Abstract
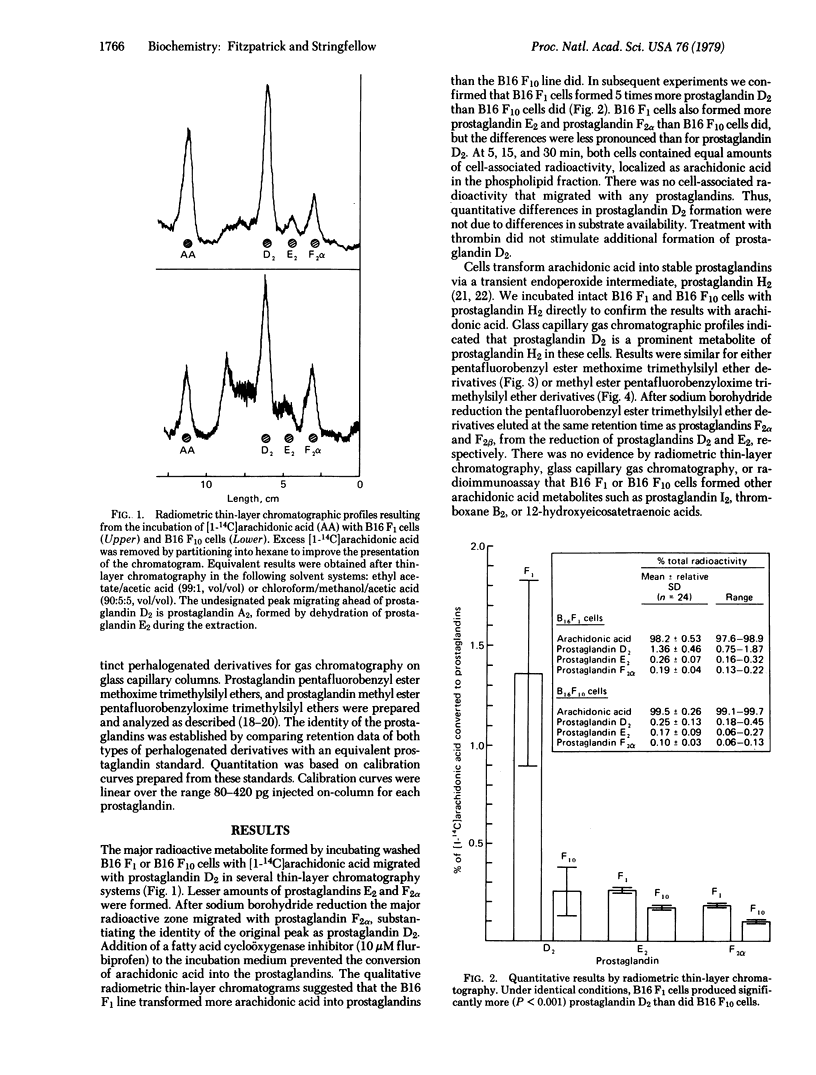
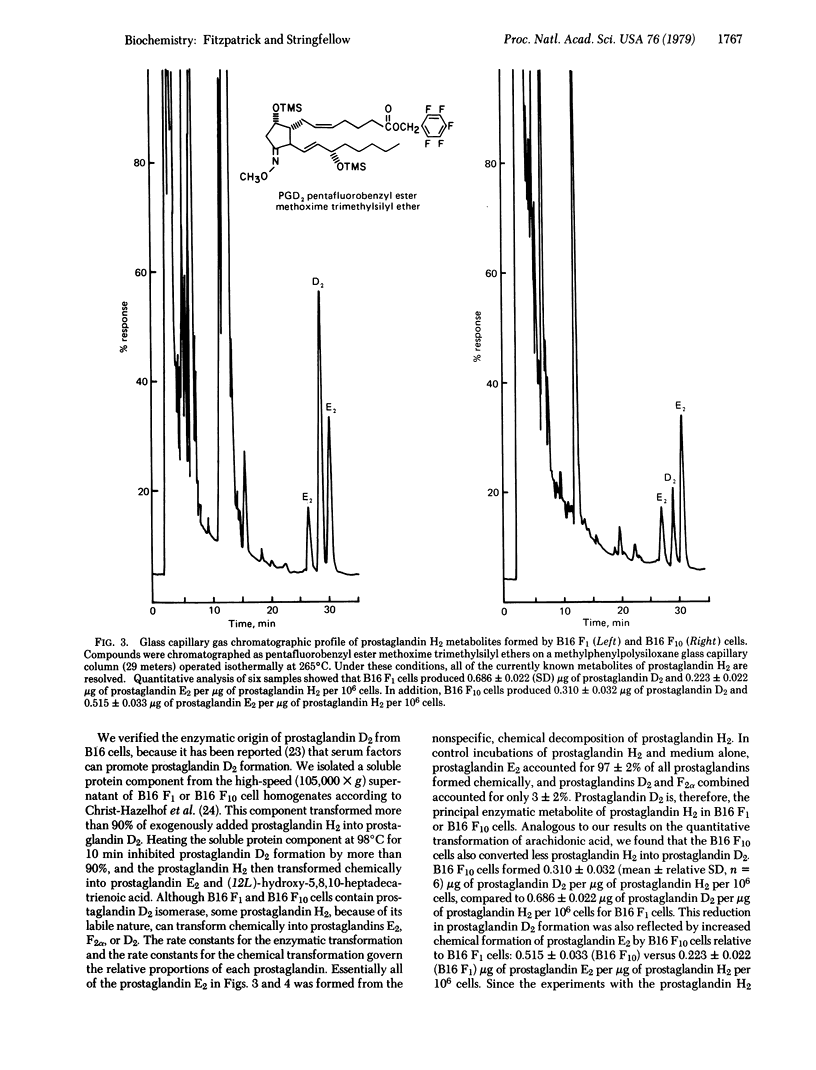
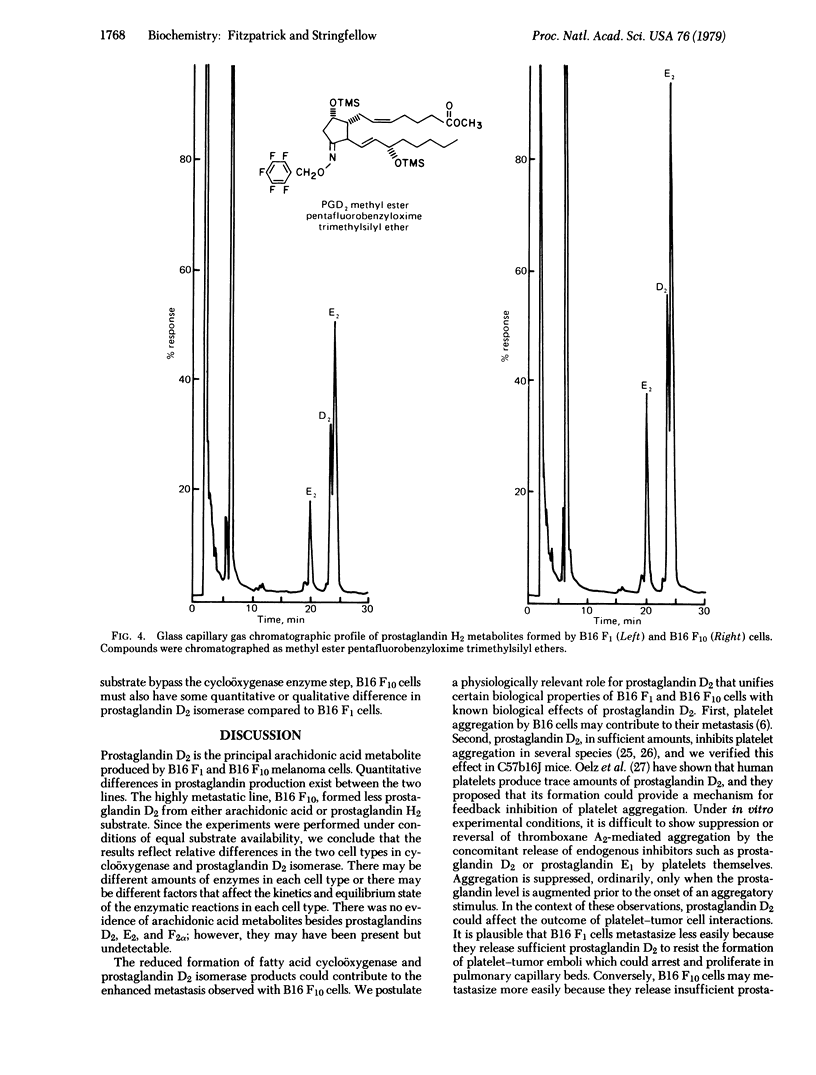
B16 malignant melanoma cell lines transform arachidonic acid and its transient metabolite, prostaglandin endoperoxide H2, into prostaglandin D2. The highly metastatic line, B16 F10, forms less prostaglandin D2 compared to the moderately metastatic parent line, B16 F1. Since platelet aggregation may be one factor involved in B16 metastasis and since prostaglandin D2 inhibits platelet aggregation, this prostaglandin could affect the outcome of platelet-tumour interactions, which may contribute ultimately to metastasis. Arachidonic acid metabolism may be another one of the intrinsic biochemical properties of tumor cells that affects their metastasis. Our results suggest that quantitative release of unusual prostaglandins must be considered in this context.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Halim M. S., Hamberg M., Sjöquist B., Anggård E. Identification of prostaglandin D2 as a major prostaglandin in homogenates of rat brain. Prostaglandins. 1977 Oct;14(4):633–643. doi: 10.1016/0090-6980(77)90190-3. [DOI] [PubMed] [Google Scholar]
- Anhut H., Bernauer W., Peskar B. A. Pharmacological modification of thromboxane and prostaglandin release in cardiac anaphylaxis. Prostaglandins. 1978 May;15(5):889–900. doi: 10.1016/0090-6980(78)90156-9. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Bieber G. F., Brown A. E., Case K. R., Gersten D. M., Kimmerer T. W., Lione A. Biochemical parameters correlated with tumour cell implantation. Nature. 1973 Dec 21;246(5434):487–489. doi: 10.1038/246487a0. [DOI] [PubMed] [Google Scholar]
