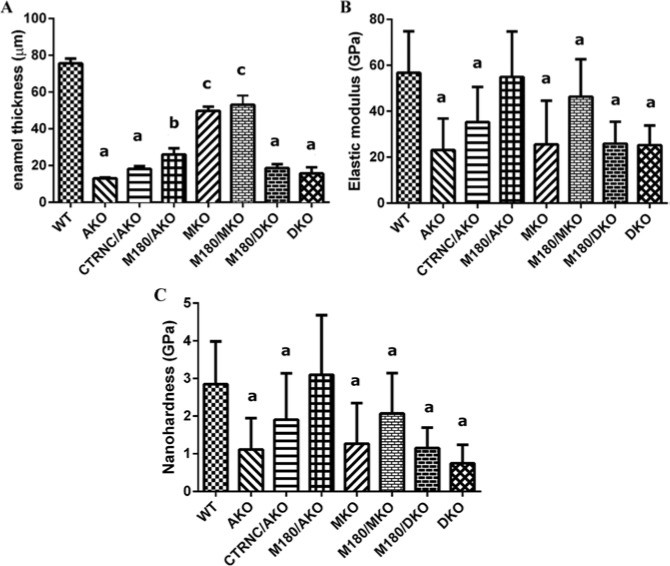
Molar enamel thickness was measured from SEM images. Enamel thickness of CTRNC/AKO, M180/AKO, and M180/DKO was 24%, 34%, and 25% that of WT, respectively (Fig. 4A).
Figure 4.
Enamel thickness and nanomechanical properties of WT, AmelxKO (AKO), CTRNCTg/AmelxKO (CTRNCTg/AKO), M180Tg/AmelxKO (M180Tg/AKO), Mmp20KO (MKO), M180Tg/Mmp20KO (M180/MKO), M180Tg/AmelxKO/Mmp20KO (M180/DKO), and AmelxKO/Mmp20KO (DKO) mouse models. (A) Molar enamel thickness, (B) enamel elastic modulus of molars, and (C) enamel hardness of molars. aSignificantly different from WT, b, and c. bSignificantly different from WT, a, and c. cSignificantly different from WT, a, andb (p < .05).
Nanomechanical Properties of Transgenic/Double-KO Enamel
Nanomechanical properties of mature enamel were determined by nanoindentation of adult molars. Molar enamel elastic modulus of CTRNC/AKO, M180/AKO, and M180/DKO was 62%, 97%, and 46% that of WT, respectively (Fig. 4B), while enamel hardness of CTRNC/AKO, M180/AKO, and M180/DKO was 67%, 108%, and 40% that of WT, respectively (Fig. 4C).
Discussion
In the current study, the phenotype of M180/DKO enamel was analyzed according to structure, thickness, and mechanical properties. In all of these analyses, the molar enamel of M180/DKO mice was significantly compromised compared with that of M180/AKO mice and was most similar to AmelxKO and CTRNC/AKO enamel, suggesting that the presence of the uncleaved C-terminus of M180 (M180/DKO) is insufficient for normal enamel structure, thickness, and mechanical properties. Presumably, the secreted amelogenin protein (with its C-terminus) could not properly participate in the formation of higher order assemblies of nanospheres and subsequent mineral crystal orientation and growth. Expression of the other major enamel protease, KLK4, begins during the maturation stage, at around post-natal day 6 (Hu et al., 2002; Simmer et al., 2011), so it is unlikely that M180Tg was cleaved during the secretory stage at post-natal day 4 by KLK4 in the absence of MMP20.
Structural analyses of molar enamel by genotype suggest that mice with molar enamel with decussating prisms (WT and M180/AKO) had significantly higher mechanical properties than the other mouse genotypes, without decussating prisms, or evidence of prisms. These data are consistent with prior suggestions that decussating enamel prism structure may be essential for maintaining hardness (Bartlett et al., 2004) and that MMP20 is necessary for decussating enamel prisms (Bartlett et al., 2011). In further agreement with analysis of the present data, prior studies of mechanical properties of AmelxKO (Li et al., 2008) and Mmp20KO enamel (Bartlett et al., 2004) found that mature enamel in both knockout models did not exhibit decussating prisms and had enamel hardness approximately 40% to 50% that of WT mice.
