Abstract
The atomic bomb survivor cancer mortality data have been used in the past to justify the use of the linear no-threshold (LNT) model for estimating the carcinogenic effects of low dose radiation. An analysis of the recently updated atomic bomb survivor cancer mortality dose-response data shows that the data no longer support the LNT model but are consistent with a radiation hormesis model when a correction is applied for a likely bias in the baseline cancer mortality rate. If the validity of the phenomenon of radiation hormesis is confirmed in prospective human pilot studies, and is applied to the wider population, it could result in a considerable reduction in cancers. The idea of using radiation hormesis to prevent cancers was proposed more than three decades ago, but was never investigated in humans to determine its validity because of the dominance of the LNT model and the consequent carcinogenic concerns regarding low dose radiation. Since cancer continues to be a major health problem and the age-adjusted cancer mortality rates have declined by only ∼10% in the past 45 years, it may be prudent to investigate radiation hormesis as an alternative approach to reduce cancers. Prompt action is urged.
Keywords: LNT Model, Radiation Hormesis, Adaptive Response, Atomic Bomb Survivors
INTRODUCTION
Though the use of the linear no-threshold (LNT) extrapolation model has become well established in radiation safety regulations and practices throughout the world in the past several decades, there continues to be a considerable amount of disagreement in the scientific community regarding the appropriateness of its use (Cameron, 1998; Cameron and Moulder, 1998; Cohen, 1998; Mossman, 1998; Strom, 1998; Tubiana, 2005; NRC, 2006; Tubiana et al., 2006; Little et al., 2009; Tubiana et al., 2009). Controlled in-vitro and animal studies have contradicted the LNT model as many of these have shown adaptive response to low dose radiation resulting in reduced mutations and cancers (Hosoi and Sakamoto, 1993; Ishii et al., 1996; Mitchel et al., 1999; Redpath et al., 2003; Elmore et al., 2005; Ina et al., 2005; Day et al., 2007; Moskalev et al., 2011; Phan, 2011; Phan et al., 2012), demonstrating a phenomenon known as radiation hormesis (Luckey, 1980; Luckey, 1991; Calabrese and Baldwin, 2003; Feinendegen, 2005; Jolly and Meyer, 2009; Sanders, 2010). For humans, the effect of radiation on cancer has been inferred by determining the cancer rates of population groups that were exposed to radiation, and comparing these to equivalent population groups not exposed to the radiation. Among all the data that are available from such human studies, the data from the atomic bomb survivors are generally considered to be the “gold standard” for estimating the cancer risk from radiation because of the large size and the non-selective nature of the radiated population group, the wide range of radiation doses involved, and the long-term systematic monitoring of their health (Hall and Brenner, 2008). For example, the atomic bomb survivor data have been used as the primary resource for estimating the carcinogenic risks from low dose radiation in reviews, e.g. (Gilbert, 2009; Suzuki and Yamashita, 2012), and these data have been used by the recent BEIR VII report to estimate radiation cancer risks for individual cancers (see Chapter 6 of the report), to reaffirm the absence of a threshold dose and support for the LNT model, and to exclude the possibility of beneficial effects of low dose radiation (see page 10 of the report) (NRC, 2006). The atomic bomb survivor data have also been used to raise concerns about the radiation dose to the public from the increasing use of diagnostic imaging (Brenner and Hall, 2007; Hall and Brenner, 2008).
EVIDENCE FOR RADIATION HORMESIS IN ATOMIC BOMB SURVIVOR DATA
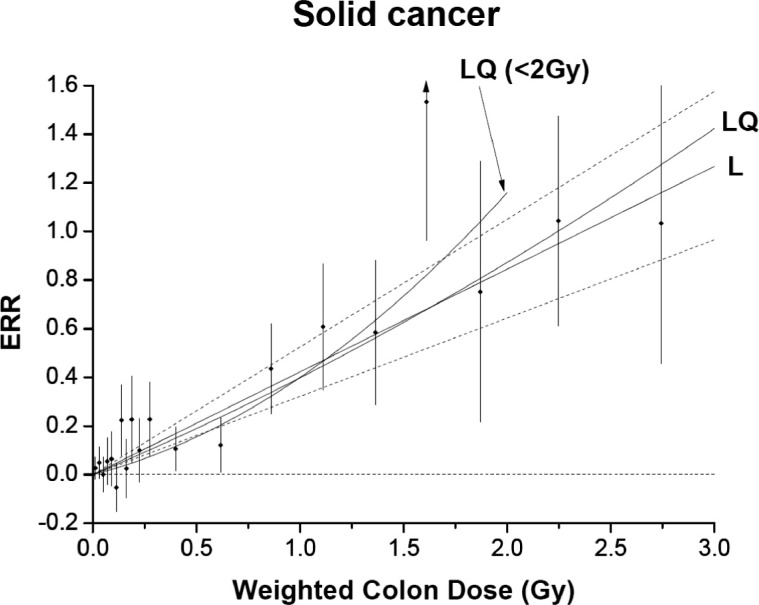
Recently an updated report was published on the mortality of atomic bomb survivors (Ozasa et al., 2012; Ozasa et al., 2013) including six additional years of follow-up compared to the previous comprehensive report (Preston et al., 2003). Figure 1 shows the results from the publication where excess relative risk (ERR) for solid cancer mortality among the survivors is plotted as a function of radiation dose, with ERR defined as (R-B)/B, where R and B are the solid cancer mortality rates of the radiated and baseline cohorts respectively. Since R and B are expected to be nearly equal for doses near zero, and the process of calculating ERR effectively involves subtraction of these two values to obtain the small difference between them, the ERR values are likely to be subject to large fluctuations in the low dose range due to the statistical errors in the cancer mortality rates. For example, the number of excess solid cancer deaths for the dose ranges of 0.005 Gy to 0.01 Gy, and 0.01 Gy to 0.02 Gy in the atomic bomb survivor cohort are 49 and 46 respectively (see Table 9 of the publication) (Ozasa et al., 2012). The total number of cancer deaths for the two dose ranges are 3653 and 789 respectively. These two dose ranges have been divided into ∼5 and ∼4 bins respectively in Figure 1, making the average excess cancers per bin (∼10 and ∼12) smaller than the average standard errors for the bins (∼27 and 14 respectively). The large variation in ERR values observed between even adjacent close dose values for doses below ∼0.3 Gy may indicate the dominance of such errors at these doses overwhelming the dose dependence of ERR.