- Christ-Hazelhof E., Nugteren D. H., Van Dorp D. A. Conversions of prostaglandin endoperoxides by glutathione-S-transferases and serum albumins. Biochim Biophys Acta. 1976 Dec 20;450(3):450–461. doi: 10.1016/0005-2760(76)90018-7. [DOI] [PubMed] [Google Scholar]
- Claesson H. E., Lindgren J. A., Hammarström S. Elevation of adenosine 3',5'-monophosphate levels in 3T3 fibroblasts by arachidonic acid: evidence for mediation by prostaglandin I2. FEBS Lett. 1977 Sep 15;81(2):415–418. doi: 10.1016/0014-5793(77)80567-x. [DOI] [PubMed] [Google Scholar]
- Cohen F., Jaffe B. M. Production of prostaglandins by cells in vitro: radioimmunoassay measurement of the conversion of arachidonic acid to PGE2 and PGF2 alpha. Biochem Biophys Res Commun. 1973 Dec 10;55(3):724–729. doi: 10.1016/0006-291x(73)91204-7. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Budmen M. B. Characterization in vivo and in vitro of tumor cells selected for resistance to syngeneic lymphocyte-mediated cytotoxicity. Cancer Res. 1976 Sep;36(9 PT1):3160–3165. [PubMed] [Google Scholar]
- Fidler I. J. Immune stimulation-inhibition of experimental cancer metastasis. Cancer Res. 1974 Mar;34(3):491–498. [PubMed] [Google Scholar]
- Fidler I. J. Inhibition of pulmonary metastasis by intravenous injection of specifically activated macrophages. Cancer Res. 1974 May;34(5):1074–1078. [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Wynalda M. A., Kaiser D. G. Oximes for high performance liquid and electron capture gas chromatography of prostaglandins and thromboxanes. Anal Chem. 1977 Jun;49(7):1032–1035. doi: 10.1021/ac50015a041. [DOI] [PubMed] [Google Scholar]
