Abstract
BACKGROUND AND PURPOSE
Naturally occurring splice variants of human CAR (hCAR), including hCAR-SV23 (insertion of amino acids SPTV) and hCAR-SV24 (APYLT), have been shown to be expressed in liver. However, little is known regarding how hCAR-SV23 and hCAR-SV24 are activated. Therefore, we investigated the mode of activation of these hCAR splice variants.
EXPERIMENTAL APPROACH
Cell-based reporter gene assays, including ligand-binding domain transactivation assays and coactivator recruitment assays, were conducted on cultured HepG2 cells transfected with various constructs and treated with 3-hydroxyflavone or a hydroxylated (galangin, datiscetin, kaempferol, morin, quercetin or myricetin) or methylated (isorhamnetin, tamarixetin, or syringetin) analogue.
KEY RESULTS
Among the flavonols investigated, only 3-hydroxyflavone increased hCAR-SV23 and hCAR-SV24 activities. 3-Hydroxyflavone did not transactivate the ligand-binding domain of these isoforms or recruit steroid receptor coactivators (SRC-1, SRC-2, or SRC-3). By comparison, 3-hydroxyflavone, galangin, datiscetin, kaempferol, quercetin, isorhamnetin and tamarixetin activated hCAR-WT, whereas none of the flavonols activated hCAR-SV25 (both SPTV and APYLT insertions). The flavonols 3-Hydroxyflavone, galangin, quercetin and tamarixetin transactivated the ligand-binding domain of hCAR-WT, but only 3-hydroxyflavone recruited SRC-1, SRC-2 and SRC-3 to the receptor.
CONCLUSION AND IMPLICATIONS
hCAR-SV23 and hCAR-SV24 can be activated by a mechanism that does not involve the ligand-binding domain of the receptor or recruitment of SRC-1, SRC-2, or SRC-3. 3-Hydroxyflavone and its structural analogues activated hCAR in an isoform-selective and chemical-specific manner. Overall, our study provides insight into a novel mode of ligand activation of hCAR-SV23 and hCAR-SV24.
Keywords: constitutive androstane receptor, splice variants, steroid receptor coactivators, flavonols, 3-hydroxyflavone
Introduction
Constitutive androstane receptor (CAR; NR1I3) is a member of the superfamily of nuclear receptors (Germain et al., 2006). It is expressed predominantly in the liver (Baes et al., 1994) and regulates the expression of many genes, including those involved in bioactivation, detoxification, and transport of drugs and other chemicals (Wang et al., 2012). Numerous naturally occurring splice variants of human CAR (hCAR) have been identified (Lamba et al., 2005; Lamba, 2008; Choi et al., 2013), including hCAR-SV23, hCAR-SV24 and hCAR-SV25 (Auerbach et al., 2003). The various nomenclatures for these hCAR isoforms are listed in Lau et al. (2011). The hCAR-SV23 splice variant has an amino acid insertion of SPTV between helices 6 and 7, whereas the hCAR-SV24 splice variant has APYLT between helices 8 and 9 and the hCAR-SV25 splice variant has both of these insertions (Auerbach et al., 2003; Arnold et al., 2004; Jinno et al., 2004). The abundance of hCAR-SV23, hCAR-SV24 and hCAR-SV25 in individual human liver samples has been estimated to be ∼6–31% (Auerbach et al., 2003; Jinno et al., 2004; DeKeyser et al., 2009; Ross et al., 2010), ∼38–42% (Jinno et al., 2004; Ross et al., 2010) and ∼2–10% (Savkur et al., 2003; Jinno et al., 2004) of total hCAR transcripts, respectively.
Very little is known regarding the mechanism by which ligands activate hCAR-SV23 and hCAR-SV24, although it has been reported that activators of hCAR-SV23 and hCAR-SV24 recruit coactivators to the ligand-binding domain (LBD) of the receptor. For example, di-(2-ethylhexyl)phthalate (DEHP) activates hCAR-SV23 and recruits steroid receptor coactivator-1 (SRC-1) (DeKeyser et al., 2009; 2011; Lau et al., 2011), steroid receptor coactivator-2 (SRC-2) (Lau et al., 2011) and steroid receptor coactivator-3 (SRC-3) (Lau et al., 2011), whereas 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime (CITCO) (Arnold et al., 2004) and artemisinin (Burk et al., 2012) activate hCAR-SV24 and recruit coactivators, such as SRC-1, SRC-2, SRC-3 and vitamin d-interacting protein (DRIP205). However, it appears that hCAR-SV23 may also be activated by a mechanism that does not involve transactivation of the LBD of hCAR-SV23 or recruitment of SRC-1, SRC-2 or SRC-3, as shown in a previous study with a complex chemical mixture (i.e. Ginkgo biloba extract) (Lau et al., 2011). Currently, it is not known whether activation of hCAR-SV24 occurs by an indirect mechanism. Comparatively, both orthosteric agonism and indirect activation have been shown for the wild-type form of hCAR (hCAR-WT). As demonstrated in experiments with a hCAR-WT agonist, CITCO binds directly to the LBD of the receptor and recruits coactivators (Maglich et al., 2003; Burk et al., 2005), indicating that it is an orthosteric agonist. In contrast, phenobarbital activates hCAR-WT (Wang et al., 2004; Faucette et al., 2007) in an indirect manner whereby it does not bind to the LBD of the receptor (Moore et al., 2000) or recruit coactivators, such as SRC-1, SRC-2 or SRC-3 (Lau et al., 2011).
Flavonoids are a class of polyphenolic chemicals (Figure 1) with a three-ring structure – a heterocyclic ring and two aromatic rings (Middleton et al., 2000). They can be divided into various subclasses based on the type of substituent in the heterocyclic ring. As shown in Figure 1, the subclasses include flavonol, flavone, flavanol, flavanone, flavanolol and isoflavone (Birt et al., 2001). Shown in Figure 2 are 3-hydroxyflavone and other flavonols with specific substitutions in the phenyl rings. As indicated previously, G. biloba extract, which contains approximately 24% w/w of flavonol glycosides, including those of quercetin, kaempferol and isorhamnetin (van Beek and Montoro, 2009), appears to activate hCAR-SV23 by an indirect mechanism (Lau et al., 2011). However, this finding may reflect the competing actions of the many chemicals in the complex mixture. Therefore, the primary objective of the present study was to use single chemical entities to determine whether hCAR-SV23 and another hCAR splice variant (hCAR-SV24) are activated by an indirect mechanism. Our experimental approach involved the systematic investigation of 3-hydroxyflavone and its hydroxylated (galangin, datiscetin, kaempferol, morin, quercetin and myricetin) and methylated (isorhamnetin, tamarixetin and syringetin) analogues. For comparative purposes, we also investigated the effects of 3-hydroxyflavone and its analogues on the activity of the hCAR-SV25 splice variant and the reference isoform hCAR-WT. The results are discussed in the context of indirect activation as a mechanism of hCAR-SV23 and hCAR-SV24 activation, and the isoform-selective and chemical-specific activation of hCAR by the various flavonols.
Figure 1.
Chemical structure of flavonoid backbone, flavone, flavonol, flavanol, flavanone, flavanolol and isoflavone.
Figure 2.
