Abstract
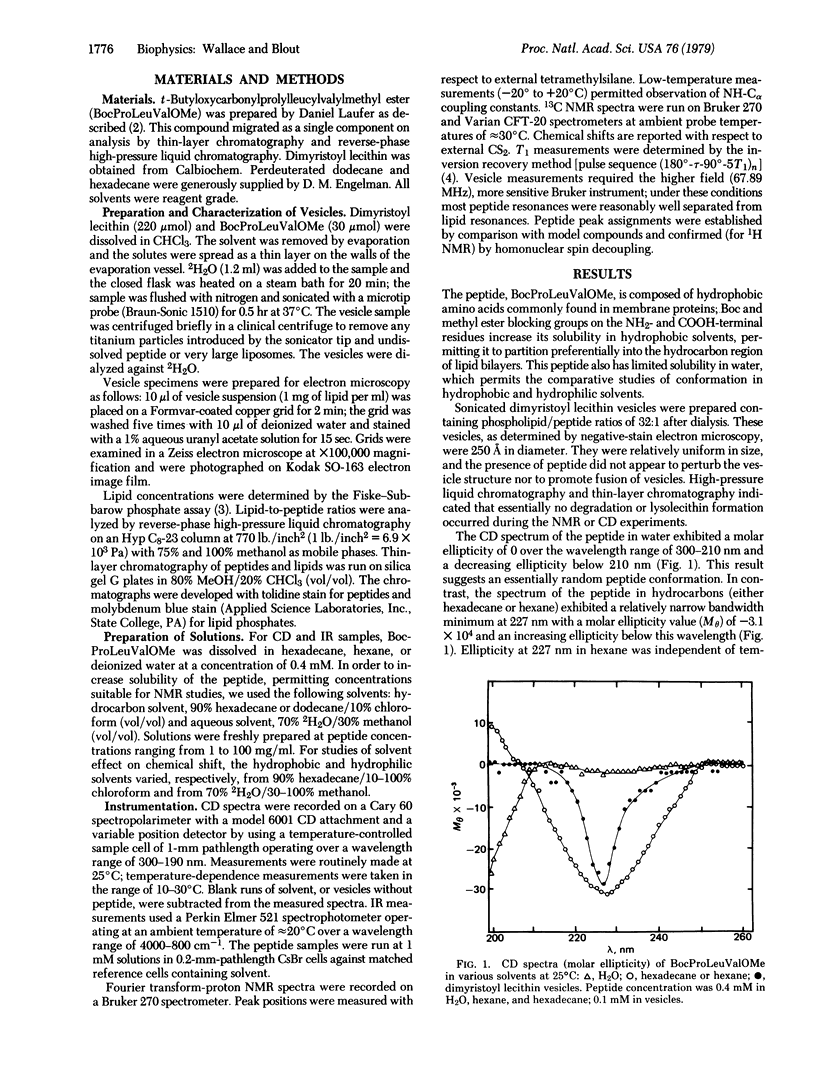
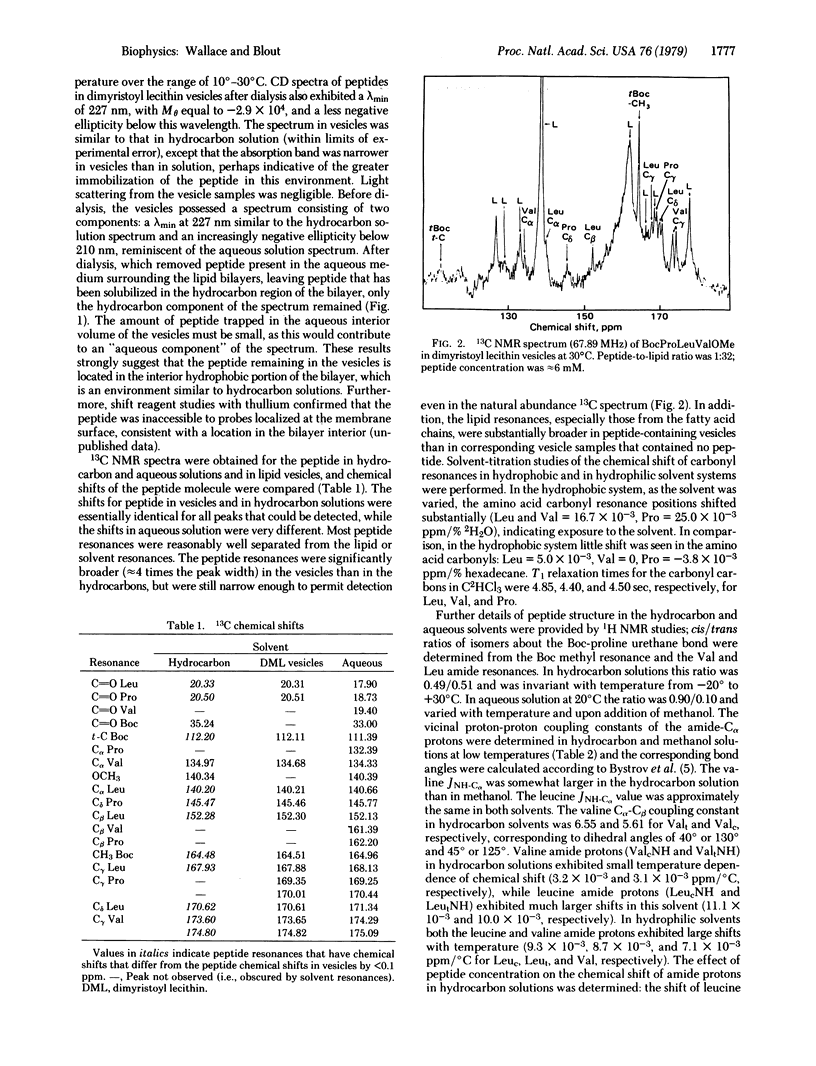
To demonstrate a method by which the conformation of membrane proteins may be determined spectroscopically in model membranes, we determined the structure of a hydrophobic oligopeptide, t-butyloxycarbonylprolylleucylvalylmethyl ester, in phospholipid vesicles by nuclear magnetic resonance, circular dichroism, and infrared spectroscopy. 13C nuclear magnetic resonance and circular dichroism techniques demonstrated that the conformation of this peptide in linear hydrocarbon solutions was essentially identical to its conformation in lipid vesicles. 1H nuclear magnetic resonance and infrared spectroscopy of the peptide in hydrocarbon solution then provided additional high-resolution information concerning the structure of the peptide as found in the hydrophobic portion of the lipid bilayer. The conformation of this peptide in hydrophobic media a differs from its structure in hydrophilic solvents, not only in bond angles and the proportion of cis/trans isomers about the X-proline bond, but also in its intermolecular associations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti E., Palumbo M., Bonora G. M., Toniolo C. On the oxy analogues to the 4 leads to 1 intramolecularly hydrogen-bonded peptide conformations. Macromolecules. 1976 May-Jun;9(3):417–420. doi: 10.1021/ma60051a006. [DOI] [PubMed] [Google Scholar]
