Abstract
BACKGROUND AND PURPOSE
Solid lipid nanoparticles containing cholesteryl butyrate (cholbut SLN) can be a delivery system for the anti-cancer drug butyrate. These nanoparticles inhibit adhesion of polymorphonuclear and tumour cells to endothelial cells and migration of tumour cells, suggesting that they may act as anti-inflammatory and anti-tumour agents. Here we have evaluated the effects of cholbut SLN on tumour cell growth using in vitro and in vivo models.
EXPERIMENTAL APPROACH
Cholbut SLNs were incubated with cultures of four tumour cell lines, and cell growth was analysed by assessing viability, clonogenic capacity and cell cycle. Effects on intracellular signalling was assessed by Western blot analysis of Akt expression. The in vivo anti-tumour activity was measured in two models of PC-3 cell xenografts in SCID/Beige mice.
KEY RESULTS
Cholbut SLN inhibited tumour cell line viability, clonogenic activity, Akt phosphorylation and cell cycle progression. In mice injected i.v. with PC3-Luc cells and treated with cholbut SLN, . in vivo optical imaging and histological analysis showed no metastases in the lungs of the treated mice. In another set of mice injected s.c. with PC-3 cells and treated with cholbut SLN when the tumour diameter reached 2 mm, analysis of the tumour dimensions showed that treatment with cholbut SLN substantially delayed tumour growth.
CONCLUSION AND IMPLICATIONS
Cholbut SLN were effective in inhibiting tumour growth in vitro and in vivo. These effects may involve, in part, inhibition of Akt phosphorylation, which adds another mechanism to the activity of this multipotent drug.
Keywords: solid lipid nanoparticles, cholesteryl butyrate, clonogenic assay, tumour growth in vivo
Introduction
Cytotoxic anticancer drugs exhibit features, such as poor specificity, high toxicity and susceptibility to induce drug resistance, which substantially limit their use. Their conventional administration often leads to their extensive and indiscriminate binding to body tissues and serum proteins in a highly unpredictable manner, and only a small fraction of the drug actually reaches the tumour site (Wong et al., 2007); this reduces their therapeutic efficacy and increases the systemic toxicity. Since the early 1990s, a number of solid lipid nanoparticles (SLN) or SLN-based systems have been successfully formulated and tested for the delivery of cytotoxic drugs (Wong et al., 2007). These SLN are particles of submicron size (50–100 nm) intended to leak out of the blood vessels and accumulate within the tumour. In terms of drug stability, drug biodistribution, pharmacokinetics and cancer activity, SLN formulations of anticancer agents have been shown to be more effective than the corresponding free drug, and they are at least comparable with other drug carrier systems such as polymeric systems and liposomes. In many cases, nanoparticles also have the potential to bypass the multidrug-resistance mechanisms that involve cell-surface pump proteins through their entry into the cells via endocytosis (Davis et al., 2008). Moreover, nanocarriers may preferentially concentrate in tumours, inflammatory sites and antigen sampling sites because of the enhanced permeability and retention observed in the vasculature of these sites. Once biodegradable nanoparticles have accumulated at the target site, they can act as local drug depots depending on the make-up of the carrier, thus providing a source of continuous supply of encapsulated therapeutic compound(s) at the solid tumour site (Singh and Lillard, 2009).
Butyrate has received close attention as a potential chemopreventive agent (Wollowski et al., 2001; Delzenne et al., 2003), especially because in vitro exposure of tumour cells to this agent induces apoptosis, inhibits proliferation and promotes differentiation (Kobayashi et al., 2003) in tumour cells derived from colorectal, breast, gastric, lung, brain and pancreatic cancers (Brioschi et al., 2008). Moreover, sodium butyrate has shown some efficacy as an anti-tumour drug in phase I and phase II clinical trials, possibly through its inhibition of histone deacetylase (HDAC) (Minucci and Pelicci, 2006). However, its short half-life limits its clinical use (Pellizzaro et al., 1999), and maintenance of therapeutic concentrations requires a continuous parenteral administration. Moreover, compliance is further reduced by several adverse events, such as abdominal cramps, nausea, diarrhoea, anaemia, headache and strong odour.
The warm microemulsion method has been used to prepare SLN containing cholesteryl butyrate (cholbut SLN) with an average diameter around 80 nm, to be used as a pro-drug of butyric acid (Gasco, 2004; Matsumura and Kataoka, 2009). Such nanoparticles had negative zeta potential, high enough in modulus to prevent their aggregation and to obtain stable nanoparticle dispersions. They were rapidly internalized and displayed an anti-tumour effect greater than that of butyrate against several tumour cell lines (Salomone et al., 2001; Serpe et al., 2004; Minelli et al., 2012).
We have already shown that cholbut SLN inhibited adhesion to human umbilical vein endothelial cells (HUVEC) and migration of several cell types, including cancer cell lines (Dianzani et al., 2006; Minelli et al., 2012). The anti-adhesion effect was exerted through actions on both the HUVEC and the cancer cells. We therefore suggested that cholbut SLN could be used as anti-tumour agents.
The aim of the research reported here was to extend these analyses by assessing the anti-proliferative potential of cholbut SLN on tumour cell lines in vitro and to investigate whether the Akt signalling pathway was involved in the effects of cholbut SLN. Activation of Akt, by phosphorylation, is known to play an important role in a variety of malignancies such as colon, breast, prostate and non-small cell lung cancer, where it is involved in mediating a range of biological responses, including cell growth, proliferation and survival (Roy et al., 2002; Asano et al., 2004).
Our results showed that cholbut SLN strikingly decreased viability and proliferation of the colon cancer cell lines HT29, HCT15, and HCT116, and the prostate cancer cell line (PC-3), in vitro by acting in a concentration- and time-dependent manner, and with activity greater than that of free butyrate. These effects were accompanied by inhibition of the Akt pathway and cell cycle arrest in the S and G2/M phase. Moreover, in vivo, cholbut SLN inhibited dissemination and growth of cancer cells in mice, supporting the potential efficacy of cholbut SLN in anti-cancer therapy.
