Abstract
Matrix metalloproteinase-20 (enamelysin, MMP20) is essential for dental enamel development. Seven different MMP20 mutations in humans cause non-syndromic enamel malformations, termed amelogenesis imperfecta, and ablation of Mmp20 in mice results in thin brittle enamel with a dysplastic rod pattern. Healthy enamel formation requires the sliding movement of ameloblasts in rows during the secretory stage of development. This is essential for formation of the characteristic decussating enamel rod pattern observed in rodents, and this is also when MMP20 is secreted into the enamel matrix. Therefore, we propose that MMP20 facilitates ameloblast movement by cleaving ameloblast cell-cell contacts. Here we show that MMP20 cleaves the extracellular domains of the E- and N-cadherin adherens junction proteins, that both E- and N-cadherin transcripts are expressed at significantly higher levels in Mmp20 null vs. wild-type (WT) mice, and that in Mmp20 ablated mice, high-level ameloblast N-cadherin expression persists during the maturation stage of development. Furthermore, we show that E-cadherin gene expression is down-regulated from the pre-secretory to the secretory stage, while N-cadherin levels are up-regulated. This E- to N-cadherin switch supports epithelial migration in other tissues and may be an important event necessary for the ameloblasts to start moving in rows that slide by one another.
Keywords: enamel biomineralization/formation, matrix metalloproteinases (MMPs), gene expression, cadherins, ameloblast, junctional complexes
Introduction
MMP20 is a tooth-specific matrix metalloproteinase that is expressed in odontoblasts and in ameloblasts during the secretory stage of dental enamel development. As the secretory stage begins, the ameloblasts elongate, form Tomes’ processes at their apical end nearest the forming enamel, and secrete large amounts of enamel matrix proteins including amelogenin, ameloblastin, and enamelin (Bartlett and Simmer, 1999). Seven different human MMP20 mutations are known to cause autosomal recessive hypomaturation or hypoplastic-hypomaturation amelogenesis imperfecta (Kim et al., 2005; Ozdemir et al., 2005; Papagerakis et al., 2008; Lee et al., 2010; Gasse et al., 2013; Wang et al., 2013). Mmp20 null mice have soft brittle enamel and a dysplastic enamel rod pattern (Bartlett et al., 2011a). MMP20 cleaves enamel matrix proteins, and we hypothesize that, in addition, MMP20 may also cleave ameloblast cell-cell junctions to facilitate ameloblast movement in rows necessary to form mammalian rod patterns (Bartlett and Smith, 2013).
Adherens junctions (AJ) mediate cell-cell interactions. The functions of AJs include: initiation and stabilization of cell-cell adhesion, regulation of the actin cytoskeleton, intracellular signaling, and transcriptional regulation. Cadherins are a major component of AJs. They are calcium-dependent single-pass transmembrane proteins mediating cell-cell adhesion through their extracellular domains (Wheelock and Johnson, 2003a), and their intracellular domains are linked to the actin cytoskeleton by catenins (Hartsock and Nelson, 2008). More than 20 types of cadherins have been identified, each expressed in various tissues (Saito et al., 2012). For example, E-cadherin, one of the most-studied members of the cadherin superfamily, is typically expressed in epithelial cells, while N-cadherin is primarily expressed in mesenchymal cells. During normal embryonic development, cadherins play critical roles in regulating cell polarity and establishing cell sorting (Patel et al., 2003; Wheelock and Johnson, 2003b; Gumbiner, 2005). Cadherin switching occurs when cadherin isoforms change expression levels and in most cases refers to the down-regulation of E-cadherin and up-regulation of N-cadherin. This E- to N-cadherin switch was observed during development such as during gastrulation, when epiblast cells ingress through the primitive streak and when neural crest cells emigrate through the neural tube (Wheelock et al., 2008).