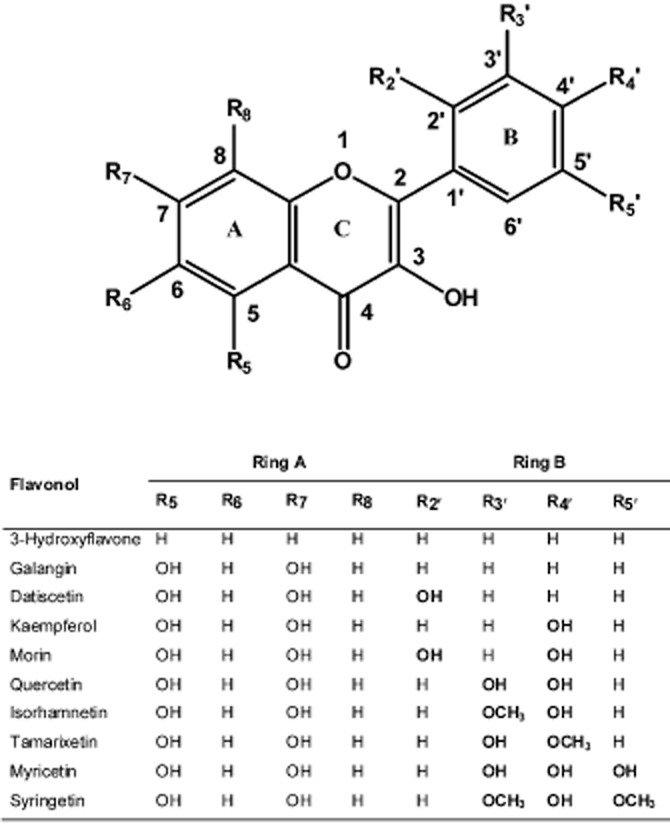
Chemical structure of specific flavonols.
Methods
Chemicals and reagents
The flavonols 3-hydroxyflavone (CAS #577-85-5), galangin (3,5,7-trihydroxyflavone; CAS #548-83-4), datiscetin (3,5,7,2′-tetrahydroxyflavone; CAS #480-15-9), kaempferol (3,5,7,4′-tetrahydroxyflavone; CAS #520-18-3), morin (3,5,7,2′,4′-pentahydroxyflavone; CAS #480-16-0), isorhamnetin (3,5,7,4′-tetrahydroxy-3′-methoxyflavone; CAS #480-19-3), tamarixetin (4′-methoxy-3,5,7,3′-tetrahydroxyflavone; CAS #603-61-2), myricetin (3,5,7,3′,4′,5′-hexahydroxyflavone; CAS #529-44-2) and syringetin (3′,5′-dimethoxy-3,5,7,4′-tetrahydroxyflavone; CAS #4423-37-4) were purchased from Indofine Chemical Company, Inc. (Hillsborough, NJ, USA). Quercetin dihydrate (3,5,7,3′,4′-pentahydroxyflavone; CAS #6151-25-3), DEHP (CAS #117-81-7) and 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP; CAS #76150-91-9) were purchased from Sigma-Aldrich (St Louis, MO, USA). The hCAR-WT agonist 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime (CITCO; CAS #338404-52-7) was obtained from Enzo Life Sciences, Inc. (Plymouth Meeting, PA, USA), and 5α-androstan-3α-ol (androstanol; CAS #7657-50-3) was from Steraloids (Newport, RI, USA). CellTiter-Glo Luminescent Cell Viability Assay, FuGENE 6 transfection reagent, and Dual-Luciferase Reporter Assay System were from Promega (Madison, WI, USA). Charcoal-stripped, heat-inactivated fetal bovine serum (Hyclone Laboratories, Logan, UT, USA) was bought from Thermo Fisher Scientific, Inc. (Nepean, ON, Canada). Minimum Essential Medium (catalog #10370), Opti-MEM and all other cell culture reagents were obtained from Life Technologies, Inc. (Carlsbad, CA, USA).
Plasmids
pCMV6-neo-hCAR-SV23, pCMV6-XL4-hCAR-SV24, pCMV6-XL5-hCAR-SV25, pCMV6-XL4-hCAR-WT, pCMV6-XL4-hRXRα, pCMV6-neo, pCMV6-XL4 and pCMV6-XL5 were purchased from OriGene Technologies, Inc. (Rockville, MD, USA). Renilla reniformis luciferase pGL4.74[hRluc/TK] was obtained from Promega. pGL3-basic-CYP2B6-PBREM/XREM-luc reporter was constructed as described previously (Wang et al., 2003). PathDetect pFR-luc trans-reporter (contains five tandem repeats of yeast GAL4 binding sites) was bought from Agilent Technologies (Santa Clara, CA, USA). The pVP16 and pM (contains the yeast GAL4 DNA-binding domain) empty vectors were provided in the Matchmaker Mammalian Two-Hybrid Assay Kit (Clontech, Mountain View, CA, USA). The pM-hCAR-SV23-LBD, pVP16-hCAR-SV23-LBD, pM-hCAR-WT-LBD, pVP16-hCAR-WT-LBD, pM-hSRC1-RID, pM-hSRC2-RID and pM-hSRC3-RID constructs were prepared as described previously (Lau et al., 2011). To construct the pM-hCAR-SV24-LBD and pVP16-hCAR-SV24-LBD plasmids, the LBD (Gln-105 to Ser-353) (Arnold et al., 2004) of hCAR-SV24 was amplified from pCMV6-XL4-hCAR-SV24 and inserted into the pVP16 or pM vector. The primers used for amplification of hCAR-SV24-LBD were 5′-GGA-GGA-ATT-CCA-ACT-GAG-TAA-GGA-GCA-AGA-A-3′ (forward) and 5′-GGG-AGG-ATC-CTC-AGC-TGC-AGA-TCT-CCT-GG-3′ (reverse). The plasmids were sequenced (Nucleic Acid Protein Service Unit at the University of British Columbia, Vancouver, BC, Canada), and the identity of each plasmid was confirmed by comparing their sequence with published sequence.
Cell culture and drug treatment
HepG2 human hepatocellular carcinoma cells (catalog #HB-8065) were purchased from the American Type Culture Collection (Manassas, VA, USA). They were grown in T75 flasks and cultured in Minimum Essential Medium supplemented with 2 mM L-glutamine, 100 U·mL−1 penicillin, 100 μg·mL−1 streptomycin and 10% v/v heat-inactivated fetal bovine serum (Life Technologies, Inc.) (Lau et al., 2010). In the cell viability and transfection experiments, HepG2 cells were cultured in the same supplemented culture medium as described above, except that 10% v/v charcoal-stripped, heat-inactivated fetal bovine serum (Hyclone Laboratories) was used. Cells were seeded onto 24-well microplates at a density of 25 000 cells/well (cell viability assay) or 100 000 cells/well (reporter gene assays) and in a volume of 0.5 mL of supplemented culture medium.
Cell viability assay
At 24 h after plating, cultured HepG2 cells were treated with 0.5 mL of fresh supplemented culture medium containing 3-hydroxyflavone, galangin, datiscetin, kaempferol, morin, quercetin, isorhamnetin, tamarixetin, myricetin, syringetin, dextran (1% w/v; negative control), Triton X-100 (0.1% v/v; positive control), or DMSO (0.1% v/v; vehicle) for 24 h, as detailed in each figure legend. At the end of the treatment period, cell viability was determined (CellTiter-Glo Luminescent Cell Viability Assay, Promega) according to manufacturer's protocol, but with minor modifications. Culture medium was aspirated and the cells were washed twice with 200 μL of phosphate-buffered saline (pH 7.4). An 125 μL of CellTiter Reagent (0.2×) was added to each well. The plate was mixed on an orbital shaker for 5 min, incubated at room temperature for 10 min to stabilize the signal, and 100 μL of the content was transferred to a 96-well white polystyrene microplate (catalog #655075, Grenier Bio-One, Monroe, NC, USA). Luminescence was measured using a GloMax 96 microplate luminometer (Promega Corporation). Net luminescence was determined by subtracting the luminescence of the background control (processed as described above but without cells) from the treated group. Cell viability was calculated by dividing the net luminescence of each treatment group with that of the vehicle-treated control group and expressed as a percentage. Four independent experiments were conducted and each experiment was performed in triplicate.