Methods
Preparation of cholbut SLN and sodium butyrate solutions
Cholbut SLN were prepared by the warm microemulsion (μE) method reported elsewhere (Minelli et al., 2012) and described in PATENT WO0030620. Briefly, warm oil-in-water μE was prepared by melting cholesterylbutyrate (Asia Talent Chemical, Shenzen, China), Epikuron 200 (Cargill, Milan, Italy) at 85°C as lipid phase, and then adding external water phase containing sodium glycocholate (PCA, Basaluzzo, Italy). Because the melting point of cholesteryl butyrate is 98°C, 2-phenylethanol (Sigma-Aldrich, Milan, Italy) was used in the process to help melting with the aim of adding its preservative effects in the final product (final concentration about 0.25% w·v−1). After stirring, the clear μE was dispersed in cold water (2/4°C) with a dispersion ratio μE/water of 1:10. The cholbut SLN dispersion was then washed by tangential flow filtration (TFF) using Vivaflow 50 membrane (RC, Cut-off 100 000 Da; Sartorius Stedim Biotech GmbH, Göttingen, Germany). For in vivo experiments, the cholbut SLN was further concentrated by TFF to obtain a concentration that was more than twice that in the original preparation. Finally, all aqueous dispersions of cholbut SLN, for in vitro or in vivo experiments, were sterilized by filtration at 0.2 μm before use, and no loss of cholbut contents was showed by HPLC analysis.
In cholbut SLN, the whole lipid matrix itself acts as a prodrug of butyrate. Because the loading efficiency of these preparations cannot be properly defined, compared with the usual situation in which a drug is incorporated in the SLN carrier, high recovery of the hydrophobic prodrug matrix was taken as the reference parameter for quality control. This always detected a minimal concentration reduction during four washing steps, possibly due to adsorption to the membranes, because no cholbut was found in the washing water. Moreover, no loss of either cholbut SLN or free butyrate was detected after the sterilizing filtration step.
Characterization of cholbut SLN formulations was performed by dynamic light scattering (DLS; Malvern Zetasizer – Nano ZS, Malvern Instruments LtD, Malvern, Worchester, UK), HPLC-UV analysis (Agilent 1260, Agilent Technologies, Santa Clara, CA, USA), field emission scanning electron microscopy {FeSEM–ZEISS (Carl Zeiss Microscopy GmbH, Jena, Germany), SUPRA 40 (Carl Zeiss Microscopy GmbH), GEMINI column [Phenomenex, Castel Maggiore (BO), Italy], SMARTSEM software (Carl Zeiss Microscopy GmbH)} and laser doppler micro-electrophoresis (LDME, Malvern Zetasizer – Nano ZS). Gel permeation chromatography (GPC) analysis have been performed for further studying size distribution, using a glass column (1 cm diameter, 25 cm height) filled with Sepharose CL-4B (Sigma-Aldrich) loaded with 1 mL cholbut SLN and eluted with PBS (pH 7.4).
Sodium butyrate solutions were freshly prepared in sterile water before each experiment, at a concentration of 5 M.
Cell culture
HT29, HCT15 and HCT116 cells from human colon adenocarcinoma were obtained from American Type Culture Collection (Manassas, VA), PC-3 from human prostate carcinoma were gifted by Dr. Pili (Roswell Park Cancer Institute, Buffalo, NY, USA). Cholbut SLN was produced by Dr. Gasco (Nanovector s.r.l. Torino, Italy). The human tumour cell lines were grown in culture dishes as a monolayer in RPMI 1640 medium plus 10% fetal calf serum (FCS), 100 U·mL−1 penicillin, 100 mg·mL−1 streptomycin, at 37°C in a 5% CO2-humidified atmosphere.
PC-3Luc cells were constructed by stably transfecting PC-3 cells with luciferase construct, as previously described (Loberg et al., 2006).
Cell viability assay
The effect of cholbut SLN on cell growth and survival was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, cells were normalized at 800 cells per 100 μL in 96-well plates. After an overnight incubation, the medium was replaced with 100 μL of culture medium with cholbut SLN or sodium butyrate (50–300 μM). After 24, 48 and 72 h of incubation, viable cells were measured, by 2,3-bis(2-methoxy-4-nitro-5sulphophenyl)-2H-tetrazolium-5-carboxanilide (Sigma-Aldrich) inner salt reagent, at UV 490 nm, as described by the manufacturer's protocol. In some experiments, cholbut SLN or NaBut from 50 to 300 μM was refilled every 24 h. The UV readings of controls (i.e. cells that had received no drug) were normalized to 100%, and the readings from cholbut SLN-treated cells were expressed as % of controls. Eight replicate wells were used to determine each data point and three different experiments were performed.
Colony-forming assay
Cells (800 per well) were seeded into six-well plates and treated with the compounds. The medium was changed after 72 h and cells were cultured for additional 10 days. Subsequently, cells were fixed and stained with a solution of 90% crystal violet (Sigma-Aldrich) and 10% methanol. Colonies were then photographed and counted with a Gel Doc equipment (Bio-Rad Laboratories, Milan, Italy).
Cell cycle analysis
PC-3 cells (1.5 × 107) were seeded and treated with titrated doses (50–300 μM) of cholbut SLN. After 24, 48 and 72 h, adherent and non-adherent cells were collected, washed in 1 × PBS and fixed in 75% ice cold ethanol and subsequently resuspended in a buffer containing 0.02 mg·mL−1 RNase A (Worthington Biochemical Corporation, Lakewood, NJ, USA), 0.05 mg·mL−1 propidium iodide (Sigma-Aldrich), 0.2% v/v Nonidet P-40 (Sigma-Aldrich), 0.1% w/v sodium citrate (Sigma-Aldrich). Samples were acquired with a FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and analysed with the software FlowJo v8.6.3 (Becton Dickinson).
In some experiments (n = 3), cholbut SLN (50–300 μM) was replenished every 24 h.
Western blot analysis
Cells, incubated with or without 100μM cholbut SLN for 8–48 h, were exposed to 0.01 μM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) for 10 min to stimulate Akt activation. They were then lysed in a buffer composed of 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% NP40, phosphatase and protease inhibitor cocktail (Sigma-Aldrich). Cell lysates were then cleared from insoluble fractions by high-speed centrifugation, and protein concentrations were determined with a commercial kit (Bio-Rad Laboratories). Proteins (40 μg per lane) were loaded on 10% SDS PAGE gels and, after electrophoresis, transferred onto nitrocellulose membranes. These were blocked by incubation for 1 h at room temperature with 5% non-fat milk dissolved in Tris-buffered saline Tween 20. The membranes were then probed overnight with primary antibodies and, after three washes, incubated for 1 h with HRP-conjugated secondary antibodies (Bio-Rad Laboratories). Bands were detected by chemiluminescence, and densitometric analysis was performed with the Multi-Analyst software (version 1.1, Bio-Rad Laboratories).
