Abstract
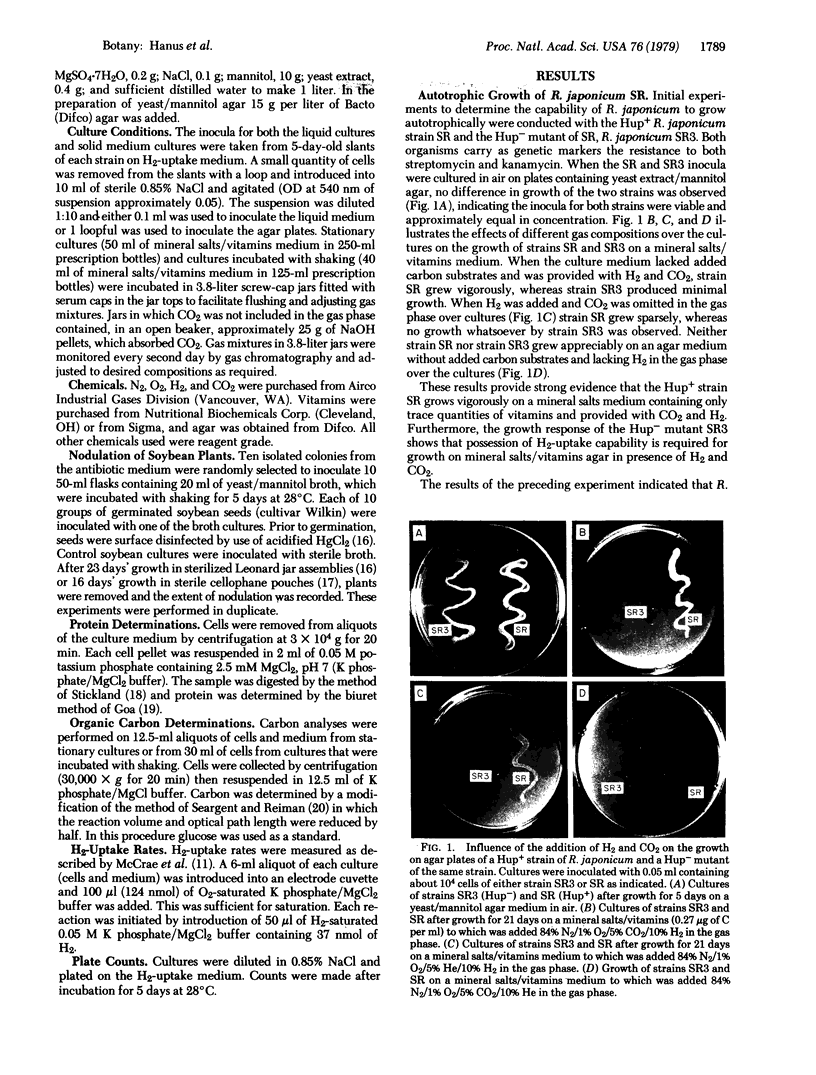
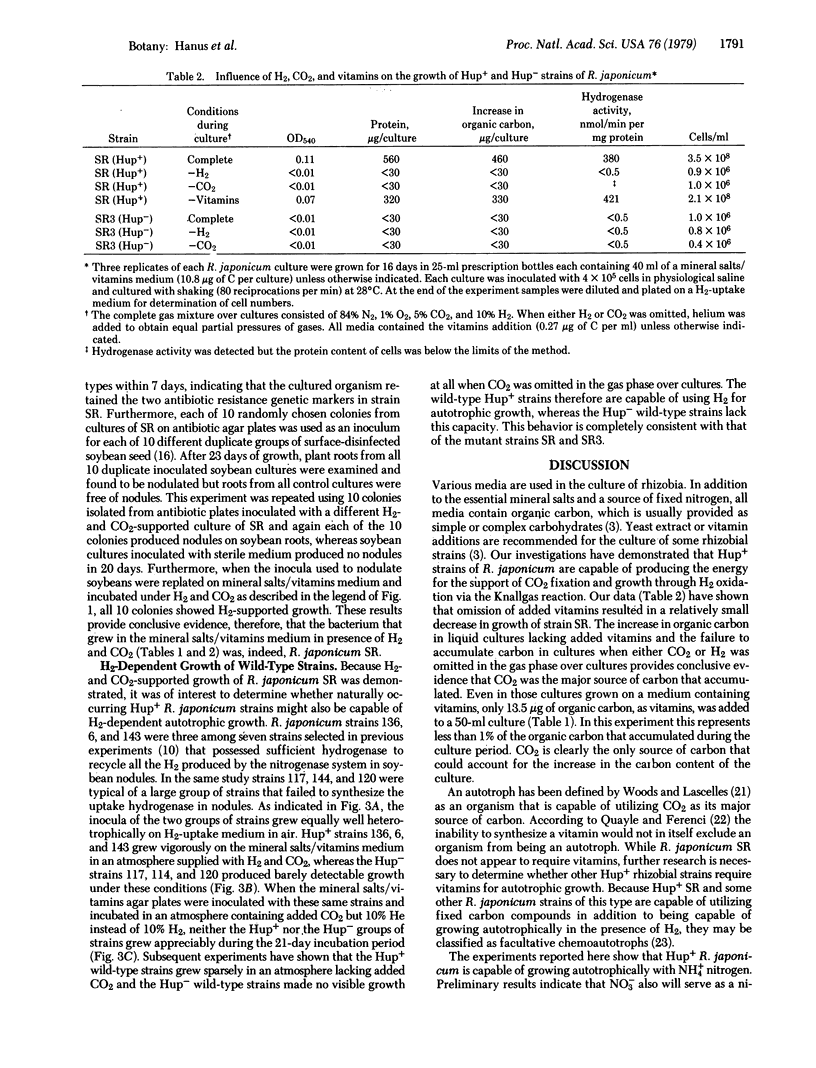
Previous research from this laboratory has demonstrated CO2-fixing and H2-uptake capacities of certain strains of Rhizobium japonicum. In this report we have shown that SR, a H2-uptake-positive (Hup+) strain of R. japonicum, is capable of autotrophic growth with H2 as the energy source. Growth occurred on mineral salts/vitamins/Noble agar, mineral salts/vitamins liquid medium (0.27 μg of C as vitamins per ml), and in mineral salts liquid medium with no added vitamins when cultures were provided with NH4Cl and incubated in an atmosphere containing H2, CO2, O2, and N2. Little or no growth occurred when either H2 or CO2 was omitted from the atmosphere or when the culture was inoculated with SR3, a Hup- mutant of SR. Growth was measured by protein synthesis, fixed organic carbon, and increase in cell number in liquid cultures. The organism that grew autotrophically was verified as R. japonicum by (i) apparent purity on streak plates; (ii) retention of the double antibiotic resistance markers; and (iii) its capability to nodulate soybeans. H2- and CO2-supported growth was demonstrated for three additional Hup+ wild-type R. japonicum strains (USDA 136, 3I1b 6, and 3I1b 143), while three Hup- wild-type strains (USDA 120, 3I1b 144, and USDA 117) were incapable of growth on the Noble agar medium containing mineral salts/vitamins in the H2/CO2/O2/N2 atmosphere. This demonstrated capability of Hup+R. japonicum strains to grow autotrophically requires revision of current concepts regarding conditions for survival and competition of these bacteria in the soil and their relationships to other microorganisms.