Transient transfection and reporter gene assays
At 5 h after plating, cultured HepG2 cells were transfected with 20 μL of a transfection master mix containing FuGENE 6 transfection reagent (3 μL/μg of DNA), serum-free Opti-MEM (20 μL/well), and various plasmids for 24 h. In the hCAR-SV23-, hCAR-SV24-, hCAR-SV25-, and hCAR-WT-dependent reporter gene assays, cells were transfected with pCMV6-neo-hCAR-SV23, pCMV6-XL4-hCAR-SV24, pCMV6-XL5-hCAR-SV25, pCMV6-XL4-hCAR-WT, or its respective empty vector (50 ng/well) together with pGL3-basic-CYP2B6-PBREM/XREM-luc reporter plasmid (50 ng/well) and pGL4.74[hRluc/TK] internal control vector (5 ng/well). pCMV6-XL4-hRXRα (10 ng/well) was added to the hCAR-SV23, hCAR-SV24, and hCAR-SV25 assays, but not the hCAR-WT assay, because of the isoform-dependent requirement for exogenous hRXRα (Auerbach et al., 2005; 2007; Lau et al., 2011).
Transfected HepG2 cells were treated with 0.5 mL of fresh supplemented culture medium containing 3-hydroxyflavone, galangin, datiscetin, kaempferol, morin, quercetin, isorhamnetin, tamarixetin, myricetin, syringetin, DEHP, CITCO, TCPOBOP, or DMSO (0.1% v/v; vehicle) for 24 h, as detailed in each figure legend. In the hCAR-WT-dependent reporter gene assay, transfected cells were co-treated with androstanol (10 μM), which is a hCAR-WT inverse agonist (Moore et al., 2000), to decrease the constitutive activity (Burk et al., 2005). At the end of the treatment period, transfected HepG2 cells were lysed and firefly luciferase and R. reniformis luciferase activities were determined using a Dual-Luciferase Reporter Assay System. Luminescence was measured using a GloMax 96 microplate luminometer (Promega Corporation). Luciferase activity was expressed as a ratio of firefly luciferase to R. reniformis luciferase activity. Three or four independent experiments were conducted and each experiment was performed in triplicate.
In the hCAR-SV23-LBD and hCAR-SV24-LBD assays, HepG2 cells were transfected with pM-hCAR-SV24-LBD (Gln-105 to Ser-353; 40 ng/well), pM-hCAR-SV23-LBD (Gln-105 to Ser-352; 40 ng/well), or the pM empty vector (40 ng/well) along with pCMV6-XL4-hRXRα (10 ng/well), pGL4.74[hRluc/TK] internal control plasmid (1 ng/well), and pFR-luc reporter plasmid (200 ng/well), using FuGENE 6 transfection reagent (3 μL/μg of DNA) diluted in 20 μL of serum-free Opti-MEM. In the hCAR-WT-LBD assay, HepG2 cells were transfected with pM-hCAR-WT-LBD (Gln-105 to Ser-348; 40 ng/well) or the pM empty vector (40 ng/well) along with pGL4.74[hRluc/TK] internal control plasmid (1 ng/well), and pFR-luc reporter plasmid (100 ng/well), using FuGENE 6 transfection reagent (3 μL/μg of DNA) diluted in 20 μL of serum-free Opti-MEM. At 24 h after transfection, cells were treated with 0.5 mL of fresh supplemented culture medium containing 3-hydroxyflavone, galangin, datiscetin, kaempferol, quercetin, isorhamnetin, tamarixetin, DEHP, CITCO, TCPOBOP, or DMSO (0.1% v/v; vehicle) for 24 h, as detailed in each figure legend. In the hCAR-WT-LBD assay, androstanol (10 μM; hCAR-WT inverse agonist) (Moore et al., 2000) was added to each treatment group to decrease the constitutive activity (Burk et al., 2005). Luciferase activity was measured and normalized as described under Transient Transfection and Reporter Gene Assays. Three independent experiments were conducted and each experiment was performed in triplicate. The plasmids used in the hCAR-SV23, hCAR-SV24, hCAR-SV25, and hCAR-WT-dependent reporter gene assays did not affect viability of HepG2 cells, as assessed in a CellTiter-Glo assay (data not shown).
Mammalian two-hybrid assay
Mammalian two-hybrid assays were performed as detailed previously (Lau et al., 2011). At 5 h after plating, cultured HepG2 cells were co-transfected with a receptor expression plasmid (40 ng/well) or its pVP16 empty vector (40 ng/well), a coactivator expression plasmid (10 ng/well), pCMV6-XL4-hRXRα (10 ng/well, except for the hCAR-WT assay), pGL4.74[hRluc/TK] internal control plasmid (1 ng/well), and pFR-luc reporter plasmid (100 ng/well). The receptor expression plasmids were pVP16-hCAR-SV23-LBD, pVP16-hCAR-SV24-LBD, and pVP16-hCAR-WT-LBD. The coactivator expression plasmids were pM-hSRC1-RID, pM-hSRC2-RID, and pM-hSRC3-RID. At 24 h after transfection, cells were treated with 0.5 mL of fresh supplemented culture medium containing 3-hydroxyflavone, galangin, datiscetin, kaempferol, quercetin, isorhamnetin, tamarixetin, DEHP, CITCO, TCPOBOP, or DMSO (0.1% v/v; vehicle) for 24 h, as detailed in each figure legend. In the hCAR-WT-dependent mammalian two-hybrid assay, androstanol (10 μM; hCAR-WT inverse agonist) (Moore et al., 2000) was added to each treatment group to decrease the constitutive activity (Burk et al., 2005). Luciferase activity was measured and normalized as described under Transient Transfection and Reporter Gene Assays. Three or four independent experiments were conducted and each experiment was performed in triplicate.
Statistical analysis
Data were analyed by one-way or two-way analysis of variance, as appropriate, and when significant differences were detected, the Student Newman-Keuls multiple comparison test was performed (SigmaPlot 11.0, Systat Software, Inc., San Jose, CA). The level of statistical significance was set a priori at P < 0.05.
Results
Effect of 3-hydroxyflavone and its structural analogues on viability of cultured HepG2 cells
To identify the range of non-cytotoxic concentrations of the ten individual flavonols in cultured HepG2 cells, the CellTiter-Glo Luminescent Cell Viability Assay was conducted. This assay measures the ATP level of metabolically active cells. As shown in Figure 3, none of the flavonols at 10 or 30 μM influenced cell viability, whereas 100 μM of 3-hydroxyflavone, galangin, kaempferol, myricetin, tamarixetin, and isorhamnetin decreased it. Dextran, which is a negative control, did not compromise cell viability, whereas Triton X-100, which is a positive control, caused a total loss of cell viability. Based on the results of this experiment, all subsequent experiments were conducted with flavonols at a concentration of 30 μM or less.
Figure 3.