- Deslauriers R., Walter R., Smith I. C. Intramolecular motion in peptide determined by 13C NMR: a spin-lattice relaxation time-study on MSH-release-inhibiting factor. FEBS Lett. 1973 Nov 15;37(1):27–32. doi: 10.1016/0014-5793(73)80419-3. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Tasumi M., Miyazawa T. 1H nuclear magnetic resonance studies of N-acetyl-L-proline N-methylamide. Molecular conformations, hydrogen bondings, and thermodynamic quantitites in various solvents. Biopolymers. 1977 Jun;16(6):1259–1270. doi: 10.1002/bip.1977.360160608. [DOI] [PubMed] [Google Scholar]
- Kopple K. D., Schamper T. J., Go A. Conformation of cyclic peptides. 8. Cyclic hexapeptides containing the L-Pro-D-Phe sequence. J Am Chem Soc. 1974 Apr 17;96(8):2597–2605. doi: 10.1021/ja00815a046. [DOI] [PubMed] [Google Scholar]
- Laufer D. A., Blout E. R. A new stepwise synthesis of an octapeptide corresponding to a sequence around the "reactive" serine of chymotrypsin. J Am Chem Soc. 1967 Mar 1;89(5):1246–1249. doi: 10.1021/ja00981a034. [DOI] [PubMed] [Google Scholar]
- Stimson E. R., Zimmerman S. S., Scheraga H. A. Conformational studies of oligopeptides containing proline and glycine. Macromolecules. 1977 Sep-Oct;10(5):1049–1060. doi: 10.1021/ma60059a032. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]