FIGURE 1.
From Ozasa et al. (Ozasa et al., 2013). Excess relative risk (ERR) for all solid cancer mortality in atomic bomb survivors in relation to radiation exposure. The black circles represent ERR and 95% CI for the dose categories, together with trend estimates based on linear (L) with 95% CI (dotted lines) and linear-quadratic (LQ) models using the full dose range, and LQ model for the data restricted to dose < 2 Gy. Figure reproduced with permission from the Radiation Research Society.
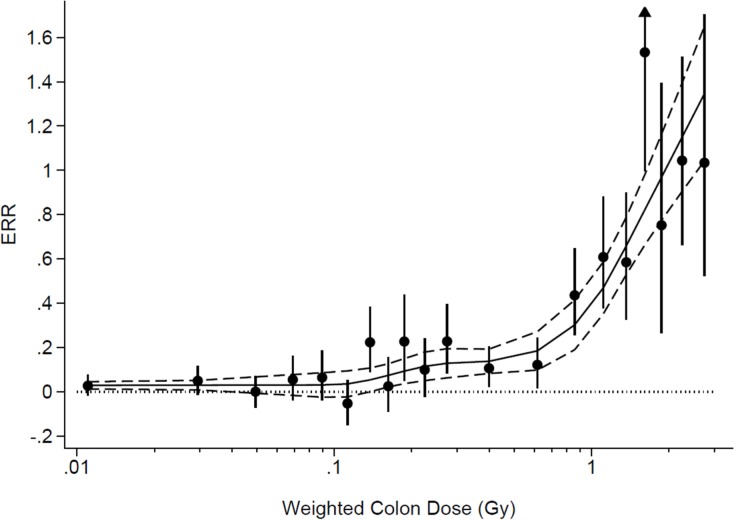
When ERR is considered over the whole dose range, the authors reported that the linear dose–response relationship provided the best fit to the solid cancer mortality data (Ozasa et al., 2012). After performing a detailed dose threshold analysis, the authors also concluded that zero dose is the best estimate for the dose threshold, apparently validating the present use of the LNT model for radiation safety purposes. This conclusion is however questionable as the functional forms they used to fit the ERR data in the dose threshold analysis may have been too restrictive, resulting in the conclusion of zero dose threshold (Doss et al., 2012). If they had used a more generalized functional form, they would not have concluded that zero dose is the best estimate of the dose threshold, as the lower bounds of the point-wise 95% CIs would have been below zero for low doses, as for example determined in the analysis shown in Figure 2. Thus, the atomic bomb survivor data do not provide evidence for the absence of a threshold dose for the carcinogenic effects of radiation.
FIGURE 2.
Excess relative risk (ERR) for all solid cancer in atomic bomb survivors in relation to radiation exposure. The black circles and error bars represent ERR and 95% CIs for the dose categories. Data from (Ozasa et al., 2013). Solid Line - fit to the ERR data using a multiple linear regression in which weighted log colon dose was entered into the model using a restricted cubic spline transformation with five knots. Regression weights were equal to the inverse of the variance of the point estimates. Dashed lines are 95% CI of the fit. Figure from performing analysis equivalent to (Doss et al., 2012) with the corrected data in (Ozasa et al., 2013). Figure provided by Brian L. Egleston.
The updated atomic bomb survivor data have also shown a reduction of ERR for doses in the range of 0.3 to 0.7 Gy when compared to the linear fit to the data (see Figure 1). This deviation from linearity or curvature of the dose response data for 0–2 Gy dose range was significant with a P value of 0.02 in the present update as compared to earlier reports where such deviation was not significant (see Table 7 of the publication) (Ozasa et al., 2012). The reason for the significant curvature was the lower than the expected cancer rates for the dose range of 0.3–0.7 Gy, for which the authors had no explanation (See page 238 of the publication) (Ozasa et al., 2012). The LNT model that the authors used cannot explain this significant reduction in cancers with increasing dose at low doses, since the fundamental idea behind the LNT model is that the higher the dose, the higher the number of mutations, and the higher the cancer incidence.
One possible reason for the inexplicable shape of the dose response curve may be the bias introduced by the method of analysis used to determine the values of ERR as a function of dose (Ozasa et al., 2012). In calculating the ERR values, the authors did not use a zero dose cohort as a baseline group since even the lowest dose cohort had some exposure to the atomic bomb radiation (See Table 1 of the report) (Ozasa et al., 2012). Instead, they fitted the cancer mortality data for all the different dose cohorts using ERR in the form of a linear (or linear plus quadratic) function of dose multiplied by an effects modification factor to account for other variables such as age, sex, etc., and extracted the ERR values from the fit to the whole dataset (see page 231 of the report) (Ozasa et al., 2012). In this procedure, the cancer mortality rates of the lowest dose cohorts effectively determined the baseline cancer mortality rate through linear extrapolation to zero dose. If the low dose radiation cohorts had reduced cancer rates compared to the baseline cancer rate due to radiation hormesis, then this procedure would introduce a negative bias in the baseline cancer rate, since the lower cancer rates at low doses (extrapolated to zero dose) would effectively be used as the baseline cancer rate during the fitting process. Many retrospective human studies have shown reduced cancer rates in the cohorts subjected to low dose radiation in comparison to the cohorts not subjected to such radiation (Hwang et al., 2006; Cohen, 2007; Vaiserman, 2010; Thompson, 2011). Let us now discuss the publication (Hwang et al., 2006) in some detail, as it reports the effect of accidental whole body low dose radiation on cancer rate in a large population group.