MMPs facilitate cell movement by cleaving the extracellular domain of cadherins, and cadherin cleavage can release p120-catenin and β-catenin from the intracellular cadherin domain. These molecules can enter the cell nucleus to alter gene expression (Munshi and Stack, 2006). Here we seek to determine if a lack of MMP20 alters cadherin expression and abundance.
Materials & Methods
Mice
All handling, care, and usage of animals were approved by The Forsyth Institute. Mice were housed in an AAALAC-approved facility. The origin of the Mmp20 null mice was as previously described (Caterina et al., 2002).
Cell Culture
Ameloblast-lineage cells (ALC) (Nakata et al., 2003) were grown in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 4.5 g/L D-glucose, 4 mM L-glutamine, and 110 mg/L sodium pyruvate (Invitrogen, Carlsbad, CA, USA), and were harvested 3 days after reaching 100% confluence to allow for sufficient E- and N-cadherin expression.
Cadherin Cleavage Assay and Immunoblotting
Cells were washed with phosphate-buffered saline and subjected to lysis in RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate) with Halt protease and phosphatase inhibitor cocktail (EDTA-free) (Pierce, Rockford, IL, USA). Each 50-μL reaction contained 0.2 μg MMP20 catalytic domain (Enzo Life Sciences, Farmingdale, NY, USA), 2 mM CaCl2, or 10 mM EDTA, and cell lysate containing E- and N-cadherin. Reactions were incubated at 37°C for 5 hrs, and products were fractionated on SDS-PAGE gels. Cadherin fragments were visualized by Western blotting, with mouse monoclonal E- or N-cadherin antibodies (BD Biosciences, Franklin Lakes, NJ, USA) targeting the cytosolic portions of cadherins, and polyclonal E-/N-cadherin antibodies (R&D Systems, Minneapolis, MN, USA) targeting their extracellular domains. To test whether MMP20 cleaves cadherin extracellular domains, we used recombinant human E- and N-cadherin Fc chimera as substrates (R&D Systems). Each 25-μL reaction contained 0.05 μg MMP20 catalytic domain, 0.5 μg recombinant cadherin extracellular domain, and cleavage assay buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM CaCl2, and 0.1% Brij-35) or 10 mM EDTA. Reactions were incubated at 37°C overnight to ensure full cleavage. Western blotting was performed with human E- or N-cadherin antibodies (R&D Systems) and anti-His(C-term) antibody (Invitrogen).
Quantitative Real-time PCR (qPCR)
mRNA from 1-, 5-, or 11-day-old mouse first molar enamel organs was reverse-transcribed to cDNA. Each qPCR reaction contained LightCycler 480 SYBR Green I Master (Roche, Basel, Switzerland), cDNA, and 0.25 μM forward and reverse primers. Primers were: Cdh1 (E-cadherin) forward (5′-CAG CCTTCTTTTCGGAAGACT-3′), reverse (5′-GGTAGACAG CTCCCTATGACTG-3′); Cdh2 (N-cadherin) forward (5′-CCA GCAGATTTCAAGGTGGAC-3′), reverse (5′-TTACAGCTAC CTGCCACTTTTC-3′); and Rn18s (18S ribosomal RNA) forward (5′-GTAACCCGTTGAACCCCATT-3′), reverse (5′-CC ATCCAATCGGTAGTAGCG-3′). Reactions were run on a Roche LightCycler 480 with the following program: 3 min at 95°C for initial denaturation, and 95°C for 15 sec, 58°C for 15 sec, and 72°C for 15 sec for 40 cycles, followed by a melting curve. We generated standard curves with each primer set using control samples prepared by pooling all cDNA samples and making a 4-fold dilution series covering all sample concentrations. Reaction efficiencies and cadherin gene expression levels normalized to the reference gene Rn18s were calculated as previously described (Pfaffl, 2001). All expression levels are presented as relative ratios to the WT day 1 data. Each time-point was obtained by duplicate qPCR analysis, and the experiment was repeated three times. Statistical significance was assessed by t tests.
