Abstract
Hormesis is a biphasic dose-response relationship, occurring when low concentrations of toxic agents elicit apparent improvements. In this work, the ability of sub-inhibitory concentrations of Tetracycline to induce hormetic response in a model organism was investigated. To this aim a reference strain of Escherichia coli, MG1655, was exposed to six decreasing doses of Tetracycline (between 0.12 and 0.00375 μg/ml), much lower than the Minimal Inhibitory Concentration (4 μg/ml). An hormetic increase was observed at the intermediate concentrations (0.015-0.03 μg/ml) of the tested range. The Colony Forming Unit number, indeed, rose up to 141% and 121% as compared to the control. At the highest (0.12 μg/ml) and lowest (0.00375 μg/ml) concentrations a slight decrease in CFU number was found. Results demonstrated that, in Escherichia coli, low concentrations of Tetracycline bias the bacterial numerical increase through a hormetic response; the dose-response curve describing this numerical increase is an U-inverted curve. Furthermore, these data confirm that hormesis is common to many - if not all - living systems, including bacteria; they underline the relevance of a deepened knowledge of both the effects and the possible consequences of exposure to low doses of contaminants.
Keywords: Hormesis, Antibiotic, Escherichia coli MG1655, Tetracycline, Biphasic dose-response, Bacterial growth
INTRODUCTION
Hormesis is a biphasic dose-response relationship, regarded as an adaptive function. The hormetic response is characterized by a mild stimulation (30–60 %) of the considered function, the endpoint, at low concentrations, and by the inhibition of the same function at higher doses. Many phylogenetically distant organisms and biological systems exposed to a wide range of stimuli show a dose-dependent biphasic response (Stebbing 1998; Chapman 2001; Calabrese and Baldwin 2001, 2002; Calabrese 2008, 2012; Calabrese and Blain 2009). It has been demonstrated that antibiotics trigger this response in plants (Migliore et al. 2010a, 2010b). The hormetic curve can be either U- or inverted U-shaped depending on the endpoint. A U-shaped curve could be observed by plotting a detrimental effect (i.e. alterations of body functions, disease incidence or mortality). An inverted U-shaped curve could be found when physiological functions, as growth or survival rate, are considered (Calabrese and Baldwin, 2002). Four types of concentration-response curves have been identified (Townsend and Luckey 1960), the most frequently observed is the β-curve (an inverted U-shaped dose-response curve; True and Oglevee 1905; Calabrese and Baldwin 1993), with a single stimulatory peak immediately below the No Observed Effect Concentration (Stebbing 1982; Calabrese et al. 1999; Chapman 2000).
The hormesis definition does not take into account the output (beneficial or detrimental) of the observed stimulation, that must be inferred from the biological or ecological context (Calabrese and Baldwin 2002; Costantini et al. 2010). In an unpredictable variable environment, the hormetic process allows a single individual to overcome a stress condition of low/medium intensity. The final effect of the hormetic response cannot be foreseen.
Although the first historical trial demonstrating hormesis was performed on lower organisms (fungi; Southam and Ehrich 1943), few studies demonstrated the hormetic response in microorganisms. Marine and freshwater luminescent bacteria exposed to metals, showed a clear hormetic phenomenon in luminescence assays (Christofi et al. 2002; Shen et al. 2009; Deng et al. 2012). However, in pathogenic and commensal bacteria the occurrence of hormetic response induced by low doses of antibiotics has not been yet demonstrated.
Tetracycline is an antimicrobial drug commonly used either in human and veterinary clinic or in intensive farming. It is frequently found in both terrestrial and aquatic environmental compartments at very low concentrations, in the order of magnitude of ppb (Boxall et al. 2002, 2006; Boxall 2004; Sarmah et al. 2006; Brambilla et al. 2007; Migliore et al. 2007, 2010a; Wu et al. 2013).
Escherichia coli is a bacterial species belonging to Enterobacteriaceae; besides being the most widely studied prokaryotic model organism, it is a commensal inhabitant of the gastrointestinal tract in humans and warm-blooded organisms; it can also behave as an opportunistic pathogen, as well as one of the most frequently isolated ones (Berg 1996).