Tumour growth in vivo
All animal care and experimental procedures complied with protocols approved by the Turin University Bioethical Committee and the Italian Ministry of Health. Female 4–5-week-old SCID/Beige mice (Charles River Laboratories, Milan, Italy) were housed under pathogen-free conditions. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 17 animals were used in the experiments described here.
In the metastasis experiments, mice were injected in the tail vein with PC-3 cells stably expressing firefly luciferase (PC-3-Luc, 1 × 106 cells per mouse) and monitored weekly for pulmonary metastases by in vivo optical imaging. In the tumour growth experiment, mice were injected subcutaneously in the left flank with 2 × 106 PC-3 cells. Tumour size was measured weekly in two dimensions using callipers. Tumour volume was calculated as follows: tumoure volume (mm3) = D × d2/2; where D represents the largest cross sectional diameter (mm) of the tumour, and d the cross sectional diameter (mm) at right angles to D.
In each experiment, the mice were treated every 2 days with i.p. injection of 220 mM·kg−1 cholbut SLN, or PBS as control.
In vivo optical imaging
At 0, 7, 14, and 21 days after tumour cell injection, mice (n = 9) were injected i.p. with 150 mg·kg−1 luciferin (PerkinElmer, Waltham, MA, USA) in sterile PBS. They were then placed in the IVIS 200 (PerkinElmer) induction chamber and anaesthetised with isoflurane (2.5%; Abbott, Abbott Park, IL, USA) with 1 L·min−1 flow of oxygen. After 10 min, the mice were placed on the heated imaging platform of the IVIS 200 imaging station, continuing the isoflurane anaesthesia during the imaging procedure. White light and luciferase activity images were acquired with a 25 s exposure. Following imaging, the mice were removed from the imaging stage and allowed to recover from anaesthesia and returned to their original housing. Images were analysed with the Living Image software (PerkinElmer). The luminescent signal was quantified as the average radiance (p·s·cm2·sr) measured in regions of interest drawn in the lungs.
Histopathological analysis
The lungs were harvested at necropsy and fixed in 10% formalin. The fixed samples were then embedded in paraffin and four non-sequential serial sections per animal were obtained. The sections were stained with hematoxylin/eosin and analysed for the presence of metastases by light microscopy.
Data analysis
Data are shown as mean ± SEM. Statistical analyses were performed with GraphPad Prism 3.0 software (La Jolla, CA, USA) using one-way ANOVA and Dunnett's test. Values of P < 0.05 were considered statistically significant.
Materials
Cholbut SLN were produced by Dr Gasco (Nanovector s.r.l. Turin, Italy). FCS (endotoxin tested) was from Hyclone Laboratories (Milan, Italy). Trypsin was from Difco Laboratories (Milan, Italy). M199, RPMI 1640, PMA, sodium butyrate and β-actin (A-1978) were purchased from Sigma-Aldrich. Phosphorylated Akt (p-Akt) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Results
Characterization of cholbut SLN
Table 1 shows the average diameter (Zave) and polydispersity index (PI) of the cholbut SLN, evaluated by DLS, and the chemical composition, evaluated by HPLC-UV analysis, at different steps of the preparation process. The zeta potential of cholbut SLN, evaluated by LDME after the final filtration step, was −49.5 mV (Figure 1). FeSEM analysis confirmed the size and spherical morphology of cholbut SLN (Figure 2). GPC analysis (data not shown) showed two main populations of particles (Zave39% = 183.75 nm, Zave61% = 39.06 nm, Zavemax = 227 nm), which could explain the relatively high values of the PI values.
Table 1.
DLS analysis (Malvern Zetasizer – Nano ZS) Size distribution by intensity and polydispersity index. Characterization of cholbut SLN at different steps of the preparation
| Cholbut SLN | Zave (nm) | Polydispersity index |
|---|---|---|
| Before washing | 87.72 | 0.235 |
| After washing (n = 4) | 79.83 | 0.254 |
| After sterilizing filtration (0.2 μm) | 78.48 | 0.248 |
Figure 1.
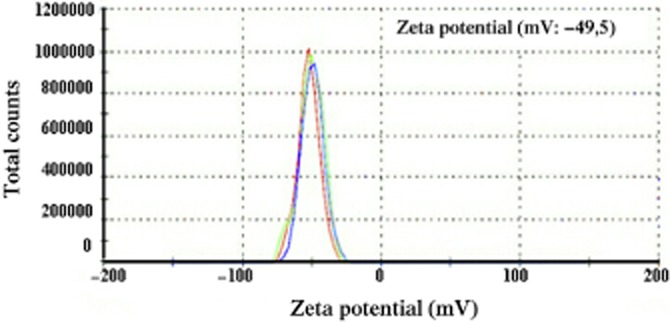
LDME. The test was performed on cholbut SLN using a Malvern Zetasizer – Nano ZS. Results are showed as total count on zeta potential (mV).
Figure 2.
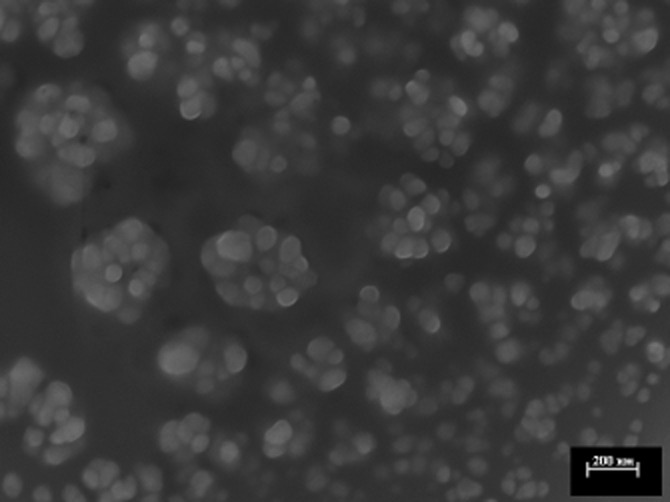
FeSEM analysis (collaboration with the Polytechnic of Turin). Image shows the morphology of cholbut SLN. White bars represent scale units of 200 nm.