Enamel Organ Protein Extraction
Enamel organs of five-day-old first molars from WT and Mmp20 null mice were extracted and homogenized in RIPA buffer with Halt protease and phosphatase inhibitor cocktail and 5 mM EDTA. Cell debris was removed by centrifugation, and the lysate was analyzed by Western blotting with polyclonal E- or N-cadherin antibodies (R&D Systems).
Immunohistochemistry (IHC)
WT and Mmp20 null mouse incisor sections were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched with 1% H2O2 in methanol for 30 min. Sections were either incubated in blocking serum overnight at 4°C, followed by one-hour incubation with 1:250 diluted mouse monoclonal E-cadherin antibody (BD Biosciences), or blocked for 30 min at RT, and incubated with rabbit polyclonal N-cadherin antibody (Abcam, Cambridge, England) at a 1:1,000 dilution overnight at 4°C. Sections without primary antibody were used as negative controls. Antibody binding was visualized with the Vector M.O.M. Immunodetection Kit for E-cadherin and the Vectastain Elite ABC Kit for N-cadherin with ImmPACT DAB peroxidase substrate (Vector Laboratories, Burlingame, CA, USA). Sections were counterstained with 0.1% Fast Green in PBS for 2 min, dehydrated, and mounted.
Results
MMP20 Cleaves the Extracellular Domains of E- and N-cadherin
ALC cells expressed both E- and N-cadherin and expressed elevated levels of E-cadherin when cells become tightly confluent. Cell lysates were added to the reactions, and products were fractioned by SDS-PAGE gels. In the absence of MMP20, anti-E- and anti-N-cadherin antibodies detected intact cadherin proteins at 120 kDa and 130 kDa, respectively. In reactions with MMP20, the intact cadherin amount became smaller, and a major 75-kDa extracellular fragment for E-cadherin and a 90-kDa extracellular fragment for N-cadherin were observed (Fig. 1). The cleavage assay was also conducted in the presence of 10 mM EDTA, demonstrating that EDTA inhibited MMP20 activity. This is consistent with results expected for a matrix metalloproteinase (MMP20).
Figure 1.
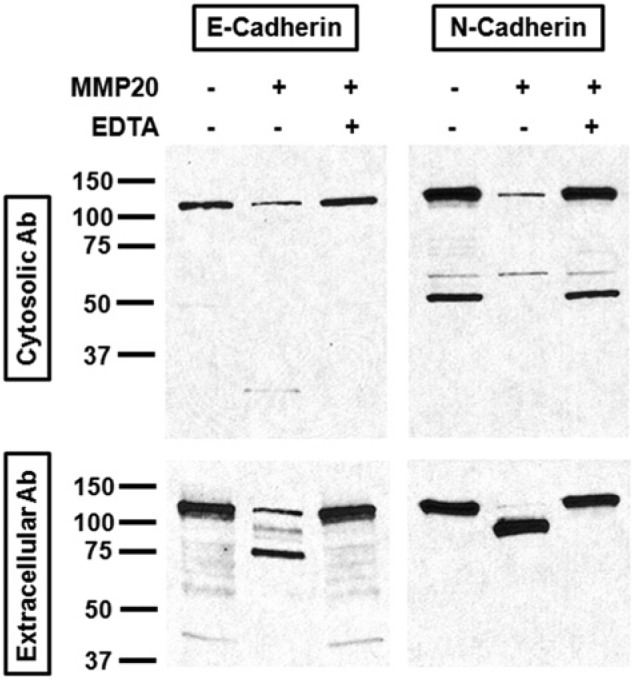
MMP20 cleaved E- and N-cadherins from ALC cell lysate. Reactions containing the MMP20 catalytic domain and ALC cell lysate were run on an SDS-PAGE gel, and cadherin proteins and fragments were detected by Western blotting. The left side represents anti-E-cadherin Western blotting and the integral protein runs at 120 kDa, whereas the right side represents anti-N-cadherin Western blotting with N-cadherin detected at 130 kDa. The gels were probed by antibodies targeting both the cytosolic domain (upper panels) and extracellular domain (lower panels) of cadherins to demonstrate cleavage. MMP20 activity was inhibited by EDTA.