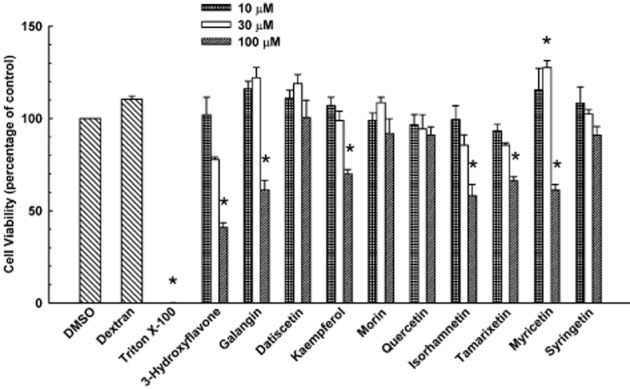
Effect of 3-hydroxyflavone and its structural analogues on viability of cultured HepG2 cells. Cultured HepG2 cells were treated with DMSO (0.1% v/v; vehicle), dextran (1% w/v), Triton X-100 (0.1% v/v), or various concentrations (10, 30, or 100 μM) of 3-hydroxyflavone, galangin, datiscetin, kaempferol, morin, quercetin, isorhamnetin, tamarixetin, myricetin, or syringetin. Cell viability was quantified as described under Methods. Data are expressed as mean ± S.E.M. for four experiments. *, significantly different from the vehicle-treated control group (P < 0.05).
Comparative effect of 3-hydroxyflavone and its structural analogues on hCAR-SV23, hCAR-SV24, hCAR-SV25, and hCAR-WT activities
A previous study indicates that certain flavonols (e.g. galangin) are capable of activating hCAR-WT in a cell-based reporter gene assay (Yao et al., 2010). To investigate whether the amino acid insertions (SPTV, APYLT, and both SPTV and APYLT) in hCAR splice variants affect their response to a class of structurally similar chemicals, we determined systematically the effects of 3-hydroxyflavone and its structural analogues on the activity of hCAR-SV23 (insertion of SPTV) and hCAR-SV24 (insertion of APYLT), and compared them to that of hCAR-SV25 (insertion of both SPTV and APYLT) and hCAR-WT (reference isoform). 3-Hydroxyflavone activated hCAR-SV23 (Figure 4A) and hCAR-SV24 (Figure 4B), whereas galangin, datiscetin, kaempferol, morin, quercetin, isorhamnetin, tamarixetin, myricetin, and syringetin had no effect on the activity of hCAR-SV23 (Figure 4A) or hCAR-SV24 (Figure 4B). None of the individual flavonols activated hCAR-SV25 (Figure 4C). As shown in Figure 4D, 3-hydroxyflavone, galangin, datiscetin, kaempferol, quercetin, isorhamnetin, and tamarixetin activated hCAR-WT. By comparison, DEHP (10 μM; positive control for hCAR-SV23) elevated hCAR-SV23 activity (Figure 4A), whereas CITCO (10 μM; positive control for hCAR-SV24 and hCAR-WT) increased the activity of hCAR-SV24 (Figure 4B) and hCAR-WT (Figure 4D), but not hCAR-SV25 (Figure 4C). TCPOBOP (0.25 μM), which is a negative control, did not affect hCAR-SV23, hCAR-SV24, hCAR-SV25, or hCAR-WT activity (Figure 4A–D). The individual flavonols increased the luciferase activities in cells transfected with the empty vector for the receptor expression plasmid and the reporter plasmid (Figure 4A–D). This increase in background luciferase activity varied widely among the flavonols investigated. Therefore, control groups in which cells transfected with an appropriate empty vector and treated with each individual chemical were included in all subsequent reporter gene assays.
Figure 4.
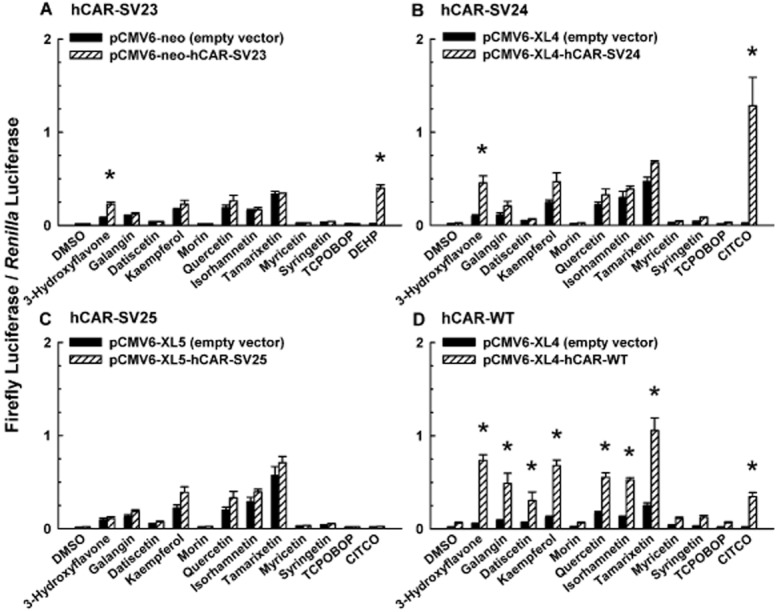
Comparative effect of 3-hydroxyflavone and its structural analogues on the transcriptional activity of hCAR-SV23, hCAR-SV24, hCAR-SV25, and hCAR-WT. Cultured HepG2 cells were transfected with pCMV6-XL4-hRXRα (except for the hCAR-WT assay), pGL4.74[hRluc/TK] internal control vector, pGL3-basic-CYP2B6-PBREM/XREM-luc, and a receptor expression plasmid or its respective empty vector, as described under Methods. The receptor expression plasmids were (A) pCMV6-neo-hCAR-SV23, (B) pCMV6-XL4-hCAR-SV24, (C) pCMV6-XL5-hCAR-SV25, and (D) pCMV6-XL4-hCAR-WT. Transfected cells were treated with DMSO (0.1% v/v; vehicle), a flavonol (3-hydroxyflavone, galangin, datiscetin, kaempferol, morin, quercetin, isorhamnetin, tamarixetin, myricetin, or syringetin; each at 30 μM), TCPOBOP (0.25 μM), CITCO (10 μM), or DEHP (10 μM). In the hCAR-WT-dependent reporter gene assay, androstanol (10 μM; inverse agonist of hCAR-WT) was added to each treatment group. Firefly luciferase and R. reniformis luciferase activities were quantified and normalized as described under Methods. Data are expressed as mean ± S.E.M. for three or four independent experiments performed in triplicate. *, significantly different from the same treatment group transfected with the empty vector and the vehicle-treated control group transfected with the same receptor expression plasmid (P < 0.05). Androstanol decreased hCAR-WT activity in the vehicle-treated control group by 64 ± 2%.
Isoform-selective activation of hCAR-SV23, hCAR-SV24, and hCAR-WT by 3-hydroxyflavone
3-Hydroxyflavone activated hCAR-SV23 (Figure 4A), hCAR-SV24 (Figure 4B), and hCAR-WT (Figure 4D). To determine whether there is an isoform-selective activation of hCAR by 3-hydroxyflavone at lesser concentrations (i.e. <30 μM), we conducted a concentration-response experiment. 3-Hydroxyflavone at a concentration of 1, 3, or 10 μM did not alter the activity of hCAR-SV23 (Figure 5A) or hCAR-SV24 (Figure 5B), whereas at 30 μM, it activated both isoforms. Comparatively, this chemical had no effect on hCAR-WT activity at 1 or 3 μM concentration, whereas it increased it at 10 and 30 μM (Figure 5C).
Figure 5.