Cholbut SLN inhibit tumour cell growth
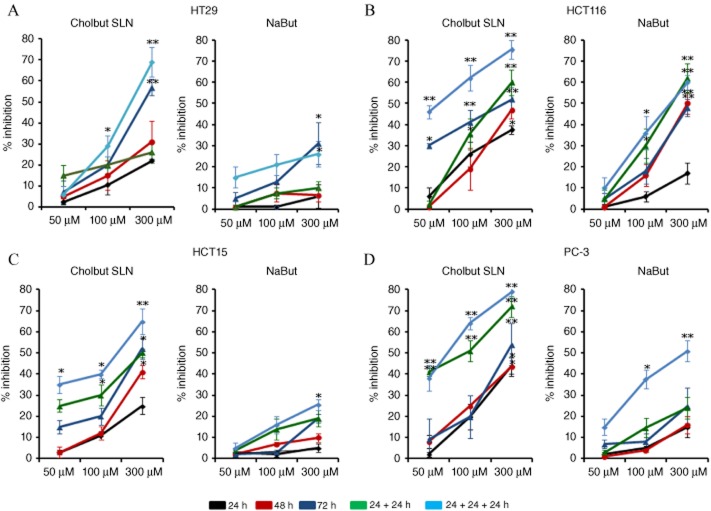
Initially, we compared the ability of cholbut SLN and free sodium butyrate to inhibit the growth of HT29, HCT116, HCT15 and PC-3 cells in vitro. Cells were cultured in the presence and absence of titrated amounts (50–300 μM) of each compound for 24–48–72 h, and the amount of viable cells was then assessed by the MTT assay. In some experiments, each compound was replenished every 24 h. Figure 3 shows the inhibition of cell proliferation induced by cholbut SLN and by sodium butyrate. The effect was concentration- and time-dependent with some differences between the four cell lines and the two substances. Sodium butyrate was active only in certain conditions. The most responsive cell line was HCT116, but only at the highest concentration and after 48–72 h of treatment, showing about 45% of growth inhibition at both these times. HCT15, HT29 and PC-3 cells were only weakly sensitive because they showed a respective growth inhibition of <5%, 15%, 20% at 48 h, and 15%, 30%, 20% at 72 h. The effects of sodium butyrate were increased in the experiments with replenishment every 24 h, especially for HCT116 and PC-3 cells, with a maximal inhibition of 35–50% obtained at 100 and 300 μM after 72 h for PC-3 and 35–60% for HCT116 cells. By contrast, all cell lines were sensitive to cholbut SLN, especially with replenishment, and the maximal inhibition reached approximately 55–80%. To investigate the possibility that cholesteryl esters may exert cell toxicity not related to sodium butyrate, we evaluated the effects of a similar SLN formulation, containing cholesteryl palmitate as the lipid matrix (50–300 μM), on cell growth of the four tumour cell lines using the MTT assay. Results showed that cholesteryl palmitate SLN did not affect cell growth even at the highest concentrations (data not shown). These results are in agreement with our previous observations showing the ineffectiveness of cholesteryl palmitate SLN in inhibiting adhesion and migration of colon cancer cells (Minelli et al., 2012).
Figure 3.
Inhibition of proliferation following cholbut SLN and sodium butyrate(NaBut) treatment. Cells (800 per well; HT29 (A), HCT116 (B), HCT15 (C), and PC-3 (D) cells) were treated with increasing concentrations (50–300 μM) of cholbut SLN or sodium butyrate for 24–72 h. In some experiments, cholbut SLN and sodium butyrate were replenished every 24 h (n = 5), without changing the culture medium (48 h culture: 24 + 24 h; 72 h culture: 24 + 24 + 24 h). Results are expressed as % inhibition of control and shown as mean ± SEM (n = 5). *P < 0.05; **P < 0.01, significantly different from control; one-way ANOVA and Dunnett's test.
To validate these findings, clonogenic survival assays were performed. Cells were seeded into 6-well plates and treated with each compound. The culture medium was changed after 72 h, and cells were cultured for an additional 10 days in the absence of the compounds. The results were similar to those obtained with the MTT assay (Figure 4). Indeed, only HCT116 and PC-3 were partly sensitive to sodium butyrate, whereas all cell lines were sensitive to cholbut SLN, whose maximal inhibition ranged 50–90%. The observation that the inhibition detected by the clonogenic assay was substantially higher than that detected by the MTT assay suggests that cells which were still viable in the MTT assay, after cholbut SLN treatment were in fact severely damaged and unable to proliferate in the clonogenic assay.
Figure 4.
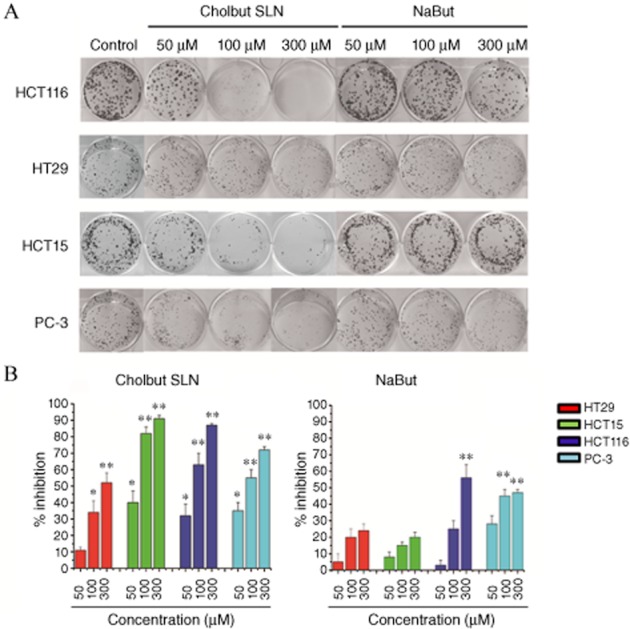
Effect of cholbut SLN and sodium butyrate(NaBut) on cell clonogenicity was tested by colony forming assay. (A) HT29, HCT116, HCT15 and PC-3 cells (500 per well) were seeded in six-well plates and treated with each drug at the indicated concentrations for 72 h. The medium was then changed and cells were cultured for additional 10 days and subsequently fixed and stained with crystal violet. (B) Summary results for the inhibition of clonogenicity. Data shown are means ± SEM (n = 5). *P < 0.05; **P < 0.01, significantly different from control; one-way ANOVA and Dunnett's test.
To assess whether inhibition of cell proliferation induced by cholbut SLN affected cell cycle progression, we analysed the cell cycle in PC-3 cells cultured in the presence and the absence of titrated amounts (50–300 μM) of cholbut SLN for 24–48–72 h (Figure 5a and 5b). Treatment with the highest concentration (300 μM) caused a significant accumulation of cells in the G2/M phase, but some effect was detected also at the lower doses. Experiments in which the compound was replenished every 24 h and the cell cycle analysed after 48 and 72 h (Figure 5c and 5d) showed a substantial increase of cells in G2/M phase already detectable at the lowest compound dose, whereas the highest dose induced accumulation of cells in the SubG1 phase.