To the best of our knowledge, this work is the first attempt to investigate the possible hormetic response induced by low doses of Tetracycline in E. coli; to this aim, an ad hoc protocol has been set up to easily quantify this response.
MATERIALS AND METHODS
Bacterial strain: Escherichia coli MG1655, was employed to study the possible hormetic effect of sub-MIC concentrations of Tetracycline.
Minimal Inhibitory concentration: the MIC value was firstly experimentally assessed with a standard dilution method, in Mueller Hinton Broth. Sub-MIC doses were tested in advance to identify the concentration range possibly inducing an hormetic effect.
Experimental design:
Antibiotic dilutions. A 16 ml volume of Mueller Hinton Broth, containing 0.24 μg/ml Tetracycline was two-fold serially diluted in the same medium, to obtain six batches of culture medium: from 0.24 to 0.0075 μg/ml.
Microbial inoculum. A 1.2 ml volume of a fresh culture of the test strain, diluted to an A600 value of 0.14 (corresponding to the to 0.5 point of McFarland nephelometric standard) was added to 60 ml of Mueller Hinton Broth. From this suspension, 8 ml amounts were added to every antibiotic batch tube, so to obtain a final volume of 16 ml and Tetracycline concentrations ranging from 0.12 to 0.00375 μg/ml.
Incubation and sampling. The tubes were then incubated at 37 °C and sampled every 30 minutes, up to five hours. At each sampling time, the A600 value of the culture was determined, and serial tenfold dilutions were performed, plating 5 μl from each one on Trypticase Soy agar plates, in triplicate. At each sampling time, the best dilutions were chosen to obtain both appreciable and accurate CFU counts. The plates were incubated at 37 °C overnight before counting CFUs.
These data, properly elaborated, give the CFU number in each culture at each sampling time. The results are the mean of six different experiments each performed in three replicates.
One-way ANOVA test was utilized to evaluate differences in cell growths. Parametric hypotheses were tested.
RESULTS
The growth curve of E. coli MG1655 in the presence/absence of sub-MIC Tetracycline concentrations, in the range 0.00375 – 0.12 μg/ml, by far lower than the MIC value observed with this E. coli strain (4 μg/ml), was determined. The hormetic dose-response curve, namely the continuum between toxic effects and apparent improvement (sensu Stebbing 1997), was identified by studying the growth curve dynamics in the presence of these sub-MIC Tetracycline concentrations.
Control growth
During time from 0 to 3.5h, the bacterial density increased from about 1×106 to 3×107 CFU/ml, then the increase became very fast (logarithmic phase) reaching a density of about 1.5×108 CFU/ml after 4.5h; this last density was maintained until the end of the experiment (5h).
Cell growth under Tetracycline
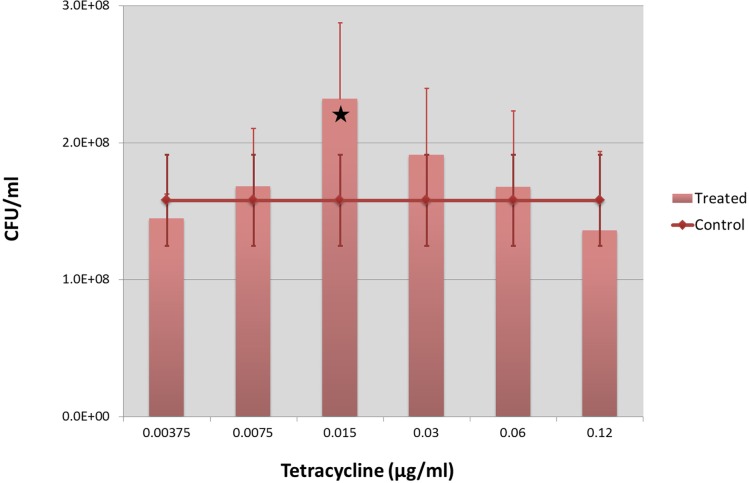
The final (5h) CFU number at different Tetracycline concentrations is shown in Figure 1. The lowest concentration (0.00375 μg/ml) caused a slight decrease (92%) of the CFU number as compared to the control. At the next higher concentration, 0.0075 μg/ml, the slight decrease in CFU number disappeared and the bacterial counts started to increase (106% of the control). The increase was evident at 0.015 μg/ml (147% of the control, ANOVA, F = 7.757, p < 0.02) and at 0.03 μg/ml (121% of the control, ANOVA, F = 1.06, n.s.). At 0.06 μg/ml the growth increase attenuated (106% of the control) and at 0.12 μg/ml the CFU number dropped to the 86% of the control. The growth dynamics of the culture exposed to 0.015 μg/ml Tetracycline, as compared to the control one is shown in Figure 2, as the mean values of 6 experiments (3 replicates each; see also Table 1).