In 1982, a number of buildings were constructed in Taiwan using steel unknowingly contaminated with Co-60, exposing thousands of residents to low dose radiation for a number of years. Detailed dose estimation performed for this population group indicates the average excess radiation dose received by the residents from the contamination was ∼0.048 Gy (Hwang et al., 2006). This radiation dose is similar to the average of the doses received by the two lowest dose cohorts in the atomic bomb survivor study (0-0.005 Gy and 0.005-0.1 Gy) (Ozasa et al., 2012). The cancer incidence for the residents has been monitored and the standardized incidence ratio (SIR) of solid cancers for the period up to 2002 has been determined to be 0.7 (with 95% CI of 0.6–0.9), based on the 82 observed and 109.5 expected solid cancers (see Table III of the publication) (Hwang et al., 2006), indicating the irradiated residents had a significantly reduced rate of solid cancers. A significant reduction in all cancers was also reported, with the SIR of 0.8 (95% CI of 0.7–1.0). A follow-up report including 3 additional years of data indicated the number of solid cancers observed had increased to 106 (Hwang et al., 2008). Adjusting for the increase in average age of the radiated population from 33.3 to 36.3 between the two reports using age-specific solid cancer incidence rates from Taiwan Cancer Registry (TCR, 2008), the expected solid cancers are projected to be 151, resulting again in SIR of ∼0.7 for solid cancers. This reduction of cancers cannot be explained using the LNT model and is consistent with the radiation hormesis model. If there were a similar reduction in cancers in the lowest dose cohorts in the atomic bomb survivors, the procedure used for analyzing the atomic bomb survivor cancer mortality data would have led to a negative bias in the baseline cancer rate. Such a bias has likely influenced the conclusions of the atomic bomb survivor studies published since 1987 when the current procedure of analysis was adopted.
If the baseline cancer rate used to compute ERR has a bias, it can be shown that the calculated ERR values can be corrected for such a bias using the following equation (Doss, 2012a),
| (1) |
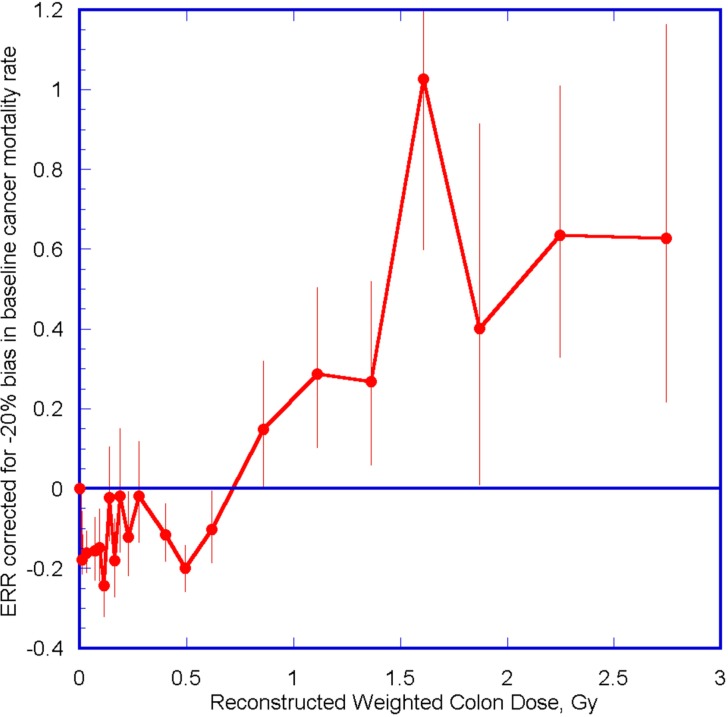
where ERR(corr) is the value of ERR corrected for the bias, and δ is the percentage bias in the baseline cancer mortality rate. This correction has been applied to the ERR values in Figure 1 assuming −20% bias in the baseline cancer rate (being a rough estimate similar to the reduction in cancers observed in the Taiwan residents study) (Hwang et al., 2006), and the resulting dose-response data are shown in Figure 3. The correction has shifted the ERRs to lower values resulting in negative ERR values for all the doses below ∼0.6 Gy (see Figure 3). Though there are large fluctuations in the corrected ERR values between even adjacent close dose values for doses below ∼0.3 Gy, the overall pattern of negative ERR values for doses below ∼0.6 Gy is indicative of the cancer preventive effect of low dose radiation that has been observed in animal and human studies (Cohen, 2007; Sanders, 2010; Thompson, 2011). Thus, the qualitative shape of the dose response curve of the atomic bomb survivor data has a plausible explanation using a radiation hormesis model when corrected for the likely bias in the baseline cancer rate, whereas there is no explanation for the observed reduction in ERR values in the 0.3–0.7 Gy dose range using the LNT model.
FIGURE 3.
Excess relative risk (ERR) for all solid cancer mortality in atomic bomb survivors corrected for −20% bias in baseline cancer mortality rate plotted as a function of colon dose. Error bars are 95% CI. The obvious requirement that ERR = 0 at zero dose has been added as an additional data point.
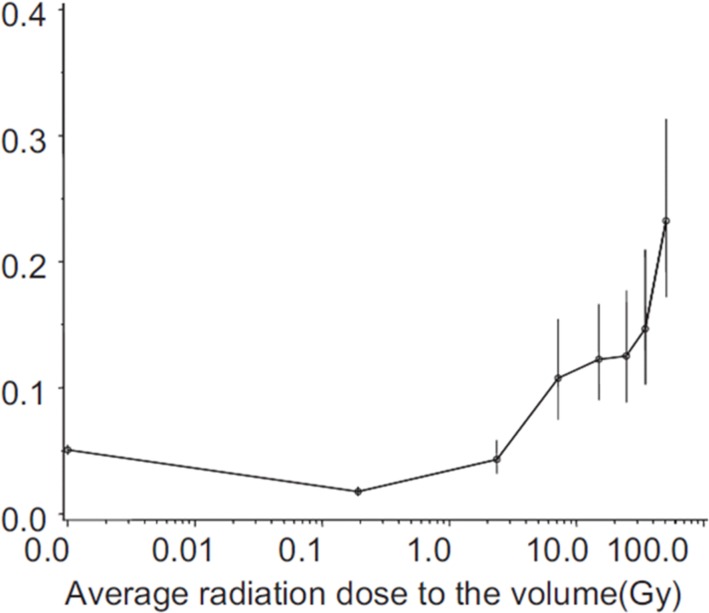
Additional support for the reduced incidence of cancers from low dose radiation in humans has been reported recently by Tubiana et al. who analyzed the incidence of second cancers in radiation therapy patients. The tissues that had received ∼0.2 Gy were found to have reduced cancers per kg compared to the unradiated tissues that had received no radiation dose (see Figure 4) (Tubiana et al., 2011).
FIGURE 4.
From (Tubiana et al., 2011). Second cancers per kg according to the mean dose received in volume in radiation therapy patients. Figure reproduced with permission from Health Physics.