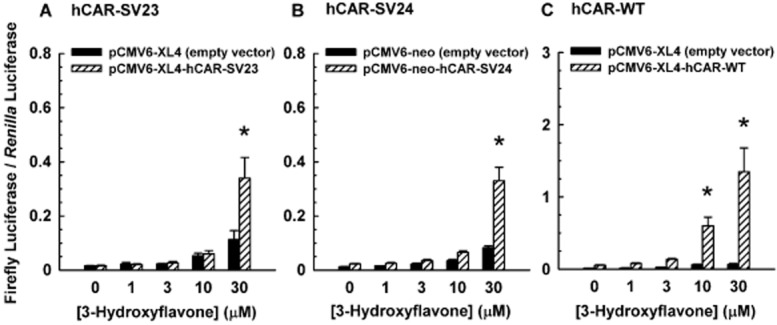
Isoform-selective activation of hCAR-SV23, hCAR-SV24, and hCAR-WT by 3-hydroxyflavone. Cultured HepG2 cells were transfected with pCMV6-XL4-hRXRα (except for the hCAR-WT assay), pGL4.74[hRluc/TK] internal control vector, pGL3-basic-CYP2B6-PBREM/XREM-luc, and a receptor expression plasmid or its respective empty vector, as described under Methods. The receptor expression plasmids were (A) pCMV6-neo-hCAR-SV23, (B) pCMV6-XL4-hCAR-SV24, and (C) pCMV6-XL4-hCAR-WT. Transfected cells were treated with DMSO (0.1% v/v; vehicle) or 3-hydroxyflavone (1–30 μM). In the hCAR-WT-dependent reporter gene assay, androstanol (10 μM; inverse agonist of hCAR-WT) was added to each treatment group. Firefly luciferase and R. reniformis luciferase activities were quantified and normalized as described under Methods. Data are expressed as mean ± S.E.M. for three or four independent experiments performed in triplicate. *, significantly different from the same treatment group transfected with the empty vector and the vehicle-treated control group transfected with the same receptor expression plasmid (P < 0.05).
Effect of 3-hydroxyflavone and/or its structural analogues on transactivation of the LBD of hCAR-SV23, hCAR-SV24, and hCAR-WT
To have an indication as to whether 3-hydroxyflavone may bind to the LBD of hCAR-SV23 and hCAR-SV24, a reporter gene assay was conducted in which cultured HepG2 cells were transfected with the LBD of hCAR-SV23 or hCAR-SV24. 3-Hydroxyflavone (30 μM) did not increase the luciferase activity in cells transfected with hCAR-SV23-LBD (Figure 6A) or hCAR-SV24-LBD (Figure 6B), whereas DEHP (10 μM; positive control for hCAR-SV23-LBD) and CITCO (10 μM; positive control for hCAR-SV24-LBD), but not TCPOBOP (0.25 μM; negative control), transactivated hCAR-SV23-LBD (Figure 6A) and hCAR-SV24-LBD (Figure 6B) respectively. By comparison, 3-hydroxyflavone, galangin, quercetin, and tamarixetin, but not datiscetin, kaempferol, or isorhamnetin, increased the luciferase activity in hCAR-WT-LBD-transfected cells (Figure 6C), whereas CITCO (10 μM; positive control) and TCPOBOP (0.25 μM; negative control) gave the expected results.
Figure 6.
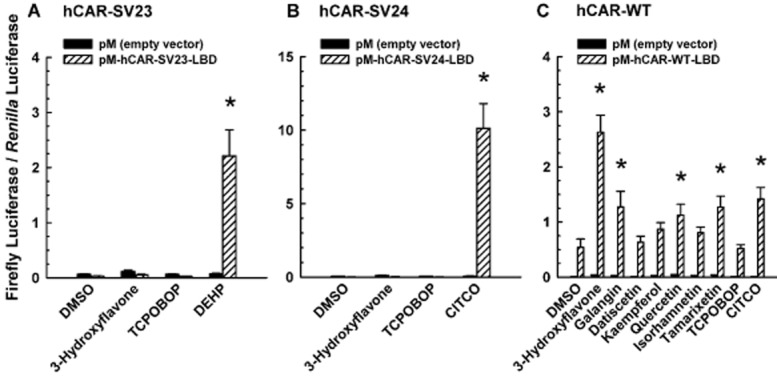
Effect of 3-hydroxyflavone and its structural analogues on transactivation of the LBD of hCAR-SV23, hCAR-SV24, and hCAR-WT. Cultured HepG2 cells were co-transfected with a receptor expression plasmid or the pM empty vector, pCMV6-XL4-hRXRα (except for the hCAR-WT assay), pGL4.74[hRluc/TK] internal control plasmid, and pFR-luc reporter plasmid, as described under Methods. The receptor expression plasmids were (A) pM-hCAR-SV23-LBD, (B) pM-hCAR-SV24-LBD, and (C) pM-hCAR-WT-LBD. Transfected cells were treated with DMSO (0.1% v/v; vehicle), a flavonol (3-hydroxyflavone, galangin, datiscetin, kaempferol, quercetin, isorhamnetin, or tamarixetin; each at 30 μM), TCPOBOP (0.25 μM), DEHP (10 μM), or CITCO (10 μM). In the hCAR-WT assay, androstanol (10 μM; inverse agonist of hCAR-WT) was added to each treatment group. Firefly luciferase and R. reniformis luciferase activities were quantified and normalized as described under Methods. Data are expressed as mean ± S.E.M. for three independent experiments performed in triplicate. *, significantly different from the same treatment group transfected with the empty vector and the vehicle-treated control group transfected with the same receptor expression plasmid (P < 0.05).
Effect of 3-hydroxyflavone and/or its structural analogues on coactivator recruitment to the LBD of hCAR-SV23, hCAR-SV24, and hCAR-WT
A mammalian two-hybrid assay was conducted to investigate whether 3-hydroxyflavone (30 μM) recruits SRC-1, SRC-2, or SRC-3. This chemical did not increase luciferase activity in cells co-transfected with hCAR-SV23 and SRC-1 (Figure 7A), SRC-2 (Figure 7B), or SRC-3 (Figure 7C). Comparatively, DEHP (10 μM; positive control) increased the activity, but TCPOBOP (0.25 μM; negative control) had no effect (Figures 7A–C). Furthermore, 3-hydroxyflavone did not affect the recruitment of SRC-1, SRC-2, or SRC-3 to the hCAR-SV23-LBD by DEHP, suggesting that 3-hydroxyflavone did not negatively influence our mammalian two-hybrid assay (data not shown). 3-Hydroxyflavone also did not recruit SRC-1 (Figure 7D), SRC-2 (Figure 7E), or SRC-3 (Figure 7F) to the LBD of hCAR-SV24, whereas the positive control (10 μM CITCO) and negative control (0.25 μM TCPOBOP) gave the expected results (Figure 7D–F). By comparison, 3-hydroxyflavone, but not galangin, datiscetin, kaempferol, quercetin, isorhamnetin, or tamarixetin, recruited SRC-1 (Figure 7G), SRC-2 (Figure 7H), and SRC-3 (Figure 7I) to the LBD of hCAR-WT. Control analysis with CITCO (10 μM; positive control) and TCPOBOP (0.25 μM; negative control) gave the expected results (Figure 7G–I).
Figure 7.