Keywords: hydrogenase, legume, chemolithotroph, soybean
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter K. R., Jennings N. T., Hanus J., Evans H. J. Hydrogen evolution and uptake by nodules of soybeans inoculated with different strains of Rhizobium japonicum. Can J Microbiol. 1978 Mar;24(3):307–311. doi: 10.1139/m78-051. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in pea root nodule bacterioids. Arch Mikrobiol. 1968;62(3):272–283. doi: 10.1007/BF00413898. [DOI] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Mutant Strains of Rhizobium japonicum with Increased Ability to Fix Nitrogen for Soybean. Science. 1978 Aug 4;201(4354):448–450. doi: 10.1126/science.201.4354.448. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A. Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- McCrae R. E., Hanus J., Evans H. J. Properties of the hydrogenase system in Rhizobium japonicum bacteroids. Biochem Biophys Res Commun. 1978 Jan 30;80(2):384–390. doi: 10.1016/0006-291x(78)90688-5. [DOI] [PubMed] [Google Scholar]
- Quayle J. R., Ferenci T. Evolutionary aspects of autotrophy. Microbiol Rev. 1978 Jun;42(2):251–273. doi: 10.1128/mr.42.2.251-273.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]
- Schubert K. R., Engelke J. A., Russell S. A., Evans H. J. Hydrogen reactions of nodulated leguminous plants: I. Effect of rhizobial strain and plant age. Plant Physiol. 1977 Nov;60(5):651–654. doi: 10.1104/pp.60.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Jennings N. T., Evans H. J. Hydrogen Reactions of Nodulated Leguminous Plants: II. Effects on Dry Matter Accumulation and Nitrogen Fixation. Plant Physiol. 1978 Mar;61(3):398–401. doi: 10.1104/pp.61.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]