LNT MODEL VS. RADIATION HORMESIS
The inability of the LNT model to explain (i) the significant reduction of cancers in the 0.3–0.7 Gy region of the atomic bomb survivor dose-response data, (ii) the reduction of cancers in Taiwan residents exposed to low dose radiation and in other radiated cohorts, and (iii) the reduction of second cancers per kg of tissue subjected to ∼20 cGy dose in radiation therapy patients is not surprising since the LNT model considers only the initial interaction of radiation that causes the oxidative damage and mutation. The LNT model completely ignores the body’s defensive adaptive responses that may be triggered by the low dose radiation (Feinendegen, 2005; Feinendegen et al., 2013). The importance of such adaptive response becomes clear when one considers the contrast between (i) the failure of anti-angiogenesis therapies which did not take into consideration the adaptive response of the tumors to the therapy and (ii) the occasional unexpected cure of metastatic lesions following radiation therapy to a primary tumor known as the abscopal effect, which is likely from the adaptive response of increased immune system activity from the stimulation by incidental low dose radiation to parts of body from the radiation therapy (Doss, 2013). The radiation hormesis concept, on the other hand, includes the consideration of the adaptive responses of the body, i.e. (i) the protective radiobiological effects of low dose radiation that tend to reduce the naturally occurring DNA damage resulting in the suppression of the endogenous carcinogenic process (Feinendegen, 2005; Scott, 2007; Liu, 2010), and (ii) the elevated immune system response (Ina and Sakai, 2005; Farooque et al., 2011) which plays a major role in preventing occult cancers from becoming clinical cancers (Koebel et al., 2007), and in suppressing metastasis (Nowosielska et al., 2010).
The atomic bomb survivor data are considered to be the best available data for estimating radiation effects by many scientists and advisory committees, and have been used to justify the continued use of the LNT model for radiation safety (NRC, 2006). Since the LNT model cannot explain even qualitatively the reduction of cancers in the 0.3–0.7 Gy dose range in the updated atomic bomb survivor cancer mortality data, the use of the LNT model should be discontinued, and the present radiation safety regulations for low dose radiation should be revised taking into account the unsuitability of the LNT model. In addition, since the radiation hormesis model can explain the shape of the atomic bomb survivor dose-response data qualitatively, the phenomenon of radiation hormesis should be investigated further to build confidence in its validity. The study of radiation hormesis and the use of low dose radiation to reduce cancers had been proposed several decades ago (Luckey, 1980; Hickey et al., 1983; Luckey, 1991), and continues to be advocated (Luckey, 1999; Cameron, 2002; Pollycove, 2007; Doss, 2012b). A proper scientific approach to decide between two competing hypotheses is to perform studies to test the predictions from the two hypotheses. Thus, the study of radiation hormesis should have been initiated in pilot human studies when it was proposed over three decades ago (Luckey, 1980; Hickey et al., 1983), considering the important beneficial consequences to human health if such studies had demonstrated reduced cancers from the low dose radiation. However, no prospective human cancer prevention studies have been conducted thus far to investigate the concept to determine its validity because of the dominance of the LNT model-based radiation safety regulations which has resulted in carcinogenic concerns regarding any dose of radiation.
Though no prospective cancer prevention studies have been performed with low dose radiation in humans so far, the effect of low dose radiation applied to the whole body (or to half the body) has been investigated for non-Hodgkin’s lymphoma patients in a clinical study, with the low dose radiation treatments being interspersed between the standard radiation therapy treatments to the primary tumor (Sakamoto, 2004). The interspersed low dose radiation treatments have resulted in reduced metastases and improved overall patient survival in comparison to the standard radiation therapy alone (see Figure 5), indicating the effectiveness of low dose radiation in reducing the adverse impact of cancers for these patients.
FIGURE 5.
From (Sakamoto, 2004). Survival of Stage I and II non-Hodgkin’s lymphoma patients following interspersed low-dose total-body or half-body irradiation between local radiation therapy treatments compared to local radiation therapy treatments only. Figure reproduced with permission from Nonlinearity in Biology Toxicology and Medicine.
RADIATION HORMESIS AS A METHOD OF REDUCING CANCERS
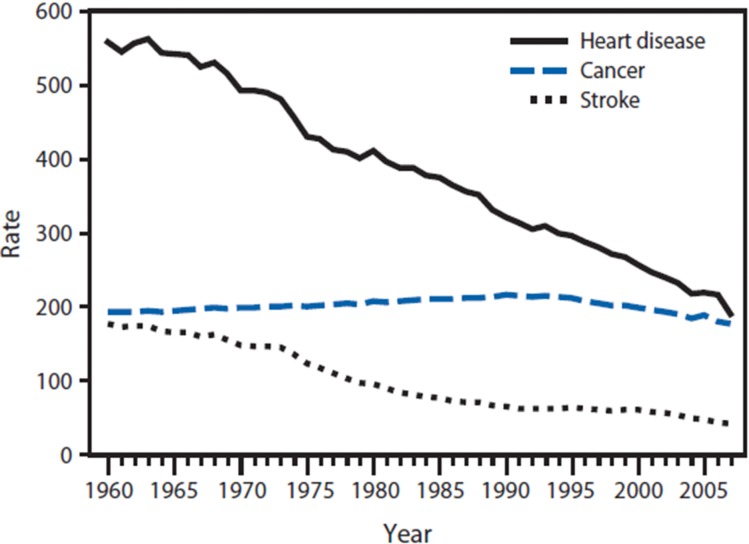
If the hormetic effect observed in the atomic bomb survivors (and other radiated population groups) would be confirmed in human studies and applied to the general population, it could result in a considerable reduction in cancer mortality. Since the traditional approaches to reduce cancers have had limited success (Faguet, 2005; Goldstein et al., 2012) and there has been only about 10% reduction in age-adjusted cancer mortality rate in the past 45 years (See Figure 6) (Remington and Brownson, 2011), it may be very worthwhile to investigate the validity of the radiation hormesis concept in humans as an alternative paradigm, as has been suggested in prior publications (Luckey, 1999; Cameron, 2002; Pollycove, 2007).
FIGURE 6.
From (Remington and Brownson, 2011). Trends in age-adjusted death rates for the leading chronic diseases in United States for 1960–2007.