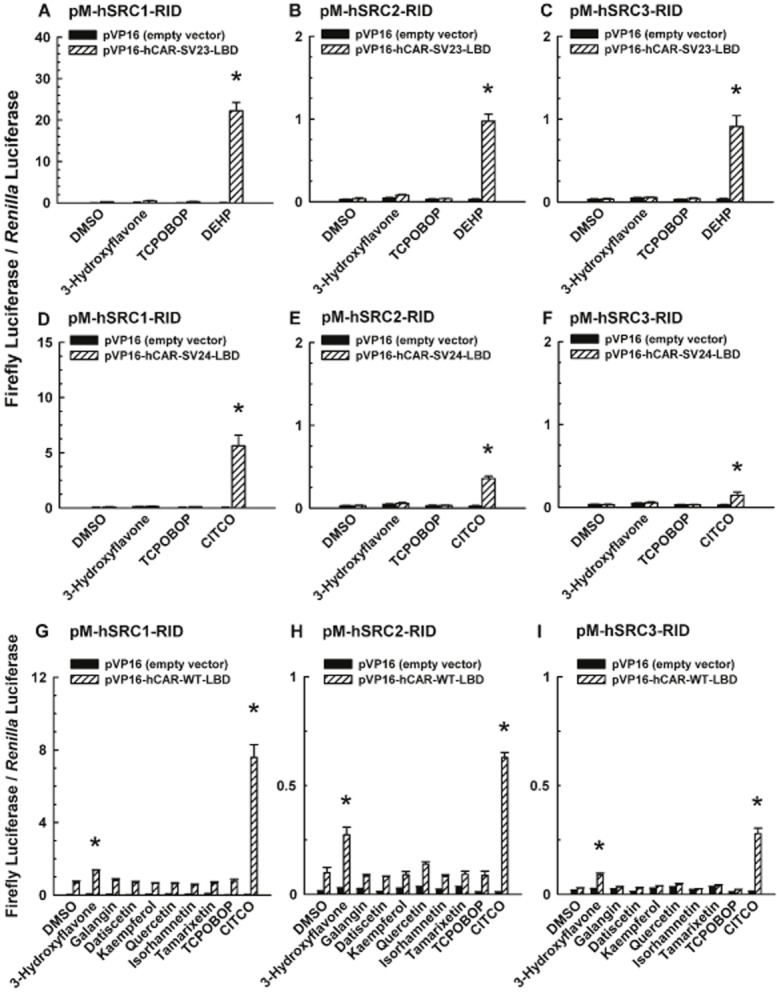
Effect of 3-hydroxyflavone and its structural analogues on coactivator recruitment to the LBD of hCAR-SV23, hCAR-SV24, and hCAR-WT. Cultured HepG2 cells were co-transfected with a receptor expression plasmid or the pVP16 empty vector, a coactivator expression plasmid, pCMV6-XL4-hRXRα (except for the hCAR-WT assay), pGL4.74[hRluc/TK] internal control plasmid, and pFR-luc reporter plasmid. The receptor expression plasmids were (A, B, C) pVP16-hCAR-SV23-LBD, (D, E, F) pVP16-hCAR-SV24-LBD, and (G, H, I) pVP16-hCAR-WT-LBD. The coactivator expression plasmids were (A, D, G) pM-hSRC1-RID, (B, E, H) pM-hSRC2-RID, and (C, F, I) pM-hSRC3-RID. Transfected cells were treated with DMSO (0.1% v/v; vehicle), a flavonol (3-hydroxyflavone, galangin, datiscetin, kaempferol, quercetin, isorhamnetin, or tamarixetin; each at 30 μM), TCPOBOP (0.25 μM), DEHP (10 μM), or CITCO (10 μM). In the hCAR-WT assay, androstanol (10 μM; inverse agonist of hCAR-WT) was added to each treatment group. Firefly luciferase and R. reniformis luciferase activities were quantified and normalized as described under Methods. Data are expressed as mean ± S.E.M. for three or four independent experiments performed in triplicate. *, significantly different from the same treatment group transfected with the empty vector and the vehicle-treated control group transfected with the same receptor expression plasmid (P < 0.05). Androstanol decreased hCAR-WT activity in the vehicle-treated control group by 43 ± 9%.
Discussion
hCAR-SV23 (SPTV between helices 6 and 7) and hCAR-SV24 (APYLT between helices 8 and 9) are splice variants of hCAR (Lamba et al., 2005; Lamba, 2008). The SPTV amino acid insertion between helices 6 and 7 (Auerbach et al., 2003) has been postulated to affect the conformation of the LBD (DeKeyser et al., 2011). By comparison, the APYLT amino acid insertion between helices 8 and 9 is thought not to affect the conformation of the LBD, but it may influence the position of the AF2 domain and compromise the heterodimerization between hCAR and RXR (Auerbach et al., 2003; Omiecinski et al., 2011). Both of these amino acid insertions result in splice variants devoid of constitutive activity (DeKeyser et al., 2011; Omiecinski et al., 2011). Whereas most of the mechanistic studies on hCAR have focused on the wild-type reference form, there is virtually no information on how ligands activate the functionally active splice variants of this receptor, such as hCAR-SV23 and hCAR-SV24. Our previous study suggested that G. biloba extract activates hCAR-SV23 by an indirect mechanism (Lau et al., 2011). However, a potential confounding factor in that study is the competing actions of the many chemicals in the complex mixture. Therefore, in the present study, we used single chemical entities to study the mode of action of the hCAR-SV23 and hCAR-SV24 splice variants.
A novel finding in the present study is that ligand activation of not only hCAR-SV23, but also hCAR-SV24, occurs by an indirect mechanism (i.e. one that does not involve orthosteric agonism). This conclusion is based on the result that 3-hydroxyflavone activated hCAR-SV23 and hCAR-SV24, but it did not transactivate the LBD of these isoforms. Consistent with this proposal, this chemical also did not recruit steroid receptor coactivators SRC-1, SRC-2, or SRC-3. How 3-hydroxyflavone activates hCAR-SV23 and hCAR-SV24 remains to be investigated, but according to our data, it activates hCAR-SV23 and hCAR-SV24 in a manner distinct from DEHP activation of hCAR-SV23 and CITCO activation of hCAR-SV24. Based on the experimental data available to date, it appears that ligand-activation of hCAR-SV23 and hCAR-SV24 occurs by at least two mechanisms: (i) direct activation that involves ligand binding to the LBD of these receptors, as suggested by the transactivation of the LBD of hCAR-SV23 by DEHP and that of hCAR-SV24 by CITCO (Lau et al., 2011 and present study), and recruitment of coactivators as shown previously in the recruitment of SRC-1, SRC-2, and SRC-3 to hCAR-SV23-LBD by DEHP (DeKeyser et al., 2009; 2011; Lau et al., 2011) and to hCAR-SV24-LBD by CITCO (Arnold et al., 2004; Li et al., 2008) and artemisinin (Burk et al., 2012); and (ii) indirect activation as shown in the current study with 3-hydroxyflavone. It remains to be investigated whether the indirect mode of ligand activation of hCAR-SV23 and hCAR-SV24 involves signal transduction mechanisms, such as those proposed for the wild-type form of rodent CAR (Bauer et al., 2004; Rencurel et al., 2006; Koike et al., 2007; Shindo et al., 2007; Osabe and Negishi, 2011).
To date, very few chemicals have been investigated for their effects on the transcriptional activity of hCAR-SV25. CITCO (Jinno et al., 2004), phenobarbital (Jinno et al., 2004), clotrimazole (Auerbach et al., 2003), and G. biloba extract (Lau et al., 2011) have been investigated, but none of them activates hCAR-SV25. Likewise, as shown in the present study, none of the flavonols activated hCAR-SV25. Therefore, it appears that a double insertion of the SPTV and APYLT amino acids in hCAR results in a non-functional isoform in hCAR-SV25. The reason for this is not clear, but based on results obtained from in silico modeling, it has been proposed that the double insertion forms two additional loops that may affect the heterodimerization surface, resulting in a loss of heterodimerization between hCAR-SV25 and RXR (Savkur et al., 2003).
