Abstract
BACKGROUND AND PURPOSE
Flavonols and terpene lactones are putatively responsible for the properties of Ginkgo biloba leaf extracts that relate to prevention and treatment of cardiovascular disease and cerebral insufficiency. Here, we characterized rat systemic and cerebral exposure to these ginkgo compounds after dosing, as well as the compounds’ pharmacokinetics.
EXPERIMENTAL APPROACH
Rats received single or multiple doses of ShuXueNing injection (prepared from GBE50 for intravenous administration) or GBE50 (a standardized extract of G. biloba leaves for oral administration). Brain delivery of the ginkgo compounds was assessed with microdialysis. Various rat samples were analysed using liquid chromatography/mass spectrometry.
KEY RESULTS
Slow terminal elimination features of the flavonols counterbalanced the influence of poor oral bioavailability on their systemic exposure levels, which also resulted in significant accumulation of the compounds in plasma during the subchronic treatment with ShuXueNing injection and GBE50. Unlike the flavonols, the terpene lactones had poor enterohepatic circulation due to their rapid renal excretion and unknown metabolism. The flavonol glycosides occurred as major forms in plasma after dosing with ShuXueNing injection, while the flavonol aglycone conjugates were predominant in plasma after dosing with GBE50. Cerebral exposure was negligible for the flavonols and low for the terpene lactones.
CONCLUSION AND IMPLICATIONS
Unlike the significant systemic exposure levels, the levels of cerebral exposure to the flavonols and terpene lactones are low. The elimination kinetic differences between the two classes of ginkgo compounds influence their relative systemic exposure levels. The information gained is relevant to linking ginkgo administration to the medicinal effects.
Keywords: Ginkgo biloba, GBE50, ShuXueNing injection, flavonols, terpene lactones, systemic exposure, cerebral exposure, pharmacokinetics, route of administration, PK markers
Introduction
Products of Ginkgo biloba leaf extracts (ginkgo extracts) have become widely used botanical medicines and dietary supplements, especially for the prevention and treatment of cardiovascular disease and cerebral insufficiency (Kleijnen and Knipschild, 1992; Zhou et al., 2004). Impaired blood flow is a common feature of many cardiovascular and cerebrovascular diseases. EGb761, a standardized ginkgo extract, stimulates up-regulation and activation of endothelial nitric oxide synthase in human umbilical vein endothelial cells, which leads to enhancement of nitric oxide generation for vasodilatation (Koltermann et al., 2007). Two-hour intravenous (i.v.) infusion of Ginaton solution, a standardized injectable ginkgo extract, improves coronary blood flow in patients with coronary artery disease (Wu et al., 2007), and oral (p.o.) ingestion of ginkgo extract for 4 weeks increases cerebral blood flow in healthy volunteers (around 60 years of age) (Mashayekh et al., 2011). In addition, EGb761 attenuates oxidized low density lipoprotein-induced functional damage in human umbilical vein endothelial cells (Ou et al., 2009), inhibits endothelial inflammation (Chen et al., 2003; 2011) and reduces lipid accumulation in foam cells (Tsai et al., 2010). The ginkgo extract also decreases blood viscosity and viscoelasticity, reduces erythrocyte malondialdehyde levels and promotes erythrocyte deformability (Huang et al., 2013). The overall therapeutic relevance of these pharmacological properties most likely depends on bioavailability of the bioactive ginkgo ingredients in the systemic circulation. For this reason, it is imperative to investigate the systemic exposure to and plasma pharmacokinetics of ginkgo compounds after administration of the botanical extract.
The neuroprotective effects of ginkgo extracts have been documented in several studies. EGb761 exerts in vitro antioxidative effect, inhibition of amyloid-β aggregation and attenuation of mitochondrion-induced apoptosis (Luo et al., 2002; Longpré et al., 2006). Administration of the ginkgo extract via p.o. normalizes reduction of neurogenesis in mouse hippocampus (Tchantchou et al., 2007). The neuroprotective action is dependent on heme oxygenase 1 in ischaemic reperfusion brain injury (Saleem et al., 2008). However, it remains open to question whether the ginkgo constituents or their metabolites reach the brain in concentrations high enough for the neuroprotective effects.