FIGURE 1.
Hormetic response to low doses of Tetracycline on Escherichia coli MG1655. CFU number of E. coli MG1655 after 5 hours in cultures under different Tetracycline concentrations, much lower than the MIC. Mean values of 6 experiments (3 replicates each) and standard deviations are reported. Significant difference (ANOVA, p < 0.02) is indicated by a star.
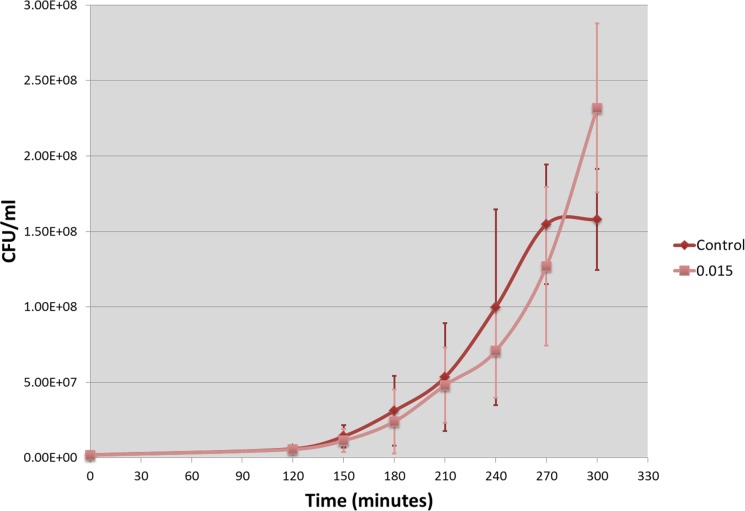
FIGURE 2.
Comparison of the growth dynamics between control and Tetracycline exposed batches. Control growth results in a sigmoidal curve (red); 0.015 μg/ml Tetracycline exposed batch, showing the best increase, grows as an exponential curve (pink). Mean values of 6 experiments (3 replicates each) and standard deviations are reported.
TABLE 1.
1×108 CFU number of E. coli MG1655 after 5 hours in cultures under different Tetracycline concentrations (as μg/ml). Mean values of 3 replicates for each experiment are reported.
| Experiment | Control | 0.00375 | 0.0075 | 0.015 | 0.03 | 0.06 | 0.12 |
|---|---|---|---|---|---|---|---|
| #1 | 1.49 | 1.50 | 2.05 | 2.29 | 1.91 | 1.84 | 1.20 |
| #2 | 1.23 | 1.56 | 1.42 | 1.72 | 1.30 | 1.12 | 0.73 |
| #3 | 1.39 | 1.20 | 1.81 | 3.00 | 1.97 | 2.20 | 2.40 |
| #4 | 1.57 | 1.40 | 1.26 | 2.00 | 1.35 | 1.60 | 1.00 |
| #5 | 1.61 | 1.31 | 1.27 | 1.91 | 1.14 | 0.96 | 1.30 |
| #6 | 2.20 | 1.70 | 2.27 | 3.00 | 2.40 | 2.33 | 1.53 |
DISCUSSION
The present study shows how low Tetracycline concentrations are able to elicit, in E. coli MG1655, an hormetic dose-response curve, namely a continuum between population dynamic reduction (toxic effect) and growth promotion (improvement; sensu Stebbing 1997). Such response is induced by sub-MIC concentrations of Tetracycline, in a range well below the MIC value (4 μg/ml). The “apparent improvement” of the growth dynamics is replaced by the bacteriostatic effect of Tetracycline at the highest concentration tested.