In view of the reported significant curvature in dose-response of the present atomic bomb survivor data (for 0–2 Gy dose range) that is not consistent with the LNT model and has provided evidence for radiation hormesis, and in view of the additional human data supporting the concept of radiation hormesis in Taiwan residents subjected to low dose radiation and in the study of second cancers in radiation therapy patients, the long period of adherence to the LNT model has likely resulted in serious adverse consequences. By our inaction in not studying radiation hormesis in humans since the time it was proposed in the 1980s, we may have contributed to a large number of preventable cancer deaths. The analysis of the atomic bomb survivor data has shown there was some reduction in the cancer mortality rate in the low dose region (see Figure 3). Since there is considerable uncertainty in quantifying this reduction as it depends on the assumed bias in the baseline cancer mortality rates, for the discussion below it will be assumed that 10% reduction in cancer mortality rates may be achieved by the application of low dose radiation in humans. Under this assumption, and using the current estimate of 577,190 annual cancer deaths in USA (Siegel et al., 2012), over 1.15 million cancer deaths may have been prevented in the past 20 years in the USA by the application of radiation hormesis. The worldwide numbers would be ∼13 times higher considering the 7.6 million annual global cancer death toll (Jemal et al., 2011). Prompt action is urged.
The main reason for not investigating the radiation hormesis hypothesis in the 1980s as well as in later years was that the use of the LNT model had become well established and there was, and there continues to be, considerable concern regarding low dose radiation among the scientists and public. One of the main factors contributing to this concern is the reported increased cancers among the atomic bomb survivors. In the recent update to the atomic bomb survivor data (Ozasa et al., 2012), the authors have estimated the total number of excess cancer deaths due to radiation exposure from the bombs to be 527 in the 58% of the 86,611 study subjects who had deceased up to the end of 2003 (See Table 9 of the publication) (Ozasa et al., 2012). Extrapolating these excess cancer deaths to the rest of the study cohort using the same proportion, the total number of excess cancer deaths in the study cohort can be projected to be ∼900. Since roughly half the survivors within 2.5 km of the bombs are included in the study cohort (Pierce et al., 1996), the final excess cancer death toll among all the atomic bomb survivors can be estimated to be ∼1800. It is ironic that we are likely having more preventable cancer deaths every day (due to not studying radiation hormesis from the fear of low dose radiation) than the total projected atomic bomb cancer death toll that is mainly responsible for the fear of low dose radiation.
ENABLING STUDY OF RADIATION HORMESIS FOR CANCER PREVENTION
Though the above discussion indicates it may be worthwhile investigating the use of radiation hormesis for preventing cancers, a prospective human study of radiation hormesis for cancer prevention is not presently feasible in view of the current recommendations of most of the advisory bodies in support of the LNT model, the current radiation safety regulations based on the LNT model, and the carcinogenic concerns regarding low dose radiation in the scientific community and in the public. Major changes are needed in these areas to enable the study of radiation hormesis for cancer prevention in humans.
The first needed change is the reversal of recommendations by the advisory bodies most of which have so far supported the use of the LNT model for the purposes of radiation safety, since governments are influenced by recommendations of such advisory bodies in formulating the radiation safety regulations and policies. The advisory bodies should be asked to review their recommendations in view of the new atomic bomb survivor data and analyses that have raised doubts about the LNT model. The new evidence observed in atomic bomb survivors against the LNT model and for radiation hormesis is much stronger than the evidence claimed for low dose radiation induced leukemias (Dus, 1957; Lewis, 1957). Partly based on such evidence, the LNT model was adopted by the advisory bodies in the 1950s (Calabrese, 2009). If the advisory bodies review the data, and endorse the change away from the LNT model, it would greatly help in rescinding the LNT model-based radiation safety regulations and reducing the concerns regarding low dose radiation among the scientists and the public.
The second change needed is the revocation of the LNT model based radiation safety regulations that have been used by governments worldwide since the 1950s implying that even the smallest amount of radiation can increase the risk of cancer. These regulations prevent prospective studies of health effects of low dose radiation by raising carcinogenic concerns regarding any low dose radiation exposure. Governments should be urged to replace the present LNT model based radiation safety paradigm with an adaptive response based model and modify the radiation safety regulations accordingly to enable the prospective study of radiation hormesis (Doss, 2012b; Doss, 2013).
The third needed change is the allaying of the carcinogenic concerns regarding low dose radiation among the scientists and the public, which were fueled by many peer-reviewed publications as well as stories in the popular media regarding the projected carcinogenic hazards from low dose radiation based on the LNT model. Though several books have been written (Luckey, 1980; Luckey, 1991; Sanders, 2010) and many articles have been published in scientific journals (Luckey, 1999; Feinendegen, 2005; Scott, 2011; Doss, 2012b; Doss, 2012a) that present arguments and data supporting radiation hormesis and against the LNT model, these do not receive much publicity in the popular media. On the other hand, sensational articles that project dangers from low dose radiation based on the LNT model, e.g. (Berrington de Gonzalez et al., 2009) or based on comparison to atomic bomb survivor data (Brenner and Hall, 2007) get wide coverage, distorting the view of the scientists and the general public about the present state of scientific knowledge, and raising their concerns regarding low dose radiation. Hence, a prolonged education program should be initiated to correct the misconceptions and inform the scientific community and the public regarding the invalidity of the LNT model and the evidence for the phenomenon of radiation hormesis, in order to reduce and eliminate the concerns regarding low dose radiation.
Successful implementation of these changes may make it feasible to conduct prospective pilot studies of radiation hormesis to confirm its validity and determine its usability for reducing cancers in the general population.
Another possible approach to reduce the concerns regarding low dose radiation is to explore applications of radiation hormesis for cancer patients in pilot clinical trials, since success in such clinical trials can reduce the carcinogenic concerns regarding low dose radiation.
APPLICATIONS OF RADIATION HORMESIS IN CANCER PATIENTS
There is a considerable amount of pre-clinical and clinical data suggesting the use of low dose radiation for treating cancer patients, either alone or as an adjuvant to the standard cancer therapies (Cuttler and Pollycove, 2003; Farooque et al., 2011). Pilot clinical trials are needed (preceded by pre-clinical studies to optimize the treatment protocols) to determine the effectiveness of low dose radiation in improving patient outcomes in cancer patients. Among the applications that can be considered are:
Treatment of early stage cancers. Since low dose radiation boosts the immune system, and the immune system plays a major role in keeping occult cancers in check, the adaptive response from low dose radiation may be sufficiently effective in treating early stage cancers reducing the need for current standard treatments such as lumpectomy, radiation therapy, and/or chemotherapy with the accompanying adverse side effects (Doss, 2013).