The flavonols and terpene lactones are believed to be responsible for most of the pharmacological effects of ginkgo extract (DeFeudis and Drieu, 2000; Drieu and DeFeudis, 2000; Tchantchou et al., 2009; Yoshitake et al., 2010). Recently, we investigated the intestinal absorption and presystemic elimination of a variety of chemical constituents present in oral GBE50, another standardized extract of G. biloba leaves (Li et al., 2012). As a result, many unchanged flavonol glycosides, unchanged terpene lactones and the flavonol aglycone conjugates were detected in the bloodstreams of dosed rats, while the other classes of ginkgo compounds (including biflavones, flavones, isoflavones, flavanols and carboxylic acids) occurred at negligible plasma levels. Full pharmacokinetic (PK) information about systemic and cerebral exposure to ginkgo flavonols and terpene lactones is critically important in establishing a link between administration of the extract and its pharmacological properties. Earlier human and animal PK studies focused on measurement of oral bioavailability (F) of the ginkgo terpene lactones (Bhattaram et al., 2002; Biber, 2003; Rossi et al., 2009), while PK assessments of ginkgo flavonols have been relatively scarce (Chen et al., 2010). These PK studies have not fully characterized the pharmacokinetics of the ginkgo compounds. More recently, brain penetration of ginkgo flavonols and terpene lactones has been studied in rats by measurement of the concentrations of these substances in brain tissue homogenate (Rossi et al., 2009; Rangel-Ordóñez et al., 2010) and by microdialysis (Lang et al., 2010). However, the data have been inconclusive. The objective of the current study was to characterize and compare systemic and cerebral exposure to flavonols and terpene lactones and to investigate influence of the route of administration on the pharmacokinetics of these ginkgo compounds. The levels of systemic exposure to flavonols and terpene lactones were found to be significant in rats, but their cerebral exposure levels were not. The two classes of ginkgo compounds had different elimination kinetics.
Methods
A detailed description of experimental procedures and materials is provided in the Supporting Information Appendix S1 Supplemental Methods section, which is available online.
GBE50 and ShuXueNing injection
GBE50, a standardized proprietary extract of G. biloba leaves, was a 200:3 solid extract manufactured by Shanghai XingLing Sci. & Tech. Pharmaceuticals (Shanghai, China). ShuXueNing injection was an injectable solution from GBE50 manufactured by Shanghai Asia Pioneer Pharmaceuticals (Shanghai, China), each millilitre of which was prepared from 3.5 mg of GBE50. Table 1 shows the content levels of flavonols and terpene lactones present in GBE50 and ShuXueNing injection. Figure 1 depicts the chemical structures of flavonols and terpene lactones.
Table 1.
Content levels of ginkgo compounds in ShuXueNing injection or GBE50
| Compound | ShuXueNing injection (μmol·mL−1) | GBE50 (μmol·10 mg−1) |
|---|---|---|
| Bilobalide (1) | 0.0002 | 0.954 |
| Ginkgolide A (2) | 0.289 | 0.551 |
| Ginkgolide B (3) | 0.100 | 0.325 |
| Ginkgolide C (4) | 0.184 | 0.316 |
| Ginkgolide J (5) | 0.091 | 0.105 |
| Quercetin 3-O-dirhamnosyl-glucoside (10) | 0.072 | 0.270 |
| Quercetin 3-O-(p-coumaroyl)-glucosyl-O-rhamnoside (11) | 0.096 | 0.617 |
| Quercetin 3-O-rhamnosyl-O-glucoside (12) | 0.174 | 0.660 |
| Quercetin 3-O-glucosyl-O-rhamnoside (13) | 0.065 | 0.354 |
| Quercetin (16) | 0.003 | 0.088 |
| t-Quercetin | 0.397 | 2.149 |
| Kaempferol 3-O-dirhamnosyl-glucoside (19) | 0.089 | 0.425 |
| Kaempferol 3-O-(p-coumaroyl)-glucosyl-O-rhamnoside (20) | 0.080 | 0.707 |
| Kaempferol 3-O-rhamnosyl-O-glucoside (21) | 0.162 | 0.655 |
| Kaempferol 3-O-glucosyl-O-rhamnoside (22) | 0.072 | 0.474 |
| Kaempferol (26) | 0.004 | 0.095 |
| t-Kaempferol | 0.441 | 2.378 |
| Isorhamnetin 3-O-dirhamnosyl-glucoside (27) | 0.002 | 0.018 |
| Isorhamnetin 3-O-rhamnosyl-O-glucoside (29) | 0.088 | 0.559 |
| Isorhamnetin 3-O-glucosyl-O-rhamnoside (30) | 0.0003 | 0.004 |
| Isorhamnetin (32) | 0.001 | 0.014 |
| t-Isorhamnetin | 0.127 | 0.665 |
The ginkgo compounds are given ID numbers in parentheses, which are consistent with those for GBE50 described in our earlier publication (Li et al., 2012). Hydrochloric acid-based hydrolysis was applied to the sample to convert the flavonol glycosides present into their aglycone forms before measurement of the content levels of t-quercetin, t-kaempferol, and t-isorhamnetin in ShuXueNing injection or GBE50.