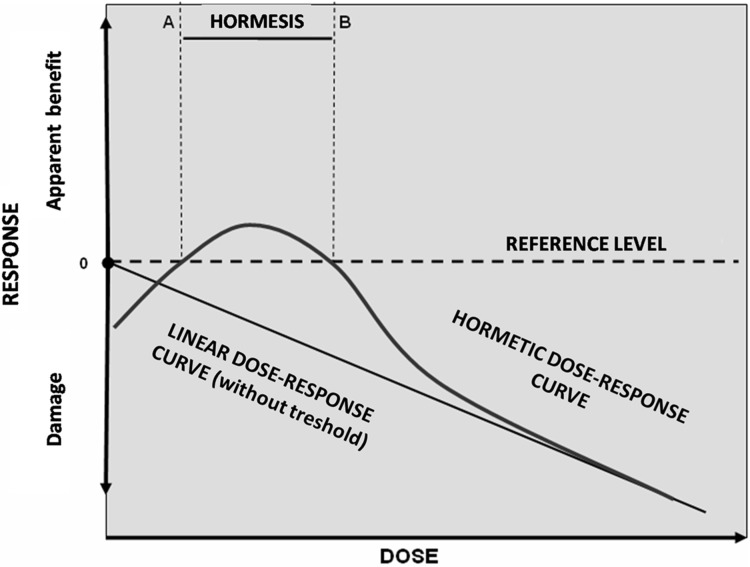
The hormetic curve has been obtained by exposing the bacterial cells to Tetracycline in the concentrations range 0.00375 – 0.12 μg/ml. The highest and statistically significant increase in cell count was found at 0.015 μg/ml, while the concentration of 0.12 μg/ml elicited only a slight bacteriostatic effect. The dose-response curve we found fit quite well with the theoretical model by Klonowski (2007), including the slight reduction above and below the hormesis concentration interval (A–B interval, in Figure 3). Concentrations of 0.0075 and 0.06 μg/ml are the A and B points of the model hormetic curve in the Figure 3: in that concentration interval, indeed, the hormetic increase has been found between 106% and 147% of the control.
FIGURE 3.
The hormetic dose-response and linear no-threshold relationships are shown. In the hormetic curve doses lower than A and higher than B cause toxic or other harmful effects, while, in the range A–B an apparent improvement is observed (by Klonowsky 2007; modified).
The overall lack of literature concerning hormetic response in bacteria does not allow to speculate too much about the underlying mechanisms; experiments performed with the typical ribosome-inhibiting Tetracyclines, at doses by far higher than those causing the response we described, demonstrated that no morphological changes occur in E. coli cells (Oliva et al. 1992). The mechanism underlying this kind of hormetic response, that is a time-limited enhancing of the number of bacterial cells, recalls an r-strategy reaction - and, indeed, E. coli is an r-strategist species - global regulation systems could therefore be activated in the presence of many, even unrelated kind of stresses including these very low antibiotic doses.
Finding a clear hormetic response in microorganisms confirms that it is common to many - if not all - living systems. The sub-inhibitory doses of Tetracycline inducing the hormetic response are comparable to the concentrations commonly detected in antibiotic contaminated soils and waters (Sarmah et al. 2006; Brambilla et al. 2007; Migliore et al. 2007). The occurrence of the hormetic response at these concentrations opens new concerns about the effects of antibiotic environmental and food contamination. The recent discoveries about the multiple roles the gut-associated microbiota plays in shaping the fitness, the health and even the behaviour of its host (for a review see Clemente et al. 2012; Cryan and Dinan 2012), pose new questions about the bias that antibiotics could exert. Besides the well-known effects of therapeutic doses, a recurrent or constant very low dosage of food-introduced antibiotics, could elicit hormetic responses. The response can be of different extent in the different microbial populations, modifying the ratios within them. The long term periods effects of this should deserve to be studied.