Use of interspersed low dose radiation as an adjuvant to standard radiation therapy. Interspersing low dose radiation of the whole body or half of the body between standard radiation therapy treatments may be effective in improving primary tumor control as well as in reducing metastatic disease as has been observed in the clinical study of non-Hodgkin’s lymphoma patients (Sakamoto, 2004).
Reduction of second cancers in radiation therapy patients. With the longer-term survival of cancer patients following improvements in cancer therapies, there are increasing concerns regarding the incidence of second cancers in the survivors (Tubiana, 2009; Yock and Caruso, 2012). The analysis of Tubiana et al. indicates there was reduced incidence of second cancers per kg in tissues exposed to radiation dose of ∼20 cGy in comparison to unexposed tissues in radiation therapy patients (See Figure 4) (Tubiana et al., 2011). This suggests second cancers may be reduced in cancer patients by subjecting them to low dose radiation exposure. Clinical trials are needed to determine if whole body radiation exposure at the level of ∼20 cGy following standard cancer therapy is effective in reducing the incidence of second cancers in these patients.
For these initial applications, low dose radiation exposure in the range of 10–20 cGy to the whole body or to half the body may be achieved conveniently using the standard radiation therapy machines which are readily available in most hospitals caring for such patients. Success in such clinical trials can reduce the current carcinogenic concerns regarding low dose radiation by demonstrating its cancer preventive nature, and enable the study of radiation hormesis for cancer prevention in larger clinical trials.
PROSPECTIVE STUDY OF RADIATION HORMESIS
Though there is evidence supporting radiation hormesis in the atomic bomb survivor data, second cancer data in radiation therapy patients and other epidemiological studies, we need to perform detailed prospective pilot studies to confirm the validity of the phenomenon, and determine the radiation doses that result in significant reduction in cancers. If the pilot studies fail to demonstrate a significant cancer preventive effect, the radiation hormesis hypothesis will need to be rejected. However, if the reduction in cancers from low dose radiation is confirmed in pilot studies, the concept should be applied to larger population groups in order to reduce the cancer rates in the wider population. At the same time, a systematic investigation should be initiated to optimize the cancer prevention by varying the low dose radiation treatment parameters. The optimum radiation dose that results in the most reduction in cancers would need to be determined from such studies.
Since the adaptive response from a single application of low dose radiation is likely to decrease with elapsed time, repeated exposure to low dose radiation at an appropriate time interval may reinforce the adaptive response and enable even higher reduction in cancer mortality rates than was observed in the atomic bomb survivors. However, this needs to be confirmed, and the optimum time interval needs to be determined. Chronic radiation should also be studied at different dose rates to assess if the cancer rates can be reduced using chronic radiation. In addition, different types of low dose radiation should be studied to determine if there are differences in the cancer preventive effect, in order to optimize the type of radiation to be used. Whereas some of these studies may show results in a relatively short time, others may require a lifetime of follow up. Thus, substantial resources will need to be allocated to the systematic study of radiation hormesis over a long period of time. The rejection of the LNT model and rescinding of LNT model-based radiation safety regulations (which would be a pre-requisite for enabling the study of radiation hormesis) can free up considerable amount of resources and manpower from reduced compliance and enforcement needs which can be redirected to the systematic study of radiation hormesis.
SUMMARY
The conclusion of zero threshold dose for carcinogenic effects of radiation in the recent updated report on the atomic bomb survivor cancer mortality data appears to be unjustified and may be the result of the restrictive functional forms that were used to fit the data. Also, the shape of the dose-response observed in the recent update of atomic bomb survivor data is clearly non-linear with the significant reduction in cancer mortality rate in the dose range of 0.3 Gy to 0.7 Gy. This raises doubts about the LNT model and possibly shows evidence for the phenomenon of radiation hormesis when a correction is applied for a likely bias in the baseline cancer mortality rate. Though the use of radiation hormesis was proposed more than three decades ago as a method of reducing cancers, no prospective human cancer prevention studies have been conducted so far to determine its validity due to carcinogenic concerns based on the LNT model. Since the current approaches to reduce cancers have had limited success with only about 10% reduction in age-adjusted cancer mortality rate in the past 45 years, it may be prudent to investigate radiation hormesis as an alternative paradigm to reduce cancers, though it is against the present radiation safety regulations and practices, which are based on the LNT model. Several major changes will need to occur before any prospective human studies of cancer prevention using radiation hormesis can be conducted, including (i) recognition of the importance of adaptive response by advisory committees and reversal of their current recommendations to use the LNT model for radiation safety, (ii) changes in the governments’ radiation safety regulations recognizing adaptive response, and (iii) allaying of concerns among the scientists and the public regarding low dose radiation through increased dissemination of information on the invalidity of the LNT model and the evidence for radiation hormesis. Low dose radiation may also be helpful in improving outcomes in cancer patients by cure of early stage cancers, as an adjuvant to standard radiation therapy to improve tumor control and reduce metastases, and to reduce the incidence of second cancers. Pilot clinical trials are needed to determine the effectiveness of low dose radiation in these applications. Success in such clinical trials can help to reduce the concerns regarding low dose radiation and enable the study of cancer prevention using radiation hormesis. Prompt action is needed.
Acknowledgments
Thanks to Brian L. Egleston for revising the analysis reported in Doss et al. (Doss et al., 2012) using the corrected data from (Ozasa et al., 2013) and generating Figure 2.
This work was supported in part by the Office of Science (BER), U.S. Department of Energy, under Award No. DE-SC0001196. The views and opinions expressed herein are those of the author and do not necessarily reflect those of his employer or the funding agency.