Figure 1.
Chemical structures of ginkgo flavonols and terpene lactones.
Experimental animals
All rat studies were conducted in compliance with the Guidance for Ethical Treatment of Laboratory Animals (Ministry of Science and Technology of China, 2006; http://www.most.gov.cn/fggw/zfwj/zfwj2006) and the experimental protocols were approved by the Institutional Animal Care and Use Committee at the Shanghai Institute of Materia Medica (Shanghai, China). For blood sampling, the femoral arteries of male Sprague-Dawley rats (260–300 g) were cannulated under pentobarbital anaesthesia. Other rats received bile duct-cannulation surgery for bile collection. After surgery, the femoral-artery-cannulated (FAC) rats and bile-duct-cannulated (BDC) rats were housed individually and allowed to regain the preoperative body weights before use.
Plasma PK studies
The FAC rats were randomly divided into three groups of four rats each to assess the plasma pharmacokinetics of flavonols and terpene lactones after a 15 min i.v. infusion of ShuXueNing injection at 1, 2 or 4 mL·kg−1. Serial blood samples (∼110 μL; 0, 5, 15 and 30 min and 1, 2, 4, 6, 8, 11 and 24 h) were collected and heparinized for plasma preparation. A similar experiment was also performed in FAC rats that received a p.o. dose of GBE50 (suspended in 0.5% w/v sodium carboxymethycellulose) at 10, 30 or 90 mg·kg−1 via gavage. In addition, a subchronic PK study was implemented for seven consecutive days. Four FAC rats received ShuXueNing injections at 1 mL·kg−1·day−1 and another four FAC rats received GBE50 at 30 mg·kg−1·day−1. Serial blood samples were collected on days 1 and 7.
Brain microdialysis
A brain microdialysis study was performed in FAC rats according to a protocol modified from an earlier method (Sun et al., 2009). After a 15 min i.v. infusion of ShuXueNing injection (4 mL·kg−1) or a p.o. dose of GBE50 (90 mg·kg−1), the dialysate samples were collected at 10 min intervals for 6 h. Blood samples were collected at 0, 5, 15 and 30 min and 1, 2, 4 and 6 h after dosing. The brain extracellular fluid (bECF) concentration was calculated using the following equation:
| (1) |
where CbECF is the bECF concentration; Cd is the measured concentration in the dialysate sample. Rin vivo is the in vivo recovery by retrodialysis, which was 16%, 13%, 11%, 9% and 11% for bilobalide, ginkgolides A, B, C and J respectively.
Excretion studies
Rats that had not undergone any surgery were randomly assigned to two groups (four rats/group) and housed singly in rat metabolic cages. Urine and faecal samples were collected before and 0–8, 8–24 and 24–32 h after a 15 min i.v. infusion dose of ShuXueNing injection (4 mL·kg−1) or p.o. dose of GBE50 (90 mg·kg−1).