Figure 5.
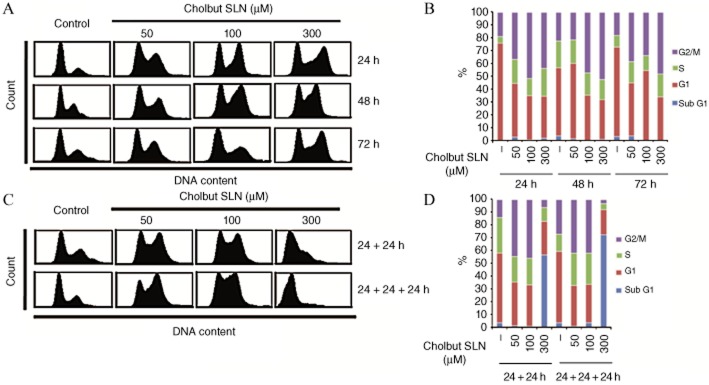
Induction of cell cycle arrest by cholbut SLN treatment. (A,B) PC-3 cells (1.5 × 105) were treated or not with 50–300 μM cholbut SLN for 24–72 h and cell cycle was then assessed by flow cytometry. (C,D) in these experiments, cholbut SLN was replenished every 24 h (48 h culture: 24 + 24 h; 72 h culture: 24 + 24 + 24 h) without changing the culture medium (A and C: representative cell cycle plots; B and D: quantification of cell cycle phases; n = 3).
We then evaluated cell death in PC-3 cells, treated with cholbut SLN (50–300 μM) for 48–72 h with replenishment, by means of annexin V/ propidium iodide staining. Figure 6 shows that treatment with 300 μM of cholbut SLN caused substantial cell death as only 60% of the cells were viable at 48 h, and this value decreased further to 30% at 72 h. By contrast, the 50 and 100 μM concentrations did not induce cell death.
Figure 6.
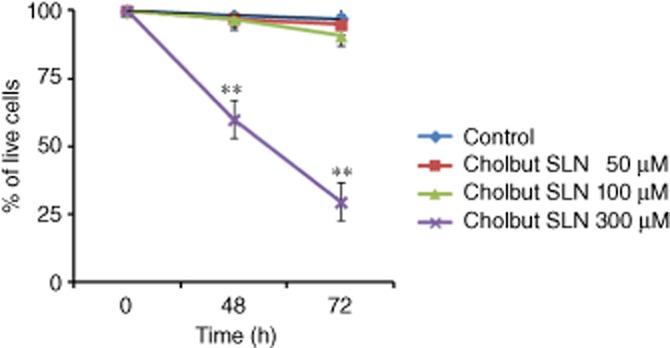
Induction of cell death following treatment with cholbut SLN. PC-3 cells (1.5 × 105) were incubated with 50–300 μM cholbut SLN with replenishment, for 48 (24 + 24 h)–72 h (24 + 24 + 24 h) and cell death was then assessed by flow cytometry. Data shown are means ± SEM (n = 3). **P < 0.01, significantly different from control; one-way ANOVA and Dunnett's test.
Cholbut SLN modulate Akt signaling
To investigate the mechanism(s) underlying cholbut SLN-mediated inhibition of cell proliferation, we evaluated Akt phosphorylation, because the Akt signalling pathway is involved in the regulation of cell proliferation, apoptosis, adhesion and migration (Kwiatkowska et al., 2011; Matsuoka et al., 2012). To this end, HCT15, HCT116, HT29 and PC-3 cells were incubated with 100 μM cholbut SLN for 8–48 h and then treated with 0.01 μM PMA for 10 min to induce Akt phosphorylation. Western blot analysis showed that Akt phosphorylation was induced by PMA in all cell lines (Figure 7), and was inhibited after treatment of the cells with cholbut SLN in a time-dependent manner.
Figure 7.
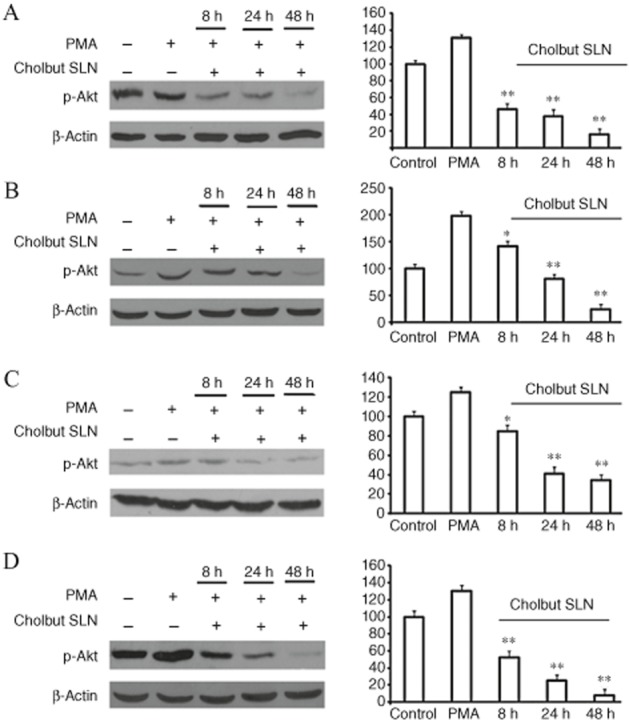
Effect of cholbut SLN on Akt phosphorylation in HCT15 (A), HCT116 (B), HT29 (C) and PC-3 (D). Cells were treated with 100 μM cholbut SLN for 8, 24 or 48 h and subsequently incubated with fresh media containing (0.01 μM) PMA to induce Akt phosphorylation, which was then evaluated by Western blot in the cell lysates. The same blots were probed with anti β-actin antibody as a control. Left: Western blot analysis from a representative experiment. Right: Densitometric analysis of Akt phosphorylation expressed in arbitrary units;data are expressed as means ± SEM (n = 3) and shown as % control. *P < 0.05; **P < 0.01, significantly different from PMA alone; one-way ANOVA and Dunnett's test.