To determine if MMP20 targeted the extracellular domain of each cadherin, we used recombinant human cadherin extracellular domains as substrates. The extracellular domains were attached to a C-terminal 6X His-Tag (Fig. 2a). Cleavage products were identified with anti-cadherin antibodies that bind specifically to extracellular domains and antibodies specific for the 6X His-Tag. In reactions without MMP20 or with both MMP20 and ETDA, only the intact cadherin bands were observed (Fig. 2b), indicating that no cleavage took place. With MMP20, the intact protein bands disappeared, and the E- and N-cadherin reactions produced predominant fragments at 75 and 90 kDa, respectively. The anti-His-Tag antibody after MMP20 cleavage revealed a 35-kDa C-terminal fragment from E-cadherin and a 37-kDa C-terminal fragment from N-cadherin. The fragments were larger than the human IgG1 Fc domain (~27 kDa) plus the 6-amino-acid (IEGRMD) linker (~0.8 kDa) and 6X His-Tag (~0.9 kDa). These results demonstrate that both cadherins were cleaved within their extracellular domains.
Figure 2.
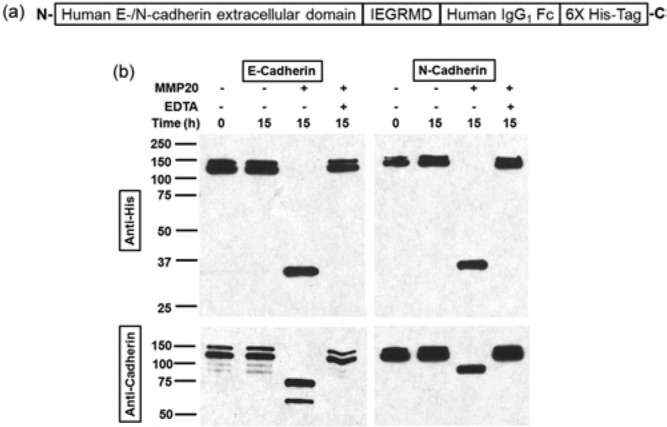
MMP20 cleaved the extracellular domain of E- and N-cadherins. (a) Schematic representation of human recombinant E-/N-cadherin extracellular domains. The N-terminal extracellular domains are linked to human IgG1 Fc fragment through a 6-amino-acid linker IEGRMD, and a 6X His-Tag is present at each C-terminus. (b) Reactions were fractionated on SDS-PAGE gels, and cadherin proteins were probed by Western blotting with both anti-cadherin antibodies and anti-His-Tag antibody. The former detects N-terminal fragments and the latter detects C-terminal fragments. Integral E- and N-cadherin recombinant proteins were run as doublets at 120 and 130 kDa, respectively. The cleaved fragments detected by the anti-His-Tag antibody were too large to contain just the linker plus IgG1 Fc fragment plus 6X His-Tag, so the cut sites were in the extracellular cadherin domains.