REFERENCES
- Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. The road to linearity: why linearity at low doses became the basis for carcinogen risk assessment. Arch Toxicol. 2009;83:203–25. doi: 10.1007/s00204-009-0412-4. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–97. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Cameron JR. Argument against the motion that the LNT model is appropriate for the estimation of risk from low-level (less than 100 mSv/year) radiation. Med Phys. 1998;25:276. doi: 10.1118/1.598207. [DOI] [PubMed] [Google Scholar]
- Cameron JR. A prospective study should be performed to test the hypothesis that an increase in background radiation to residents in the gulf states will increase their longevity. For the proposition. Med Phys. 2002;29:1511–2. doi: 10.1118/1.1489045. [DOI] [PubMed] [Google Scholar]
- Cameron JR, Moulder JE. Proposition: radiation hormesis should be elevated to a position of scientific respectability. Med Phys. 1998;25:1407–10. doi: 10.1118/1.598312. [DOI] [PubMed] [Google Scholar]
- Cohen B. The Cancer Risk from Low-Level Radiation. In: Tack D, Gevenois P, editors. Radiation Dose from Adult and Pediatric Multidetector Computed Tomography. Berlin: Springer-Verlag; 2007. [Google Scholar]
- Cohen BL. Argument against the motion that low levels of radon in homes should be considered harmful. Med Phys. 1998;25:277–8. doi: 10.1118/1.598207. [DOI] [PubMed] [Google Scholar]
- Cuttler JM, Pollycove M. Can Cancer Be Treated with Low Doses of Radiation? Journal of American Physicians and Surgeons. 2003;8:108–11. [Google Scholar]
- Day TK, Hooker AM, Zeng G, Sykes PJ. Low dose X-radiation adaptive response in spleen and prostate of Atm knockout heterozygous mice. Int J Radiat Biol. 2007;83:523–34. doi: 10.1080/09553000701420582. [DOI] [PubMed] [Google Scholar]
- Doss M. Evidence Supporting Radiation Hormesis in Atomic Bomb Survivor Cancer Mortality Data. Dose Response. 2012a;10:584–592. doi: 10.2203/dose-response.12-023.Doss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss M. Shifting the Paradigm in Radiation Safety. Dose Response. 2012b;10:562–583. doi: 10.2203/dose-response.11-056.Doss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss M. The importance of adaptive response in cancer prevention and therapy. Medical Physics. 2013;40:030401. doi: 10.1118/1.4773027. [DOI] [PubMed] [Google Scholar]
- Doss M, Egleston BL, Litwin S. Comments on “Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases” (Radiat Res 2012; 177:229–43) Radiat Res. 2012;178:244–5. doi: 10.1667/rr3039.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus G. Loaded Dice. Science. 1957;125:963. doi: 10.1126/science.125.3255.963. [DOI] [PubMed] [Google Scholar]
- Elmore E, Lao XY, Ko M, Rightnar S, Nelson G, Redpath J. Neoplastic transformation in vitro induced by low doses of 232 MeV protons. Int J Radiat Biol. 2005;81:291–7. doi: 10.1080/09553000500140324. [DOI] [PubMed] [Google Scholar]
- Faguet GB. The war on cancer : an anatomy of failure, a blueprint for the future. Dordrecht: Springer; 2005. [Google Scholar]
- Farooque A, Mathur R, Verma A, Kaul V, Bhatt AN, Adhikari JS, Afrin F, Singh S, Dwarakanath BS. Low-dose radiation therapy of cancer: role of immune enhancement. Expert Rev Anticancer Ther. 2011;11:791–802. doi: 10.1586/era.10.217. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;78:3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Pollycove M, Neumann RD. Hormesis by Low Dose Radiation Effects: Low-Dose Cancer Risk Modeling Must Recognize Up-Regulation of Protection. In: Baum RP, editor. Therapeutic Nuclear Medicine. Springer; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ES. Ionising radiation and cancer risks: what have we learned from epidemiology? Int J Radiat Biol. 2009;85:467–82. doi: 10.1080/09553000902883836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I, Madar S, Rotter V. Cancer research, a field on the verge of a paradigm shift? Trends Mol Med. 2012;18:299–303. doi: 10.1016/j.molmed.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–78. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- Hickey RJ, Bowers EJ, Clelland RC. Radiation hormesis, public health, and public policy: a commentary. Health Phys. 1983;44:207–19. doi: 10.1097/00004032-198303000-00001. [DOI] [PubMed] [Google Scholar]
- Hosoi Y, Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol. 1993;26:177–9. doi: 10.1016/0167-8140(93)90101-d. [DOI] [PubMed] [Google Scholar]
- Hwang SL, Guo HR, Hsieh WA, Hwang JS, Lee SD, Tang JL, Chen CC, Chang TC, Wang JD, Chang WP. Cancer risks in a population with prolonged low dose-rate gamma-radiation exposure in radiocontaminated buildings, 1983–2002. Int J Radiat Biol. 2006;82:849–58. doi: 10.1080/09553000601085980. [DOI] [PubMed] [Google Scholar]
- Hwang SL, Hwang JS, Yang YT, Hsieh WA, Chang TC, Guo HR, Tsai MH, Tang JL, Lin IF, Chang WP. Estimates of relative risks for cancers in a population after prolonged low-dose-rate radiation exposure: a follow-up assessment from 1983 to 2005. Radiat Res. 2008;170:143–8. doi: 10.1667/RR0732.1. [DOI] [PubMed] [Google Scholar]
- Ina Y, Sakai K. Further study of prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice: effects of whole-life irradiation. Radiat Res. 2005;163:418–23. doi: 10.1667/rr3316. [DOI] [PubMed] [Google Scholar]
- Ina Y, Tanooka H, Yamada T, Sakai K. Suppression of thymic lymphoma induction by lifelong low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat Res. 2005;163:153–8. doi: 10.1667/rr3289. [DOI] [PubMed] [Google Scholar]
- Ishii K, Hosoi Y, Yamada S, Ono T, Sakamoto K. Decreased incidence of thymic lymphoma in AKR mice as a result of chronic, fractionated low-dose total-body X irradiation. Radiat Res. 1996;146:582–5. [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jolly D, Meyer J. A brief review of radiation hormesis. Australas Phys Eng Sci Med. 2009;32:180–7. doi: 10.1007/BF03179237. [DOI] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- Lewis EB. Leukemia and ionizing radiation. Science. 1957;125:965–72. doi: 10.1126/science.125.3255.965. [DOI] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de Gonzalez A. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology. 2009;251:6–12. doi: 10.1148/radiol.2511081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SZ. Biological effects of low level exposures to ionizing radiation: theory and practice. Hum Exp Toxicol. 2010;29:275–81. doi: 10.1177/0960327109363967. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Hormesis with ionizing radiation. Boca Raton, Fla: CRC Press; 1980. [Google Scholar]
