Abstract
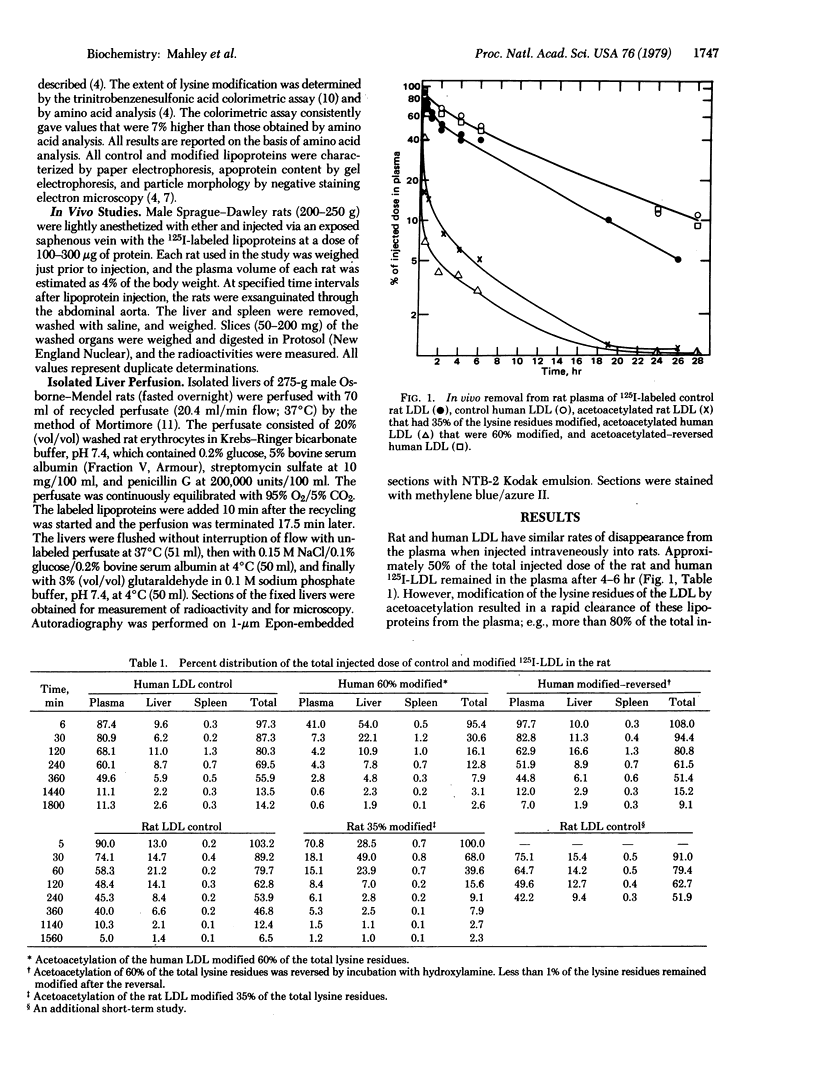
Selective chemical modification of lysine residues of lipoproteins by acetoacetylation dramatically altered the metabolism of the lipoproteins without significantly altering other physical or chemical properties. Modification of 30-60% of the total lysine residues of iodinated rat or human low-density lipoproteins (125I-LDL) resulted in a rapid removal of these acetoacetylated lipoproteins from the plasma of rats. Within minutes after intravenous injection into intact rats, greater than 80% of the total injected dose disappeared from the plasma. The rapidly cleared acetoacetylated LDL appeared in the liver, and within 6-30 min as much as 50-80% of the total injected dose of modified LDL could be accounted for in the liver. Furthermore, it was possible to demonstrate in the isolated perfused rat liver that the Kupffer cells were responsible for the lipoprotein uptake. Human high-density lipoproteins (HDL3) were also rapidly removed from the plasma after acetoacetylation. In striking contrast, acetoacetylation (30-60%) of two E apoprotein-containing lipoproteins (rat HDL1 and dog HDLc) retarded their removal from the plasma. The accelerated removal of modified LDL and HDL3, in contrast to the retarded removal of modified HDL1 and HDLc, suggests that the recognition and removal process is specific for a property acquired by only certain lipoproteins after acetoacetylation. Moreover, these results suggest that lysine residues of the E apoprotein may play a functional role in the recognition process for the normal clearance of HDL1 and HDLc, a process that is interfered with after acetoacetylation.
Keywords: lipoprotein catabolism, protein modification, hepatic uptake, liver metabolism
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Windmueller H. G., Levy R. I. Metabolic fate of rat and human lipoprotein apoproteins in the rat. J Lipid Res. 1973 Jul;14(4):446–458. [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W., Weisgraber K. H., Bersot T. P. Apoprotein (E--A-II) complex of human plasma lipoproteins. II. Receptor binding activity of a high density lipoprotein subfraction modulated by the apo(E--A-II) complex. J Biol Chem. 1978 Sep 10;253(17):6289–6295. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Pitas R. E., Weisgraber K. H., Brown J. H., Gross E. Inhibition of lipoprotein binding to cell surface receptors of fibroblasts following selective modification of arginyl residues in arginine-rich and B apoproteins. J Biol Chem. 1977 Oct 25;252(20):7279–7287. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. H., Fry D. L. Canine hyperlipoproteinemia and atherosclerosis. Accumulation of lipid by aortic medial cells in vivo and in vitro. Am J Pathol. 1977 Apr;87(1):205–226. [PMC free article] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971 Aug 10;246(15):4825–4833. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Weisgraber K. H., Mahley R. W., Assmann G. The rat arginine-rich apoprotein and its redistribution following injection of iodinated lipoproteins into normal and hypercholesterolemic rats. Atherosclerosis. 1977 Oct;28(2):121–140. doi: 10.1016/0021-9150(77)90150-2. [DOI] [PubMed] [Google Scholar]