MMP20 Regulates E- and N-cadherin Levels in vivo
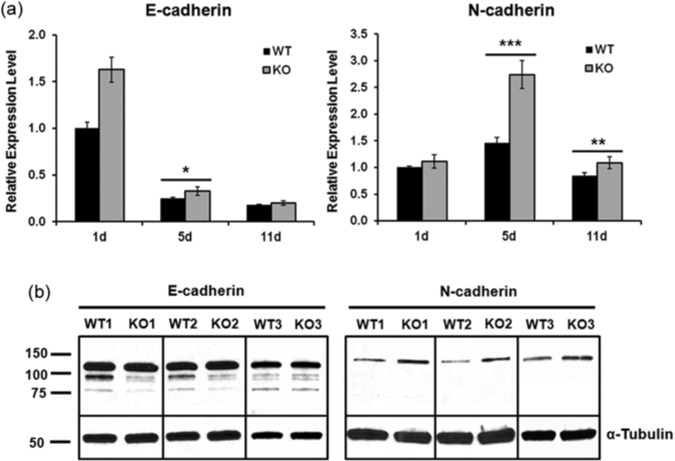
qPCR was performed on enamel organs isolated from first molars of post-natal day 1 (pre-secretory), day 5 (secretory), and day 11 (maturation) mice for assessment of E- and N-cadherin expression levels. E-cadherin gene expression level was significantly down-regulated from the pre-secretory to the secretory stage, while N-cadherin was up-regulated (Fig. 3a), indicating that cadherin switching occurred during the secretory stage, when MMP20 was secreted. Unexpectedly, the Mmp20 ablated mice expressed significantly more E- and N-cadherin transcripts than WT for all but the E-cadherin maturation stage (Fig. 3a). The difference between genotypes was greatest for N-cadherin expressed during the secretory stage. This suggests that cleavage of cadherin extracellular domains by MMP20 served to regulate cadherin expression indirectly. Perhaps this occurred through the subsequent release of catenins when the cleaved cadherin intracellular domains were removed.
Figure 3.
E- and N-cadherins were over-expressed in Mmp20 ablated mice. (a) Pools of tissue consisting of first molar enamel organs from five 1-day-old, five 5-day-old, and four 11-day-old mice for each genotype were collected for mRNA extraction and qPCR analysis. qPCR results demonstrate that, during the secretory stage, E- and N-cadherin transcripts were significantly increased in first molar enamel organs from Mmp20 ablated mice vs. WT controls. During the maturation stage, N-cadherin transcripts from ablated mice were also significantly increased over those of controls (*p < .05; **p < .005; ***p < .0001). In addition, an E- to N-cadherin switch at the transcript level was observed from the pre-secretory (day 1) to the secretory stage (day 5). (b) Total protein extracts from 5-day-old (secretory stage) first molar enamel organs of six WT and Mmp20 null mice were analyzed by Western blotting, and 3 of each are shown in this Fig. E-/N-cadherins were probed by antibodies recognizing their extracellular domains. α-Tubulin was used as a loading control to ensure that the same amount of total protein was loaded in each lane. Higher levels (approximately three-fold) of full-length N-cadherin were observed in Mmp20 null samples compared with WT controls (p = .040).
Since the qPCR results showed the biggest difference between genotypes in cadherin expression during the secretory stage, these enamel organs were collected from WT and Mmp20 null mice, and total protein was extracted in RIPA buffer. E- and N-cadherin levels were analyzed by Western blotting. Intact E- and N-cadherins were detected in both WT and null samples (Fig. 3b). N-cadherin protein levels were elevated approximately three-fold in Mmp20 ablated enamel organs when compared with WT.