As shown in the present study, many of the flavonols increased the background luciferase activity in HepG2 cells transfected with pCMV6-neo, pCMV6-XL4, or pCMV6-XL5 (i.e. empty vector control for the various receptor expression plasmids) and the pGL3-basic-CYP2B6-PBREM/XREM-luc reporter plasmid. The extent of the increase varied among the flavonols, with tamarixetin giving the greatest increase (15 to 35-fold). This shows that single chemical entities and not just a complex mixture, such as G. biloba extract (Lau et al., 2011), can increase background luciferase activity. Previously, it was suggested that the reporter plasmid (pGL3-basic-CYP2B6-PBREM/XREM-luc) interacted with an endogenous receptor(s) present in HepG2 cells treated with G. biloba extract, resulting in an increase in background luciferase activity (Lau et al., 2011). Therefore, it is important to include all the background control groups in luciferase reporter gene assays. In a previous study, quercetin, kaempferol, and tamarixetin were reported to activate hCAR-SV24 (Li et al., 2009), whereas these chemicals had no effect in the present study. Possible reasons for the discrepancy may include the greater concentrations used previously (Li et al., 2009) and the inclusion of all the empty vector control groups in the present study.
Another conclusion in our study is that structural analogues of 3-hydroxyflavone activate hCAR in an isoform-selective manner. This is demonstrated by the activation of hCAR-WT, but not hCAR-SV23 or hCAR-SV24, by galangin, datiscetin, kaempferol, quercetin, isorhamnetin, and tamarixetin, as investigated at a concentration of 30 μM. Similarly, 3-hydroxyflavone at a concentration of 10 μM activated hCAR-WT, but not hCAR-SV23 or hCAR-SV24. The lack of response of these flavonols to the SPTV insertion is similar to that reported previously for phenobarbital (Lau et al., 2011), but different from that for di-isononyl phthalate, which activates hCAR-SV23 but not hCAR-WT (DeKeyser et al., 2011). As a comparison, the lack of response of these flavonols to the APYLT insertion is similar to that reported previously for DEHP (DeKeyser et al., 2011; Lau et al., 2011), but different from that for pheniramine, which activates hCAR-SV24 but not hCAR-WT (Dring et al., 2010). Overall, our data obtained with the 3-hydroxyflavone and its structural analogues support the notion that the SPTV and APYLT amino acid insertions modify the ligand activation profile of hCAR isoforms, such as hCAR-SV23, hCAR-SV24, and hCAR-WT (Dring et al., 2010; Anderson et al., 2011; Lau et al., 2011).
Flavonols activated each of the hCAR-SV23, hCAR-SV24, and hCAR-WT isoforms in a chemical-specific manner. Only 3-hydroxyflavone, but none of its structural analogues at the concentrations (≤30 μM) investigated in the present study, activated hCAR-SV23 and hCAR-SV24. Therefore, the addition of polar hydroxyl or methoxy group to the 3-hydroxyflavone scaffold interferes with the activation mechanism of hCAR-SV23 and hCAR-SV24. In the case of hCAR-WT, (i) both 3-hydroxyflavone and galangin increased hCAR-WT activity; (ii) galangin (no hydroxylation in ring B), datiscetin (mono-hydroxylated analogue in C2′), kaempferol (mono-hydroxylated analogue in C4′), and quercetin (di-hydroxylated analogue in C3′ and C4′) activated hCAR-WT, whereas morin (di-hydroxylated analogue in C2′ and C4′) and myricetin (tri-hydroxylated analogue in C3′, C4′, and C5′) had no effect on the receptor; (iii) quercetin (no methoxy substituent), isorhamnetin (methoxy group in C3′), and tamarixetin (methoxy group in C4′) increased hCAR-WT activity to a similar extent; and (iv) isorhamnetin increased hCAR-WT activity, but syringetin (C3′ and C5′ methoxy groups in ring B) had no effect. Taken together, the number of hydroxyl and methoxy groups did not abolish hCAR-WT activation, but the addition of hydroxyl and methoxy groups at the C2′ or C5′ position of the flavonol moiety appears to be unfavourable for the activation of hCAR-WT (Table 1).
Table 1.
Structure–activity relationship in the activation of hCAR-SV23, hCAR-SV24 and hCAR-WT by 3-hydroxyflavone and its structural analogues
| Flavonol | Ring A | Ring B | Activation of hCAR-SV23 | Activation of hCAR-SV24 | Activation of hCAR-WT | ||||
|---|---|---|---|---|---|---|---|---|---|
| R5 | R7 | R2′ | R3′ | R4′ | R5′ | ||||
| 3-Hydroxyflavone | H | H | H | H | H | H | Yes | Yes | Yes |
| Galangin | OH | OH | H | H | H | H | No | No | Yes |
| Datiscetin | OH | OH | OH | H | H | H | No | No | Yes |
| Kaempferol | OH | OH | H | H | OH | H | No | No | Yes |
| Morin | OH | OH | OH | H | OH | H | No | No | No |
| Quercetin | OH | OH | H | OH | OH | H | No | No | Yes |
| Isorhamnetin | OH | OH | H | OCH3 | OH | H | No | No | Yes |
| Tamarixetin | OH | OH | H | OH | OCH3 | H | No | No | Yes |
| Myricetin | OH | OH | H | OH | OH | OH | No | No | No |
| Syringetin | OH | OH | H | OCH3 | OH | OCH3 | No | No | No |
In conclusion, hCAR-SV23 and hCAR-SV24 can be activated by a mechanism that does not involve the LBD of the receptor or recruitment of the coactivators SRC-1, SRC-2, and SRC-3, as shown in our experiments with 3-hydroxyflavone. This chemical activated hCAR-SV23 and hCAR-SV24 in a manner distinct from the mode of activation of hCAR-SV23 by DEHP and of hCAR-SV24 by CITCO. Furthermore, 3-hydroxyflavone and its structural analogues activate hCAR in an isoform-selective and chemical-specific manner. The presence of SPTV in hCAR-SV23 and APYLT in hCAR-SV24 negated receptor activation by several of the flavonol analogues investigated in the present study. Overall, our study provides insight into a novel mode of ligand activation of hCAR-SV23 and hCAR-SV24.
Acknowledgments
This research was supported by the Canadian Institutes of Health Research [Grant MOP-84581]. T.K.H.C. received a Senior Scholar Award from the Michael Smith Foundation for Health Research. The authors thank Dr. Guixiang Yang for constructing the various plasmids.
Glossary
- CAR
constitutive androstane receptor
- CAS
Chemical Abstracts Service
- CITCO
6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime
- DEHP
di-(2-ethylhexyl)phthalate
- DMSO
dimethyl sulfoxide
- hCAR
human constitutive androstane receptor
- hRXRα
human retinoid X receptor alpha
- hSRC-1
human steroid receptor coactivator-1
- hSRC-2
human steroid receptor coactivator-2
- hSRC-3
human steroid receptor coactivator-3
- LBD
ligand-binding domain
- RXRα
retinoid X receptor alpha
- SRC
steroid receptor coactivator
- SV
splice variant
- TCPOBOP
1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene
- WT
wild type
Conflict of interest
None.