In vivo experiments
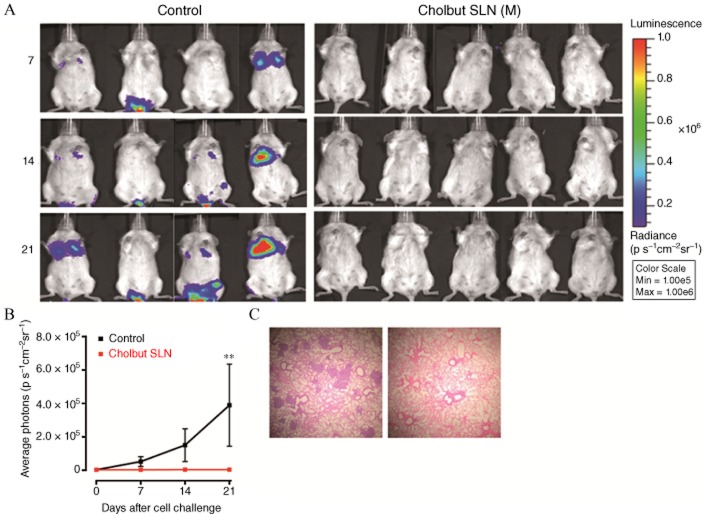
To assess the effect of cholbut SLN in vivo, mice were injected i.v. with 1 × 106 PC-3-Luc cells and treated every 2 days with i.p. injection of 220 mM·kg−1 cholbut SLN (n = 5) or PBS as control (n = 4). The dose was chosen according to preliminary experiments that demonstrated that cholbut SLN did not induce any in vivo toxicity in mice upon delivery either p.o. or i.v. (oral LD50 ≥ 1000 mg·kg−1; i.v. LD50 ≥ 400 mg·kg−1; Minelli et al., 2012). At time 0, and 7, 14 and 21 days after cell injection, the mice were i.p. injected with luciferin and analysed by in vivo optical imaging to evaluate the tumour cell growth in the lungs. Qualitative (8a) and quantitative (Figure 8b) analyses showed that a progressively increasing luminescent signal was present in the lungs of three out of four control mice, while no signal was detected in the lungs of the mice treated with cholbut SLN. To refine these data, histological analysis of the lungs was performed in the mice killed at day 28. Results showed disseminated tumour foci in the lungs of the control mice (Figure 8c, left panel), while no metastases were detected in the lungs of the mice treated with cholbut SLN (Figure 8c, right panel).
Figure 8.
Effect of cholbut SLN on tumour cell dissemination in vivo. Mice were injected i.v. with 1 × 106 PC-3-Luc cells and treated with 220 mM·kg−1 cholbut SLN (n = 5) or PBS (control group, n = 4). (A) At 0, and 7, 14, and 21 days after cell injection, mice were. injected i.p with luciferin and pulmonary metastases were macroscopically detectable and documented by in vivo optical imaging. (B) The luminescent signal was quantified as the average radiance (p s-1cm-2sr-1) measured in regions of interest drawn in lungs. Data shown are means ± SEM. **P < 0.01, significantly different from control; one-way ANOVA and Dunnett's test. (C) Non-sequential serial sections were taken from lungs of animals and stained with haematoxylin/eosin to highlight pulmonary metastasis. Representative histological analysis of the lungs in control mice (left panel) and in treated mice (right panel).
To assess further the effects of cholbut SLN on tumour growth in vivo, mice were injected s.c. with PC-3 cells and treated with either cholbut SLN or PBS starting when the tumour diameter reached 2 mm. Analysis of tumour dimensions showed that treatment with cholbut SLN significantly delayed and reduced the tumour growth compared with that in the control mice (Figure 9). Meanwhile, there was no significant difference in body weight between the treated and the control group (data not shown), suggesting that the administration of cholbut SLN caused no significant toxicity to the mice.
Figure 9.
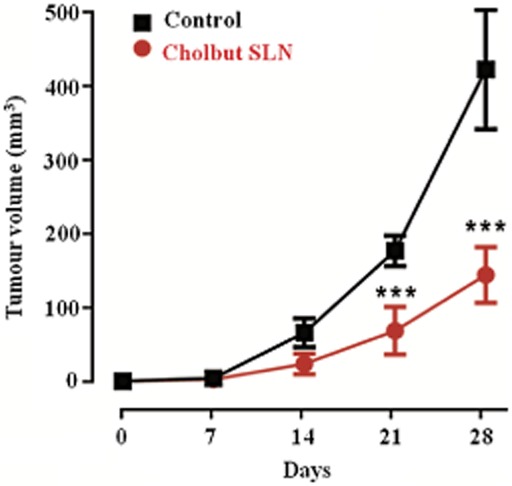
Effect of cholbut SLN on local tumour growth in vivo. Mice were injected s.c. with PC-3 cells and treated with either 220 mM·kg−1 cholbut SLN (n = 4) or PBS (control group, n = 3) starting when the tumour diameter reached 2 mm3 (size of s.c. tumours was measured weekly by callipers). Data shown are means ± SEM. ***P < 0.001, significantly different from control; one-way ANOVA and Dunnett's test.
Discussion and conclusion
Cancer progression is a multi-step process that enables tumour cells to avoid the growth checkpoints of the organism, giving rise to a primary tumour mass that may invade the surrounding tissues and even spread to distant sites, leading to metastases. If detected in the early stages, solid cancers are often locally circumscribed and can be treated successfully by surgical removal and radiotherapy of the primary tumour. The mortality of more than 90% of cancer patients is mostly due to the development of metastases (Hayot et al., 2006). Cell movements through the tissues play a crucial role in cancer invasion and dissemination. Therefore, an effective anti-tumour therapy should be effective in inhibiting both the growth and invasiveness of tumour cells (Dianzani et al., 2010).
In earlier work, we showed that cholbut SLN may have an effect in tumour cell dissemination because it inhibited adhesion of tumour cell lines to vascular endothelial cells and their migratory activity, which suggested that cholbut SLN may work as an anti-metastatic compound (Minelli et al., 2012). The present work further strengthens findings on the anti-tumour effectiveness of cholbut SLN by showing its ability to inhibit cancer cell growth both in vitro and in vivo.
Our present data show that cholbut SLN inhibited tumour cell growth in vitro at higher concentrations than those effective on tumour cell adhesion and migration. The effects was dose- and time-dependent and clearly more potent than those displayed by sodium butyrate, which was ineffective on tumour cell adhesion and migration in our previous work. Moreover, sodium butyrate was effective in vitro only in some tumour cell lines, whereas cholbut SLN were active on all cell lines tested. Wilson et al., 2010 demonstrated that cancer cell sensitivity to sodium butyrate correlated with its capacity to inhibit HDAC activity. The effects of cholbut SLN would be independent of HDAC inhibition because our previous work showed that these concentrations of cholbut SLN did not inhibit HDAC (Minelli et al., 2012). Moreover, cholbut SLN was equally effective in cell lines whose growth is either sensitive (HCT116) or resistant (HT29) to HDAC inhibitors (Wilson et al., 2010). Inhibition of cell growth was due to cycle arrest in the G2/M or SubG1 phase and induction of significant cell death.