Localization of Cadherin Proteins to the Ameloblasts of the Enamel Organ
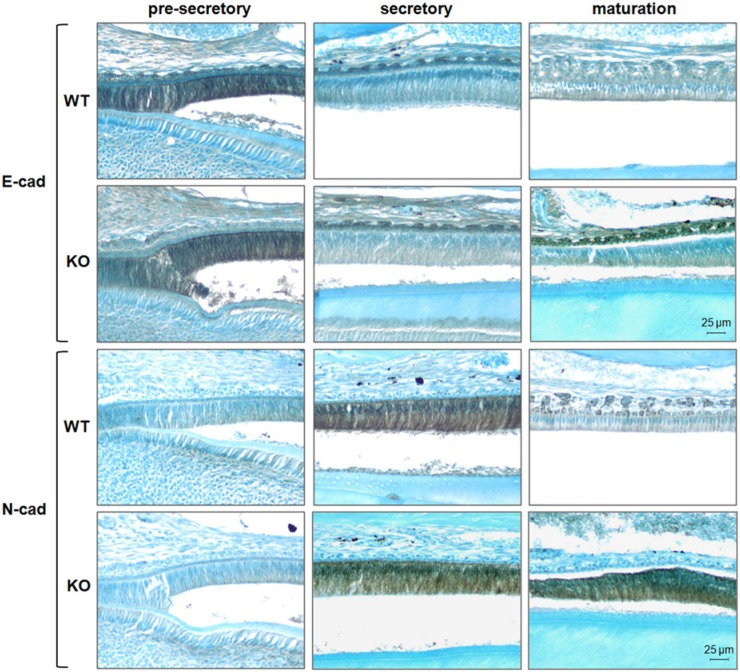
Mouse incisors erupt continuously, and therefore contain every enamel developmental stage. We performed immunohistochemistry on incisors from both WT and Mmp20 null mice to visualize cadherin protein location at each stage. Both E- and N-cadherin were localized to the ameloblasts of the enamel organ. However, their patterns of expression differed. Pre-secretory ameloblasts expressed E-cadherin strongly, and expression was greatly diminished in the secretory stage. Conversely, N-cadherin had little to no expression in pre-secretory ameloblasts but was strongly expressed in the secretory stage (Fig. 4). This confirms our qPCR result as the hallmark of a cadherin switch that may allow for ameloblast movement during the secretory stage.
Figure 4.
Localization of E- and N-cadherins to the ameloblast layer of mouse enamel organs. Thin sections of pre-secretory, secretory, and maturation-stage enamel organs were stained by immunohistochemical procedures with E- and N-cadherin antibodies. A strong E- to N-cadherin switch from the pre-secretory to the secretory stage was observed in WT and Mmp20 ablated ameloblasts. In WT samples, staining of both cadherins declined in the maturation stage, while in Mmp20 null samples, strong staining for N-cadherin persisted into the maturation stage. All images were taken at 200X magnification.
Interestingly, the strong E-cadherin staining observed in the Mmp20 null papillary layer (maturation stage) located above the ameloblasts was absent in the WT section. In several sections of maturation-stage ameloblasts from Mmp20 ablated mice, N-cadherin expression was not down-regulated. These shortened maturation-stage ameloblasts sometimes appeared to elongate and proceed back to the secretory stage (Fig. 4, lower right panel), where they may again be expected to express N-cadherin. Alternatively, a lack of N-cadherin cleavage during the secretory stage may result in its accumulation into the maturation stage.
Discussion
MMPs were previously shown to cleave cadherins. For example, MMP-3, -7, and -9 cleave the extracellular domain of E-cadherin to facilitate cell movement, cell invasion, and cell proliferation (Lochter et al., 1997; McGuire et al., 2003; Sancéau et al., 2003; Cowden Dahl et al., 2008; Lynch et al., 2010). MMP-2, -9, -12, and -28 cleave the extracellular domains of various other cadherins (Illman et al., 2006; Dwivedi et al., 2009; Hartland et al., 2009). Here we added to the data demonstrating that MMPs cleave cadherins by showing that MMP20 cleaves E-and N-cadherin. Previously, we showed that porcine MMP20 cleaves human recombinant E-cadherin (Bartlett et al., 2011b), and we further this result by demonstrating that MMP20 cleaves mouse E- and N-cadherin isolated from ALC cells and by definitively demonstrating that the cleavage sites are in the extracellular domain of the cadherins. The finding that MMP20 also cleaves N-cadherin is significant, because the ameloblast E- to N-cadherin switch occurs at the beginning of the secretory stage, when ameloblasts start moving to form mammalian rod patterns. It follows that N-cadherin cleavage and re-attachment would be necessary for ameloblast movement.