- Luckey TD. Radiation hormesis. Boca Raton, Fla: CRC Press; 1991. [Google Scholar]
- Luckey TD. Nurture with ionizing radiation: a provocative hypothesis. Nutr Cancer. 1999;34:1–11. doi: 10.1207/S15327914NC340101. [DOI] [PubMed] [Google Scholar]
- Mitchel RE, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat Res. 1999;152:273–9. [PubMed] [Google Scholar]
- Moskalev AA, Plyusnina EN, Shaposhnikov MV. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: the role of cellular stress-resistance mechanisms. Biogerontology. 2011;12:253–63. doi: 10.1007/s10522-011-9320-0. [DOI] [PubMed] [Google Scholar]
- Mossman KL. The linear no-threshold debate: where do we go from here? Med Phys. 1998;25:279–84. doi: 10.1118/1.598208. [DOI] [PubMed] [Google Scholar]
- Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Immunological mechanism of the low-dose radiation-induced suppression of cancer metastases in a mouse model. Dose Response. 2010;8:209–26. doi: 10.2203/dose-response.09-016.Nowosielska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Committee to Assess Health Risks from Exposure to Low Level of Ionizing Radiation. Washington, D.C.: National Academies Press; 2006. Health risks from exposure to low levels of ionizing radiation : BEIR VII Phase 2, National Research Council (U.S.) [PubMed] [Google Scholar]
- Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177:229–43. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. ERRATA for Volume 177, number 3 (2012) in the article “Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases”. Radiat Res. 2013;179:e0040–e0041. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- Phan N. 2011. Understanding The Biological Effects And Cancer Risk Of Medical Diagnostic Computed Tomography. PhD Thesis, McMaster University. [Google Scholar]
- Phan N, De Lisio M, Parise G, Boreham DR. Biological effects and adaptive response from single and repeated computed tomography scans in reticulocytes and bone marrow of C57BL/6 mice. Radiat Res. 2012;177:164–75. doi: 10.1667/rr2532.1. [DOI] [PubMed] [Google Scholar]
- Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950–1990. Radiat Res. 1996;146:1–27. [PubMed] [Google Scholar]
- Pollycove M. Radiobiological Basis of Low-Dose Irradiation in Prevention and Therapy of Cancer. Dose-Response. 2007;5:26–38. doi: 10.2203/dose-response.06-112.Pollycove. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Lu Q, Lao X, Molloi S, Elmore E. Low doses of diagnostic energy X-rays protect against neoplastic transformation in vitro. Int J Radiat Biol. 2003;79:235–40. doi: 10.1080/0955300031000096306. [DOI] [PubMed] [Google Scholar]
- Remington PL, Brownson RC. Fifty years of progress in chronic disease epidemiology and control. MMWR Surveill Summ. 2011;60(Suppl 4):70–7. [PubMed] [Google Scholar]
- Sakamoto K. Radiobiological basis for cancer therapy by total or half-body irradiation. Nonlinearity Biol Toxicol Med. 2004;2:293–316. doi: 10.1080/15401420490900254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CL. Radiation hormesis and the linear-no-threshold assumption. Heidelberg: Springer; 2010. [Google Scholar]
- Scott B. Low-dose-radiation activated natural protection and LNT. Health Phys. 2011;100:337–9. doi: 10.1097/hp.0b013e3182059442. [DOI] [PubMed] [Google Scholar]
- Scott BR. Low-dose radiation-induced protective process and implications for risk assessment, cancer prevention, and cancer therapy. Dose Response. 2007;5:131–49. doi: 10.2203/dose-response.05-037.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Strom DJ. The LNT model is appropriate for the estimation of risk from low-level (less than 100 mSv/year) radiation, and low levels of radon in homes should be considered harmful to health. Med Phys. 1998;25:273–5. doi: 10.1118/1.598207. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Yamashita S. Low-dose radiation exposure and carcinogenesis. Jpn J Clin Oncol. 2012;42:563–8. doi: 10.1093/jjco/hys078. [DOI] [PubMed] [Google Scholar]
- TCR 2008. Cancer Incidence Rate in Taiwan, 1998–2002 (Cancer incidence rate is calculated based on Cancer Incidence in Five Continents - Vol.IX) [Online]. Available: http://tcr.cph.ntu.edu.tw/uploadimages/CI5_V9_Site_e.pdf [Accessed May 4, 2013].
- Thompson RE. Epidemiological Evidence for Possible Radiation Hormesis from Radon Exposure: A Case-Control Study Conducted in Worcester, MA. Dose Response. 2011;9:59–75. doi: 10.2203/dose-response.10-026.Thompson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiana M. Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: the joint report of the Academie des Sciences (Paris) and of the Academie Nationale de Medecine. Int J Radiat Oncol Biol Phys. 2005;63:317–9. doi: 10.1016/j.ijrobp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tubiana M. Can we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother Oncol. 2009;91:4–15. doi: 10.1016/j.radonc.2008.12.016. discussion 1–3. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A, Averbeck D, Masse R. The debate on the use of linear no threshold for assessing the effects of low doses. J Radiol Prot. 2006;26:317–24. doi: 10.1088/0952-4746/26/3/N01. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Diallo I, Chavaudra J, Lefkopoulos D, Bourhis J, Girinsky T, Brider A, Hawkins M, Haddy N, El-Fayech C, Adjadj E, Clero E, de Vathaire F. A new method of assessing the dose-carcinogenic effect relationship in patients exposed to ionizing radiation. A concise presentation of preliminary data. Health Phys. 2011;100:296–9. doi: 10.1097/hp.0b013e31820a1b35. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13–22. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman AM. Radiation hormesis: historical perspective and implications for low-dose cancer risk assessment. Dose Response. 2010;8:172–91. doi: 10.2203/dose-response.09-037.Vaiserman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yock TI, Caruso PA. Risk of second cancers after photon and proton radiotherapy: a review of the data. Health Phys. 2012;103:577–85. doi: 10.1097/HP.0b013e3182609ba4. [DOI] [PubMed] [Google Scholar]