- Gasic G. J., Gasic T. B., Galanti N., Johnson T., Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973 May;11(3):704–718. doi: 10.1002/ijc.2910110322. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Sun F. F., Miller O. V., Johnson R. A. Prostaglandins H1 and H2. Convenient biochemical synthesis and isolation. Further biological and spectroscopic characterization. Prostaglandins. 1977 Jun;13(6):1043–1053. doi: 10.1016/0090-6980(77)90132-0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Fredholm B. B. Isomerization of prostaglandin H2 into prostaglandin D2 in the presence of serum albumin. Biochim Biophys Acta. 1976 Apr 22;431(1):189–183. doi: 10.1016/0005-2760(76)90273-3. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1973 Mar;70(3):899–903. doi: 10.1073/pnas.70.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S. Prostaglandin production by normal and transformed 3T3 fibroblasts in cell culture. Eur J Biochem. 1977 Mar 15;74(1):7–12. doi: 10.1111/j.1432-1033.1977.tb11360.x. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Samuelsson B., Bjursell G. Prostaglandin levels in normal and transformed baby-hamster-kidney fibroblasts. Nat New Biol. 1973 May 9;243(123):50–51. [PubMed] [Google Scholar]
- Hilgard P., Gordon-Smith E. C. Microangiopathic haemolytic anaemia and experimental tumour-cell emboli. Br J Haematol. 1974 Apr;26(4):651–659. doi: 10.1111/j.1365-2141.1974.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc Natl Acad Sci U S A. 1976 May;73(5):1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Polsky-Cynkin R., Levine L. Stimulation of prostaglandin biosynthesis by vasoactive substances in methylcholanthrene-transformed mouse BALB/3T3. J Biol Chem. 1976 Feb 10;251(3):776–780. [PubMed] [Google Scholar]
- Marcus A. J. The role of lipids in platelet function: with particular reference to the arachidonic acid pathway. J Lipid Res. 1978 Sep;19(7):793–826. [PubMed] [Google Scholar]