References
- Anderson LE, Dring AM, Hamel LD, Stoner MA. Modulation of constitutive androstane receptor (CAR) and pregnane X receptor (PXR) by 6-arylpyrrolo[2,1-d][1,5]benzothiazepine derivatives, ligands of peripheral benzodiazepine receptor (PBR) Toxicol Lett. 2011;202:148–154. doi: 10.1016/j.toxlet.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold KA, Eichelbaum M, Burk O. Alternative splicing affects the function and tissue-specific expression of the human constitutive androstane receptor. Nucl Recept. 2004;2:1–16. doi: 10.1186/1478-1336-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach SS, Ramsden R, Stoner MA, Verlinde C, Hassett C, Omiecinski CJ. Alternatively spliced isoforms of the human constitutive androstane receptor. Nucleic Acids Res. 2003;31:3194–3207. doi: 10.1093/nar/gkg419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach SS, Stoner MA, Su S, Omiecinski CJ. Retinoid X receptor-α-dependent transactivation by a naturally occurring structural variant of human constitutive androstane receptor (NR1I3) Mol Pharmacol. 2005;68:1239–1253. doi: 10.1124/mol.105.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach SS, DeKeyser JG, Stoner MA, Omiecinski CJ. CAR2 displays unique ligand binding and RXRα heterodimerization characteristics. Drug Metab Dispos. 2007;35:428–439. doi: 10.1124/dmd.106.012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Wolfram N, Kahl GF, Hirsch-Ernst KI. Transcriptional regulation of CYP2B1 induction in primary rat hepatocyte cultures: repression by epidermal growth factor is mediated via a distal enhancer region. Mol Pharmacol. 2004;65:172–180. doi: 10.1124/mol.65.1.172. [DOI] [PubMed] [Google Scholar]
- Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Burk O, Arnold KA, Nussler AK, Schaeffeler E, Efimova E, Avery BA, et al. Antimalarial artemisinin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol. 2005;67:1954–1965. doi: 10.1124/mol.104.009019. [DOI] [PubMed] [Google Scholar]
- Burk O, Piedade R, Ghebreghiorghis L, Fait JT, Nussler AK, Gil JP, et al. Differential effects of clinically used derivatives and metabolites of artemisinin in the activation of constitutive androstane receptor isoforms. Br J Pharmacol. 2012;167:666–681. doi: 10.1111/j.1476-5381.2012.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EJ, Jang YJ, Cha EY, Shin JG, Lee SS. Identification and characterization of novel alternative splice variants of human constitutive androstane receptor in liver samples of Koreans and Caucasians. Drug Metab Dispos. 2013;41:888–896. doi: 10.1124/dmd.112.049791. [DOI] [PubMed] [Google Scholar]
- DeKeyser JG, Stagliano MC, Auerbach SS, Prabhu KS, Jones AD, Omiecinski CJ. Di(2-ethylhexyl) phthalate is a highly potent agonist for the human constitutive androstane receptor splice variant CAR2. Mol Pharmacol. 2009;75:1005–1013. doi: 10.1124/mol.108.053702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKeyser JG, Laurenzana EM, Peterson EC, Chen T, Omiecinski CJ. Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor. Toxicol Sci. 2011;120:381–391. doi: 10.1093/toxsci/kfq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dring AM, Anderson LE, Qamar S, Stoner MA. Rational quantitative structure-activity relationship (RQSAR) screen for PXR and CAR isoform-specific nuclear receptor ligands. Chem Biol Interact. 2010;188:512–525. doi: 10.1016/j.cbi.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80. doi: 10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58:685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- Jinno H, Tanaka-Kagawa T, Hanioka N, Ishida S, Saeki M, Soyama A, et al. Identification of novel alternative splice variants of human constitutive androstane receptor and characterization of their expression in the liver. Mol Pharmacol. 2004;65:496–502. doi: 10.1124/mol.65.3.496. [DOI] [PubMed] [Google Scholar]
- Koike C, Moore R, Negishi M. Extracellular signal-regulated kinase is an endogenous signal retaining the nuclear constitutive active/androstane receptor (CAR) in the cytoplasm of mouse primary hepatocytes. Mol Pharmacol. 2007;71:1217–1221. doi: 10.1124/mol.107.034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba J, Lamba V, Schuetz E. Genetic variants of PXR (NR1I2) and CAR (NR1I3) and their implications in drug metabolism and pharmacogenetics. Curr Drug Metab. 2005;6:369–383. doi: 10.2174/1389200054633880. [DOI] [PubMed] [Google Scholar]
- Lamba JK. Pharmacogenetics of the constitutive androstane receptor. Pharmacogenomics. 2008;9:71–83. doi: 10.2217/14622416.9.1.71. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Yang G, Rajaraman G, Baucom CC, Chang TKH. Human pregnane X receptor agonism by Ginkgo biloba extract: assessment of the role of individual ginkgolides. J Pharmacol Exp Ther. 2010;335:771–780. doi: 10.1124/jpet.110.172338. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Yang G, Chang TKH. Isoform-selective activation of human constitutive androstane receptor by Ginkgo biloba extract: functional analysis of the SV23, SV24, and SV25 splice variants. J Pharmacol Exp Ther. 2011;339:704–715. doi: 10.1124/jpet.111.186130. [DOI] [PubMed] [Google Scholar]
- Li L, Chen T, Stanton JD, Sueyoshi T, Negishi M, Wang H. The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol. 2008;74:443–453. doi: 10.1124/mol.108.046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Stanton JD, Tolson AH, Luo Y, Wang H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm Res. 2009;26:872–882. doi: 10.1007/s11095-008-9788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem. 2003;278:17277–17283. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- Omiecinski CJ, Coslo DM, Chen T, Laurenzana EM, Peffer RC. Multi-species analyses of direct activators of the constitutive androstane receptor. Toxicol Sci. 2011;123:550–562. doi: 10.1093/toxsci/kfr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osabe M, Negishi M. Active ERK1/2 protein interacts with the phosphorylated nuclear constitutive active/androstane receptor (CAR; NR1I3), repressing dephosphorylation and sequestering CAR in the cytoplasm. J Biol Chem. 2011;286:35763–35769. doi: 10.1074/jbc.M111.284596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, et al. Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol. 2006;70:1925–1934. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]
- Ross J, Plummer SM, Rode A, Scheer N, Bower CC, Vogel O, et al. Human constitutive androstane receptor (CAR) and pregnane X receptor (PXR) support the hypertrophic but not the hyperplastic response to the murine nongenotoxic hepatocarcinogens phenobarbital and chlordane in vivo. Toxicol Sci. 2010;116:452–466. doi: 10.1093/toxsci/kfq118. [DOI] [PubMed] [Google Scholar]
- Savkur RS, Wu Y, Bramlett KS, Wang M, Yao S, Perkins D, et al. Alternative splicing within the ligand binding domain of the human constitutive androstane receptor. Mol Genet Metab. 2003;80:216–226. doi: 10.1016/j.ymgme.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Shindo S, Numazawa S, Yoshida T. A physiological role of AMP-activated protein kinase in phenobarbital-mediated constitutive androstane receptor activation and CYP2B induction. Biochem J. 2007;401:735–741. doi: 10.1042/BJ20061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek TA, Montoro P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J Chromatogr A. 2009;1216:2002–2032. doi: 10.1016/j.chroma.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, et al. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem. 2003;278:14146–14152. doi: 10.1074/jbc.M212482200. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Moore R, Sueyoshi T, Negishi M, LeCluyse E. Human constitutive androstane receptor mediates induction of CYP2B6 gene expression by phenytoin. J Biol Chem. 2004;279:29295–29301. doi: 10.1074/jbc.M400580200. [DOI] [PubMed] [Google Scholar]
- Wang YM, Ong SS, Chai SC, Chen T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol. 2012;8:803–817. doi: 10.1517/17425255.2012.685237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Yasuoka A, Kamei A, Kitagawa Y, Tateishi N, Tsuruoka N, et al. Dietary flavonoids activate the constitutive androstane receptor (CAR) J Agric Food Chem. 2010;58:2168–2173. doi: 10.1021/jf903711q. [DOI] [PMC free article] [PubMed] [Google Scholar]