Analysis of the nanomechanical property data from molar enamel in this study indicates an almost complete recovery of mechanical properties to WT values in M180/AKO mice, even though enamel density, volume, thickness, and structure were not completely rescued. Conversely, when the M180 transgene was overexpressed in the double-KO mice, the mechanical property rescue of M180/AKO enamel was eliminated, since the mechanical properties of M180/DKO molar enamel did not differ significantly from those of AKO. Interestingly, while M180/DKO molar hardness was 40% that of WT, AmelxKO mice lacking the C-terminus of M180Tg (but retaining MMP20 activity) had molar hardness 67% that of WT. This suggests that MMP20 has additional important functions beyond cleaving M180 at the C-terminus that could affect the enamel phenotype in Mmp20KO mice, including cleaving M180 at the N-terminus to form tyrosine-rich amelogenin peptide (TRAP) (Ryu et al., 1999), and processing of other enamel proteins, ameloblastin (Chun et al., 2010), and enamelin (Yamakoshi et al., 2006), during the secretory stage.
In conclusion, analysis of the data from the present study suggests that the presence of the C-terminus on M180 and MMP20 expression is sufficient to rescue enamel structural defects and mechanical properties, but not thickness, of AKO enamel. This is consistent with the previous report of a mouse model which overexpressed 2 Amelx splice variants (M180Tg and LRAPTg) in AKO mice, since the presence of both M180Tg and LRAPTg increased enamel thickness beyond that of the M180Tg alone (Gibson et al., 2011). We have provided further evidence that both Amelx and Mmp20 are required for enamel prism structure, mechanical properties, and thickness, and that processing of full-length amelogenin by MMP20 has a crucial role in enamel formation.
Acknowledgments
The authors thank Hui Xue from the University of Pennsylvania for assistance with protein analysis and Zhorro Nikolov from the Centralized Research Facilities at Drexel University for assistance with nanoindentation.
Footnotes
This research was supported by the National Institutes of Health (NIDCR grants DE019968, DE011089, DE016276, DE022624).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bartlett JD, Simmer JP. (1999). Proteinases in developing dental enamel. Crit Rev Oral Biol Med 10:425-441. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Beniash E, Lee DH, Smith CE. (2004). Decreased mineral content in MMP-20 null mouse enamel is prominent during the maturation stage. J Dent Res 83:909-913. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Skobe Z, Lee DH, Wright JT, Kulkarni AB, Gibson CW.(2006). A developmental comparison of matrix-metalloproteinase-20 and amelogenin null mouse enamel. Eur J Oral Sci 114(Suppl 1):18-23. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Skobe Z, Nanci A, Smith CE. (2011). MMP20 promotes a smooth enamel surface, a strong DEJ, and a decussating enamel rod pattern. Eur J Oral Sci 119(Suppl 1):199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniash E, Simmer JP, Margolis HC. (2005). The effect of recombinant mouse amelogenins on the formation and organization of hydroxyapatite crystals in vitro. J Struct Biol 149:182-190. [DOI] [PubMed] [Google Scholar]
- Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, et al. (2002). Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 277:49598-49604. [DOI] [PubMed] [Google Scholar]
- Chen E, Yuan ZA, Wright JT, Hong SP, Li Y, Collier PM, et al. (2003).The small bovine amelogenin LRAP fails to rescue the amelogenin null phenotype. Calcif Tissue Int 73:487-495. [DOI] [PubMed] [Google Scholar]
- Chun YH, Yamakoshi Y, Yamakoshi F, Fukae M, Hu JC, Bartlett JD, et al. (2010). Cleavage site specificity of MMP-20 for secretory-stage ameloblastin. J Dent Res 289:785-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner MF, Nix WD. (1986). A method for interpreting the data from depth-sensing indentation instruments. J Mater Res 1:601-609. [Google Scholar]
- Fang PA, Conway JF, Margolis HC, Simmer JP, Beniash E. (2011). Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc Natl Acad Sci USA 108:14097-14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham AG, Moradian-Oldak J, Diekwisch TG, Lyaruu DM, Wright JT, Bringas P, Jr, et al. (1995). Evidence for amelogenin “nanospheres” as functional components of secretory-stage enamel matrix. J Struct Biol 115:50-59. [DOI] [PubMed] [Google Scholar]