- Nicolson G. L., Winkelhake J. L. Organ specificity of blood-borne tumour metastasis determined by cell adhesion? Nature. 1975 May 15;255(5505):230–232. doi: 10.1038/255230a0. [DOI] [PubMed] [Google Scholar]
- Nishizawa E. E., Miller W. L., Gorman R. R., Bundy G. L., Svensson J., Hamberg M. Prostaglandin d2 as a potential antithrombotic agent. Prostaglandins. 1975 Jan;9(1):109–121. doi: 10.1016/s0090-6980(75)80122-5. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H., Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim Biophys Acta. 1973 Dec 20;326(3):448–461. doi: 10.1016/0005-2760(73)90145-8. [DOI] [PubMed] [Google Scholar]
- Oelz O., Oelz R., Knapp H. R., Sweetman B. J., Oates J. A. Biosynthesis of prostaglandin D2. 1. Formation of prostaglandin D2 by human platelets. Prostaglandins. 1977 Feb;13(2):225–234. doi: 10.1016/0090-6980(77)90004-1. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Silver M. J., Ingerman C. M., Kocsis J. J. Prostaglandin D2 inhibits the aggregation of human platelets. Thromb Res. 1974 Sep;5(3):291–299. doi: 10.1016/0049-3848(74)90168-6. [DOI] [PubMed] [Google Scholar]
- Warren B. A. Environment of the blood-borne tumor embolus adherent to vessel wall. J Med. 1973;4(3):150–177. [PubMed] [Google Scholar]
- Warren B. A., Vales O. The adhesion of thromboplastic tumour emboli to vessel walls in vivo. Br J Exp Pathol. 1972 Jun;53(3):301–313. [PMC free article] [PubMed] [Google Scholar]
- de Deckere E. A., Nugteren D. H., Ten Hoor F. Prostacyclin is the major prostaglandin released from the isolated perfused rabbit and rat heart. Nature. 1977 Jul 14;268(5616):160–163. doi: 10.1038/268160a0. [DOI] [PubMed] [Google Scholar]