Our results support the experimental evidences on the role of antibiotics as trigger of several responses in bacterial cells (Linares et al. 2006; Fajardo and Martinez 2008), other than the protection from competitors. On the basis of these results, further studies will be crucial, being this field of study still largely unexplored and being the effects and consequences of exposure to low doses, not yet considered.
Acknowledgments
This work was financially supported by the APAT (Agency for Environmental Protection, now ISPRA), with a grant to L.M. The authors are grateful to Dr. Carlo Gianfico for his kind and valuable help on many occasions.
REFERENCES
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4(11):430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- Boxall ABA. The environmental side effects of medication. How are human and veterinary medicines in soils and water bodies affecting human and environmental health? EMBO Reports. 2004;5(12):1110–1116. doi: 10.1038/sj.embor.7400307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxall ABA, Fogg L, Blackwell PA, Kay P, Pemberton EJ, Environment Agency . Review of Veterinary Medicines in the Environment. R&D Technical Report P6-012/8/TR. Environment Agency; Rio House, Waterside Drive, Aztec West, Almondsbury, BRISTOL: 2002. [Google Scholar]
- Boxall ABA, Johnson P, Smith EJ, Sinclair CJ, Stutt E, Levy LS. Uptake of veterinary medicines from soils into plants. J Agric Food Chem. 2006;54:2288–2297. doi: 10.1021/jf053041t. [DOI] [PubMed] [Google Scholar]
- Brambilla G, Patrizi M, De Filippis SP, Bonazzi G, Mantovi P, Barchi D, Migliore L. Oxytetracycline as environmental contaminant in arable lands. Anal Chim Acta. 2007;586:326–329. doi: 10.1016/j.aca.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008;27(7):1451–1474. doi: 10.1897/07-541. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis: Improving Predictions in the Low-Dose Zone. In: Luch A, editor. Molecular, Clinical and Environmental Toxicology. Vol. 3: Environmental Toxicology, (Experientia Supplementum Vol. 101) Berlin: Springer; Basel AG: 2012. pp. 551–564. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L. Possible examples of chemical hormesis in a previously published study. J Appl Toxicol. 1993;13:169–172. doi: 10.1002/jat.2550130305. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose response and their centrality in toxicology. Trend Pharmacol Sci. 2001;22(6):285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Defining Hormesis. Human Exper Toxicol. 2002;21:91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Blain RB. Hormesis and plant biology. Environ Pollut. 2009;157:42–48. doi: 10.1016/j.envpol.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L, Holland C. Hormesis: A highly generalised and reproducible phenomenon with important implications for risk assessment. Risk Anal. 1999;19:261–281. doi: 10.1023/a:1006977728215. [DOI] [PubMed] [Google Scholar]
- Chapman PM. Whole effluent toxicity testing. Usefulness, level of protection, and risk assessment. Environ Toxicol Chem. 2000;19:3–13. [Google Scholar]
- Chapman PM. The implication of hormesis to ecotoxicology and ecological risk assessment. Hum Exp Toxicol. 2001;20(10):499–505. doi: 10.1191/096032701718120337. [DOI] [PubMed] [Google Scholar]
- Christofi N, Hoffmann C, Tosh L. Hormesis responses of free and immobilized light-emitting bacteria. Ecotox Envir Saf. 2002;52(3):227–231. doi: 10.1006/eesa.2002.2203. [DOI] [PubMed] [Google Scholar]