Cholbut SLN inhibited the ERK and p38 pathways involved in cell growth, migration and adhesion (Minelli et al., 2012). Now, we have extended those findings by showing that the cholbut SLN also inhibited the Akt pathway, which is overexpressed in a number of cancers, including colon, pancreatic, ovarian and some steroid hormone-sensitive breast cancers (Roy et al., 2002; Asano et al., 2004). This effect is intriguing because the Akt pathway is critical for prostate cancer invasion (Shukla et al., 2007), promotes androgen-independent progression and is essential for neuroendocrine differentiation of prostate cancer (Li et al., 2007; Wu and Huang, 2007). Moreover, Akt phosphorylation correlates with cell growth, differentiation and adhesion (Lai et al., 2010), as well as with the invasion grade, vessel infiltration, metastasis to lymph nodes and tumour stage in human colon carcinomas (Itoh et al., 2002; Khaleghpour et al., 2004). Our data indicated that the cholbut SLN did affect the signalling machinery of tumour cells, but a wider analysis is needed for a fuller description of these effects.
High concentrations of butyrate (about 2–3 mM) are present in the colon lumen. Because they reach pharmacological effectiveness, colon cancer cells can grow only upon development of escape mechanisms. Among these is a decrease of the intracellular concentration of butyrate through down-regulation of monocarboxylate transporter 1 (MCT1) and sodium-coupled MCT1 (Davis et al., 2008), which are involved in the transport of butyrate and other short chain fatty acid anions. Therefore, cholbut SLN may overcome this resistance by providing butyrate uptake in the tumour cell through a transporter-independent mechanism. Indeed, upon treatment with cholbut SLN, substantial uptake of butyrate is already detectable after 15 min, and it then persists inside the cell for several days (Brioschi et al., 2008; Minelli et al., 2012). Development of multidrug resistance in human tumours is one of the main obstacles to the success of cancer chemotherapy. This phenomenon is often associated with increased expression of the mdr1 gene, which encodes P-glycoprotein (Pgp). As an energy-dependent efflux pump, this protein is capable of extruding certain drugs from cells, leading to decreased drug concentrations within the cells and reduced efficacy of drugs (Stein et al., 1996). The human colon carcinoma cell lines HCT116 and HCT15 were selected in this work also because they normally express the Pgp-mediated type of multidrug resistance. However, HCT15 displays a greater multidrug resistance than HCT116 (Iwahshi et al., 1991; Lee et al., 2010), which might explain the higher activity of sodium butyrate on HCT116 than on HCT15. Therefore, the observation that chobut SLN inhibited HC116 and HCT15 cells similarly supports the hypothesis that these SLN escape the transmembrane efflux pumps responsible for drug elimination.
The in vivo data supported the in vitro data and show that cholbut SLN have significant anti-tumour activity against PC-3 cells by inhibiting local tumour growth after s.c. injection of the cell line. Moreover, this SLN inhibited lung metastasis after i.v. injection of the cell line, which could be due to the effects of cholbut SLN on adhesion, migration and growth of tumour cells. In the light of these results, cholbut SLN might be used in neoadjuvant chemotherapy, allowing potentially curative surgery or after surgical resection to inhibit local relapses and development of metastasis.
Inflammation is present in most cancer tissues, including those that have no precancerous lesions, and a constant inflammatory state may be necessary to maintain and promote cancer progression to a fully malignant phenotype by supporting tumour remodelling, neoangiogenesis, metastasis dissemination, and even modulation of the anticancer innate immune response from a protective M1-type response to a tumour-promoting M2-type response (Wang et al., 2009). Accordingly, the previously reported anti-inflammatory activity of cholbut SLN (Dianzani et al., 2006), may add value to the anti-cancer activity of this compound because it might help to reset the innate immune response. Because SCID mice display a severe defect of the adaptive immune response, but maintain a weak innate immune response (Mosier et al., 1993; Thomsen et al., 2008), this anti-inflammatory effect might have had a role in our in vivo experiments.
In conclusion, cholbut SLN seem to be an effective delivery system for cholesteryl butyrate but because it decreased the active compound concentration, tumour refractoriness and toxic effects, and expanded the compound's mechanisms of actions, enabling it to act on several aspects of anti-tumour activity, such as tumour cell growth, adhesion, migration and recruitment of inflammatory cells.
Acknowledgments
This research has been supported by Associazione Italiana Ricerca sul Cancro (AIRC, Milan), Regione Piemonte (Piattaforme Innovative – IMMONC, Converging Technologies NANO-IGT, Fondazione Amici di Jean (Turin), PRIN Project 2009 (MIUR, Roma) and Progetti di Ricerca di Ateneo San Paolo 2011.
Glossary
- Cholbut SLN
cholesteryl butyrate solid lipid nanoparticles
- DLS
dynamic light scattering
- EPR
enhanced permeability and retention
- FCS
fetal calf serum
- FeSEM
field emission scanning electron microscopy
- GPC
gel permeation chromatography
- HDAC
histone deacetylase
- LDME
laser doppler micro-electrophoresis
- MCT1
monocarboxylate transporter 1
- Pgp
P-glycoprotein
- PMA
phorbol 12-myristate 13-acetate
- SLN
solid lipid nanoparticles
Conflict of interest
P Gasco is an employee of Nanovector s.r.l.