- Gibson CW, Yuan ZA, Hall B, Longenecker G, Chen E, Thyagarajan T, et al. (2001). Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 276:31871-31875. [DOI] [PubMed] [Google Scholar]
- Gibson CW, Li Y, Suggs C, Kuehl MA, Pugach MK, Kulkarni AB, Wright JT.(2011). Rescue of the murine amelogenin null phenotype with two amelogenin transgenes. Eur J Oral Sci 119(Suppl 1):70-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JC, Sun X, Zhang C, Liu S, Bartlett JD, Simmer JP. (2002). Enamelysin and kallikrein-4 mRNA expression in developing mouse molars. Eur J Oral Sci 110:307-315. [DOI] [PubMed] [Google Scholar]
- Iijima M, Moradian-Oldak J. (2004). Interactions of amelogenins with octacalcium phosphate crystal faces are dose dependent. Calcif Tissue Int 74:522-531. [DOI] [PubMed] [Google Scholar]
- Li Y, Suggs C, Wright JT, Yuan ZA, Aragon M, Fong H, et al. (2008). Partial rescue of the amelogenin null dental enamel phenotype. J Biol Chem 283:15056-15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Papagerakis P, Yamakoshi Y, Hu JC, Bartlett JD, Simmer JP. (2008). Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem 389:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis HC, Beniash E, Fowler CE. (2006). Role of macromolecular assembly of enamel matrix proteins in enamel formation. J Dent Res 85:775-793. [DOI] [PubMed] [Google Scholar]
- Moradian-Oldak J, Paine ML, Lei YP, Fincham AG, Snead ML. (2000). Self-assembly properties of recombinant engineered amelogenin proteins analyzed by dynamic light scattering and atomic force microscopy. J Struct Biol 131:27-37. [DOI] [PubMed] [Google Scholar]
- Moradian-Oldak J, Bouropoulos N, Wang L, Gharakhanian N. (2002). Analysis of self-assembly and apatite binding properties of amelogenin proteins lacking the hydrophilic C-terminal. Matrix Biol 21:197-205. [DOI] [PubMed] [Google Scholar]
- Nagano T, Kakegawa A, Yamakoshi Y, Tsuchiya S, Hu JC, Gomi K, et al. (2009). Mmp-20 and Klk4 cleavage site preferences for amelogenin sequences. J Dent Res 88:823-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver WC, Pharr GM. (1992). An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res 7:1564-1583. [Google Scholar]
- Paine ML, Zhu DH, Luo W, Bringas P, Jr, Goldberg M, White SN, et al. (2000). Enamel biomineralization defects result from alterations to amelogenin self-assembly. J Struct Biol 132:191-200. [DOI] [PubMed] [Google Scholar]
- Pugach MK, Li Y, Suggs C, Wright JT, Aragon MA, Yuan ZA, et al. (2010). The amelogenin C-terminus is required for enamel development. J Dent Res 89:165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu OH, Fincham AG, Hu CC, Zhang C, Qian Q, Bartlett JD, Simmer JP. (1999). Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J Dent Res 78:743-750. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Hu JC. (2002). Expression, structure, and function of enamel proteinases. Connect Tissue Res 43:441-449. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Hu CC, Lau EC, Sarte P, Slavkin HC, Fincham AG. (1994). Alternative splicing of the mouse amelogenin primary RNA transcript. Calcif Tissue Int 55:302-310. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Richardson AS, Smith CE, Hu Y, Hu JC. (2011). Expression of kallikrein-related peptidase 4 in dental and non-dental tissues. Eur J Oral Sci 119(Suppl 1):226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE. (1998). Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128-161. [DOI] [PubMed] [Google Scholar]
- Wiedemann-Bidlack FB, Kwak SY, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. (2011). Effects of phosphorylation on the self-assembly of native full-length porcine amelogenin and its regulation of calcium phosphate formation in vitro. J Struct Biol 173: 250-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JT, Hart PS, Aldred MJ, Seow K, Crawford PJ, Hong SP, et al. (2003). Relationship of phenotype and genotype in X-linked amelogenesis imperfecta. Connect Tissue Res 44(Suppl 1):72-78. [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JC, Fukae M, Yamakoshi F, Simmer JP. (2006). How do enamelysin and kallikrein 4 process the 32-kDa enamelin? Eur J Oral Sci 114(Suppl 1):45-51. [DOI] [PubMed] [Google Scholar]