- Clemente JC, Ursell LK, Parfrey WL, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D, Metcalfe NB, Monaghan P. Ecological processes in a hormetic framework. Ecol Letters. 2010;13:1435–1447. doi: 10.1111/j.1461-0248.2010.01531.x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Deng Z, Lin Z, Zou X, Yao Z, Tian D, Wang D, Yin D. Model of hormesis and its toxicity mechanism based on Quorum Sensing: a case study on the toxicity of Sulfonamides to Photobacterium phosphoreum. Environ Sci Technol. 2012;46:7746–7754. doi: 10.1021/es203490f. [DOI] [PubMed] [Google Scholar]
- Fajardo A, Martínez JL. Antibiotics as signals that trigger specific bacterial responses. Curr Opin Microbiol. 2008;11:161–167. doi: 10.1016/j.mib.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Klonowski W. From conformons to human brains: an informal overview of nonlinear dynamics and its application in biomedicine. Nonlinear Biomed Phys. 2007;1:5. doi: 10.1186/1753-4631-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares JF, Gustafsson I, Baquero F, Martinez JL. Antibiotics as intermicrobial signaling agents instead of weapons. PNAS. 2006;103(51):19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L, Godeas F, De Filippis SP, Bonazzi G, Mantovi P, Barchi D, Brambilla G. Effetti della contaminazione da tetracicline sul mais (Zea mays L.): rischi del trasferimento di farmaci con la fertirrigazione. Proceedings of AIOL-SItE Conference; Ancona (Italy). 2007. http://www.ecologia.it/congressi/XVII/atti/atti_postcongressuali_XVII.pdf. [Google Scholar]
- Migliore L, Godeas F, De Filippis SP, Mantovi P, Bonazzi G, Barchi D, Testa C, Rubattu N, Brambilla G. Hormetic effect(s) of tetracyclines as environmental contaminant on Zea mays. Envir Pollut. 2010a;158(1):129–134. doi: 10.1016/j.envpol.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Migliore L, Rotini A, Cerioli NL, Cozzolino S, Fiori M. Phytotoxic antibiotic Sulfadimethoxine elicits a complex hormetic response in the weed Lythrum salicaria L. Dose Response. 2010b;8(4):414–427. doi: 10.2203/dose-response.09-033.Migliore. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva B, Gordon G, McNicholas P, Ellestad G, Chopra I. Evidence that Tetracycline analogs whose primary target is not the bacterial ribosome cause lysis of Escherichia coli. Antim Agents Chemother. 1992;36:913–919. doi: 10.1128/aac.36.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah AK, Meyer MT, Boxall ABA. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006;65:725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Shen K, Shen C, Lu Y, Tang X, Zhang C, Chen X, Shi J, Lin Q, Chen Y. Hormesis response of marine and freshwater luminescent bacteria to metal exposure. Biol Res. 2009;42:183–187. [PubMed] [Google Scholar]
- Southam CM, Ehrlich J. Effects of extracts of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–524. [Google Scholar]
- Stebbing ARD. Hormesis - the stimulation of growth by low levels of inhibitors. Sci Total Environ. 1982;22:213–234. doi: 10.1016/0048-9697(82)90066-3. [DOI] [PubMed] [Google Scholar]
- Stebbing ARD. A theory for growth hormesis. BELLE Newsl. 1997 Sep 6 [Google Scholar]
- Stebbing ARD. A theory for growth Hormesis. Mutation Res. 1998;403:249–258. doi: 10.1016/s0027-5107(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Townsend JF, Luckey TD. Hormoligosis in pharmacology. J Am Med Assoc. 1960;173:44–48. doi: 10.1001/jama.1960.73020190007010. [DOI] [PubMed] [Google Scholar]
- True RH, Oglevee CS. The effect of the presence of insoluble substances on the toxic actions of poisons. Bot Gazette. 1905;39:1–21. [Google Scholar]
- Wu L, Pan X, Chen L, Huang Y, Teng Y, Luo Y, Christie P. Occurrence and distribution of heavy metals and tetracyclines in agricultural soils after typical land use change in east China. Environ Sci Pollut Res. 2013 2013 Feb 14; doi: 10.1007/s11356-013-1532-1. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]