References
- Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- Brioschi A, Zara GP, Calderoni S, Gasco MR, Mauro A. Cholesterylbutyrate solid lipid nanoparticles as a butyric acid prodrug. Molecules. 2008;13:230–254. doi: 10.3390/molecules13020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- Delzenne N, Cherbut C, Neyrinck A. Prebiotics: actual and potential effect in inflammatory and malignant colonic disease. Curr Opin Clin Nutr Metab Care. 2003;6:581–586. doi: 10.1097/00075197-200309000-00013. [DOI] [PubMed] [Google Scholar]
- Dianzani C, Cavalli R, Zara GP, Gallicchio M, Lombardi G, Gasco MR, et al. Cholesteryl butyrate solid lipid nanoparticles inhibit adhesion of human neutrophils to endothelial cells. Br J Pharmacol. 2006;148:648–656. doi: 10.1038/sj.bjp.0706761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani C, Minelli R, Mesturini R, Chiocchetti A, Barrera G, Boscolo S, et al. B7h triggering inhibits umbelical vascular endothelial cell adhesiveness to tumor cell lines and polymorphonuclear cells. J Immunol. 2010;185:3970–3979. doi: 10.4049/jimmunol.0903269. [DOI] [PubMed] [Google Scholar]
- Gasco MR. 2004. Solid lipid nanospheres suitable to a fast internalization into cells. EP 1133286, (24/11/2004) – U.S. 6.685.960 (3/02/2004)
- Hayot C, Debeir O, Van Ham P, Van Damme M, Kiss R, Decaestecker C. Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicol Appl Pharmacol. 2006;211:30–40. doi: 10.1016/j.taap.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94:3127–3134. doi: 10.1002/cncr.10591. [DOI] [PubMed] [Google Scholar]
- Iwahshi T, Okochi E, Ono K, Sugawara I, Tsuruo T, Mori S. Establishment of multidrug resistant human colorectal carcinoma HCT-15 cell lines and their properties. Anticancer Res. 1991;11:1309–1312. [PubMed] [Google Scholar]
- Khaleghpour K, Li Y, Banville D, Yu Z, Shen SH. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241–248. doi: 10.1093/carcin/bgg195. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Tan EM, Fleming SE. Sodium butyrate inhibits cell growth and stimulates p21WAF1/CIP1 protein in human colonic adenocarcinoma cells independently of p53 status. Nutr Cancer. 2003;46:202–211. doi: 10.1207/S15327914NC4602_14. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska A, Kijewska M, Lipko M, Hibner U, Kaminska B. Downregulation of Akt and FAK phosphorylation reduces invasion of glioblastoma cells by impairment of MT1-MMP shuttling to lamellipodia and downregulates MMPs expression. Biochim Biophys Acta. 2011;1813:655–667. doi: 10.1016/j.bbamcr.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS, Wu SH, et al. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC and MAPK signaling pathway. J Agric Food Chem. 2010;58:2935–2942. doi: 10.1021/jf9036694. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim SJ, Choi H, Kim YH, Lim IT, Yang HM, et al. Identification of CKD-516: a potent tubulin polymerization inhibitor with marked antitumor activity against murine and human solid tumors. J Med Chem. 2010;53:6337–6354. doi: 10.1021/jm1002414. [DOI] [PubMed] [Google Scholar]
- Li B, Sun A, Youn H, Hong Y, Terranova PF, Thrasher JB, et al. Conditional Akt activation promotes androgen-independent progression of prostate cancer. Carcinogenesis. 2007;28:572–583. doi: 10.1093/carcin/bgl193. [DOI] [PubMed] [Google Scholar]
- Loberg RD, Day LL, Dunn R, Kalikin LM, Pienta KJ. Inhibition of decay-accelerating factor (CD55) attenuates prostate cancer growth and survival in vivo. Neoplasia. 2006;8:69–78. doi: 10.1593/neo.05679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009;100:572–579. doi: 10.1111/j.1349-7006.2009.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Yashiro M, Nishioka N, Hirakawa K, Olden K, Roberts JD. PI3K/Akt signalling is required for the attachment and spreading, and growth in vivo of metastatic scirrhous gastric carcinoma. Br J Cancer. 2012;106:1535–1542. doi: 10.1038/bjc.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli R, Serpe L, Pettazzoni P, Minero V, Barrera G, Gigliotti C, et al. Cholesteryl butyrate solid lipid nanoparticles inhibit the adhesion and migration of colon cancer cells. Br J Pharmacol. 2012;166:587–601. doi: 10.1111/j.1476-5381.2011.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Mosier DE, Stell KL, Gulizia RJ, Torbett BE, Gilmore GL. Homozygous scid/scid; beige/beige mice have low levels of spontaneous or neonatal T cell-induced B cell generation. J Exp Med. 1993;177:191–194. doi: 10.1084/jem.177.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzaro C, Coradini D, Morel S, Ugazio E, Gasco MR, Daidone MG. Cholesteryl butyrate in solid lipid nanospheres as an alternative approach for butyric acid delivery. Anticancer Res. 1999;19:3921–3926. [PubMed] [Google Scholar]
- Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, et al. Akt proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- Salomone B, Ponti R, Gasco MR, Ugazio E, Quaglino P, Osella-Abate S, et al. In vitro effects of cholesteryl butyrate solid lipd nanospheres as a butyric acid prodrug on melanoma cells: evaluation of antiproliferative activity and apoptosis induction. Clin Exp Metastasis. 2001;18:663–673. doi: 10.1023/a:1013186331662. [DOI] [PubMed] [Google Scholar]
- Serpe L, Laurora S, Pizzimenti S, Ugazio E, Ponti R, Canaparo R, et al. Cholesteryl butyrate solid lipid nanoparticles as a butyric acid pro-drug: effects on cell proliferation, cell cycle distribution and c-myc expression in human leukemic cells. Anticancer Drugs. 2004;15:525–536. doi: 10.1097/01.cad.0000127329.83568.15. [DOI] [PubMed] [Google Scholar]
- Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- Singh R, Lillard JW., Jr Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein U, Walther W, Shoemaker RH. Reversal of multidrug resistance by transduction of cytokine genes into human colon carcinoma cells. J Natl Cancer Inst. 1996;88:1383–1392. doi: 10.1093/jnci/88.19.1383. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Galvani S, Canivet C, Kamar N, Böler T. Reconstitution of immunodeficient SCID/beige mice with human cells: applications in preclinical studies. Toxicology. 2008;246:18–23. doi: 10.1016/j.tox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Wang F, Arun P, Friedman J, Chen Z, Van Waes C. Current and potential inflammation targeted therapies in head and neck cancer. Curr Opin Pharmacol. 2009;9:389–395. doi: 10.1016/j.coph.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, Chueh AC, Tögel L, Corner GA, Ahmed N, Goel S, et al. Apoptotic sensitivity of colon cancer cells to histone deacetylase inhibitors is mediated by an Sp1/Sp3-activated transcriptional program involving immediate-early gene induction. Cancer Res. 2010;70:609–620. doi: 10.1158/0008-5472.CAN-09-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollowski I, Rechkemmer G, Pool-Zobel BL. Protective role of probiotics and prebiotics in colon cancer. Am J Clin Nutr. 2001;73:451S–455S. doi: 10.1093/ajcn/73.2.451s. [DOI] [PubMed] [Google Scholar]
- Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59:491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Wu C, Huang J. PI3 kinase-Akt-mTOR pathway is essential for neuroendocrine differentiation of prostate cancer. J Biol Chem. 2007;282:3571–3583. doi: 10.1074/jbc.M608487200. [DOI] [PubMed] [Google Scholar]