The results demonstrating that Mmp20 ablated mice have increased E- and N-cadherin transcript levels within their enamel organs were unexpected. Cell-cell adhesion is a highly dynamic process, and extensive remodeling occurs among junctional complexes (Shen et al., 2011). Perhaps when a cadherin is cleaved and the intracellular domain is disassembled, some of the released catenins migrate to the nucleus to play a regulatory role by suppressing cadherin expression for the purpose of facilitating cell movement. Further research is necessary to identify this mechanism.
The E- and N-cadherin protein levels (Western blots) from WT and Mmp20 null mouse enamel organs did show an approximately three-fold increase in N-cadherin for ablated mice in the secretory stage, which was likely the product of increased expression levels and decreased N-cadherin cleavage. The Western blot results were significant because immunodetection of proteins from multiple tissues can be inefficient. It is not practical to remove ameloblasts from first molar enamel organs, so the stratum intermedium, stellate reticulum, and outer enamel epithelium were also present in the protein extraction. We did not see a protein-level difference for E-cadherin, perhaps because of interference from the multiple tissues and/or its reduced expression due to the cadherin switch that occurs at the beginning of the secretory stage.
The immunohistochemical results demonstrated a pronounced E- to N-cadherin switch from the pre-secretory to the secretory stage of enamel development. This occurred regardless of the presence of MMP20. However, the most striking result showed that, in the Mmp20 ablated mice, but not WT, N-cadherin protein persisted in the ameloblasts during the maturation stage. Previously, we showed that Mmp20 null ameloblasts abnormally extend, retract, and later re-extend their Tomes’ processes (Bartlett et al., 2011b), indicating that the ameloblasts did not fully enter the maturation stage. Therefore, one interpretation of persistent N-cadherin expression in Mmp20 ablated maturation-stage ameloblasts is that the ameloblasts have not fully entered the maturation stage and therefore still express N-cadherin. This was reflected by the elongation of the Mmp20 ablated maturation-stage ameloblasts to a more “secretory stage-like” morphology. Alternatively, the lack of N-cadherin cleavage by MMP20 may have inhibited cadherin turnover and increased N-cadherin expression (qPCR and Western blot results), causing the accumulation of N-cadherin that persisted into the maturation stage.
In summary, we show that MMP20 cleaves the extracellular domains of the E- and N-cadherin adherens junction proteins, that both E- and N-cadherin transcripts are expressed at significantly higher levels in Mmp20 null vs. wild-type (WT) mice, that a profound cadherin switch occurs from E- to N-cadherin when ameloblasts progress from the pre-secretory stage into the secretory stage of enamel development, and that, in Mmp20 ablated mice, high-level ameloblast N-cadherin expression persists during the maturation stage of development. Analysis of these data suggests that, in addition to the fundamental role of MMP20 in cleaving enamel matrix proteins, MMP20 may also cleave the junctional complexes necessary for ameloblast cell movement.
Acknowledgments
We thank Dr. Toshihiro Sugiyama for his generous gift of ALC cells and Justine Dobeck for providing mouse incisor sections and thoughtful discussions.
Footnotes
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health (grant RO1DE016276).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bartlett JD, Simmer JP. (1999). Proteinases in developing dental enamel. Crit Rev Oral Biol Med 10:425-441. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Smith CE. (2013). Modulation of cell-cell junctional complexes by matrix metalloproteinases. J Dent Res 92:10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JD, Skobe Z, Nanci A, Smith CE. (2011a). Matrix metalloproteinase 20 promotes a smooth enamel surface, a strong dentino-enamel junction, and a decussating enamel rod pattern. Eur J Oral Sci 119(Suppl 1):199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JD, Yamakoshi Y, Simmer JP, Nanci A, Smith CE. (2011b). MMP20 cleaves E-cadherin and influences ameloblast development. Cells Tissues Organs 194:222-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, et al. (2002). Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 277:49598-49604. [DOI] [PubMed] [Google Scholar]
- Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, et al. (2008). Matrix metalloproteinase 9 is a mediator of epidermal growth factor–dependent E-cadherin loss in ovarian carcinoma cells. Cancer Res 68:4606-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi A, Slater SC, George SJ. (2009). MMP-9 and -12 cause N-cadherin shedding and thereby β-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res 81:178-186. [DOI] [PubMed] [Google Scholar]
- Gasse B, Karayigit E, Mathieu E, Jung S, Garret A, Huckert M, et al. (2013). Homozygous and compound heterozygous MMP20 mutations in amelogenesis imperfecta. J Dent Res 92:598-603. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. (2005). Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6:622-634. [DOI] [PubMed] [Google Scholar]
- Hartland SN, Murphy F, Aucott RL, Abergel A, Zhou X, Waung J, et al. (2009). Active matrix metalloproteinase-2 promotes apoptosis of hepatic stellate cells via the cleavage of cellular N-cadherin. Liver Int 29:966-978. [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. (2008). Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illman SA, Lehti K, Keski-Oja J, Lohi J. (2006). Epilysin (MMP-28) induces TGF-β mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci 119(Pt 18):3856-3865. [DOI] [PubMed] [Google Scholar]
- Kim JW, Simmer JP, Hart TC, Hart PS, Ramaswami MD, Bartlett JD, et al. (2005). MMP-20 mutation in autosomal recessive pigmented hypomaturation amelogenesis imperfecta. J Med Genet 42:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Seymen F, Kang HY, Lee KE, Gencay K, Tuna B, et al. (2010). MMP20 hemopexin domain mutation in amelogenesis imperfecta.J Dent Res 89:46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. (1997). Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139:1861-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CC, Vargo-Gogola T, Matrisian LM, Fingleton B. (2010). Cleavage of E-cadherin by matrix metalloproteinase-7 promotes cellular proliferation in nontransformed cell lines via activation of RhoA. J Oncol 2010:530745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JK, Li Q, Parks WC. (2003). Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 162:1831-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi HG, Stack MS. (2006). Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev 25:45-56. [DOI] [PubMed] [Google Scholar]
- Nakata A, Kameda T, Nagai H, Ikegami K, Duan Y, Terada K, et al. (2003). Establishment and characterization of a spontaneously immortalized mouse ameloblast-lineage cell line. Biochem Biophys Res Commun 308:834-839. [DOI] [PubMed] [Google Scholar]
- Ozdemir D, Hart PS, Ryu OH, Choi SJ, Ozdemir-Karatas M, Firatli E, et al. (2005). MMP20 active-site mutation in hypomaturation amelogenesis imperfecta. J Dent Res 84:1031-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagerakis P, Lin HK, Lee KY, Hu Y, Simmer JP, Bartlett JD, et al. (2008). Premature stop codon in MMP20 causing amelogenesis imperfecta. J Dent Res 87:56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SD, Chen CP, Bahna F, Honig B, Shapiro L. (2003). Cadherin-mediated cell–cell adhesion: sticking together as a family. Curr Opin Struct Biol 13:690-698. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2001). A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Tucker DK, Kohlhorst D, Niessen CM, Kowalczyk AP. (2012). Classical and desmosomal cadherins at a glance. J Cell Sci 125(Pt 11):2547-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancéau J, Truchet S, Bauvois B. (2003). Matrix metalloproteinase-9 silencing by RNA interference triggers the migratory-adhesive switch in Ewing’s sarcoma cells. J Biol Chem 278:36537-36546. [DOI] [PubMed] [Google Scholar]
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. (2011). Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73:283-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SK, Hu Y, Simmer JP, Seymen F, Estrella NM, Pal S, et al. (2013). Novel KLK4 and MMP20 mutations discovered by whole-exome sequencing. J Dent Res 92:266-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. (2003a). Cadherin-mediated cellular signaling. Curr Opin Cell Biol 15:509-514. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. (2003b). Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol 19:207-235. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. (2008). Cadherin switching. J Cell Sci 121(Pt 6):727-735. [DOI] [PubMed] [Google Scholar]