Three BDC rats received a 15 min i.v. infusion dose of ShuXueNing injection (4 mL·kg−1), and another three BDC rats received a p.o. dose of GBE50 (90 mg·kg−1). Bile was collected before and 0–10, 10–20, 20–40 and 40–50 min and 0.8–1.2, 1.2–1.8, 1.8–2.2, 2.2–3, 3–3.8, 3.8–4.2, 4.2–5.8, 5.8–6.2, 6.2–7.8, 7.8–8.2, 8.2–10.8, 10.8–11.2, 11.2–23.8 and 23.8–24.2 h after dosing.
Tissue distribution study
Rats under isoflurane anaesthesia were killed by bleeding from the abdominal aorta at 0, 15 (i.v. only), 30 min, 1 (i.v. only), 2 (p.o. only), 4 and 8 (p.o. only) h (three rats/time point) after a 15 min i.v. infusion dose of ShuXueNing injection (4 mL·kg−1) or a p.o. dose of GBE50 (90 mg·kg−1). The heart, lungs, brain, liver and kidneys were excised and homogenized in fourfold volumes of ice-cold saline.
Analysis of ginkgo flavonols and terpene lactones in biological samples
A Thermo Fisher TSQ Quantum mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) interfaced via an electrospray ionization probe with an Agilent 1100 liquid chromatograph (Agilent Technologies, Waldbronn, Germany) was used to measure concentrations of the ginkgo flavonols and terpene lactones in biosamples. Analytical assays were performed as described by Zhao et al. (2008). Hydrochloric acid (HCl; 4 M) was used to treat the rat samples to release the flavonol aglycones from the glycosides and conjugated metabolites. Accordingly, the measured flavonol levels are expressed as concentrations of total quercetin (t-quercetin), total kaempferol (t-kaempferol) and total isorhamnetin (t-isorhamnetin). To assess brain penetration, the brain dialysate samples were centrifuged at 16 060× g for 10 min. The high-speed, rapid ultrafiltration method described by Guo et al. (2006) was used to isolate unbound ginkgo compounds in plasma for measurement of unbound plasma concentration and plasma protein binding. The glycosides and metabolites of ginkgo flavonols in rat plasma, bile and urine were measured and profiled using an AB-SCIEX API 4000 Q Trap mass spectrometer (AB SCIEX, Toronto, Canada) interfaced via a Turbo V ion source with a Waters Acquity UPLC separation module (Waters, Milford, MA, USA).
Assessment of blood to plasma ratios
Rat blood-to-plasma ratios (B/P ratios) were determined for terpene lactones (bilobalide and ginkgolides A, B, C and J), flavonol glycosides (10–13, 19–22, 27, 29 and 30) and flavonol aglycone conjugates (M16G-1–M16G-4, M16S-3, M26G-1–M26G-3, M32G-1–M32G-3 and M32S-2). Blood samples were collected from rats that received a 15 min i.v. infusion dose of ShuXueNing injection (4 mL·kg−1) and those that received a p.o. dose of GBE50 (90 mg·kg−1). The blood samples were centrifuged to yield plasma and erythrocyte fractions. The B/P ratio was calculated using the following equation:
| (2) |
where CE and CP are erythrocyte and plasma concentrations respectively. The mean hematocrit value of the rats used was 0.44 ± 0.02.
PK data analysis
Plasma PK parameters were estimated by a noncompartmental method using a Thermo Kinetica software package (Thermo Fisher Scientific, Philadelphia, PA, USA). Dose proportionality assessment was conducted by the regression of log-transformed data (the power model) with criteria that were calculated according to the method described by Smith et al. (2000). To assess the extent of brain penetration, a Kp value was calculated using the following equation:
| (3) |
where AUCu,p is the area under the unbound plasma concentration-time curve.
Chemicals and reagents
Pure ginkgo compounds were obtained from the National Institutes for Food and Drug Control (Beijing, China), Tauto Biotech (Shanghai, China) and Extrasynthese (Genay, France). Organic solvents and other reagents were obtained from Sinopharm Chemical Reagent (Shanghai, China). Pentobarbital was obtained from Shanghai Westang Biotechnology (Shanghai, China).
Results
Plasma pharmacokinetics of ginkgo flavonols and terpene lactones after acute or subchronic daily administration of ShuXueNing injection
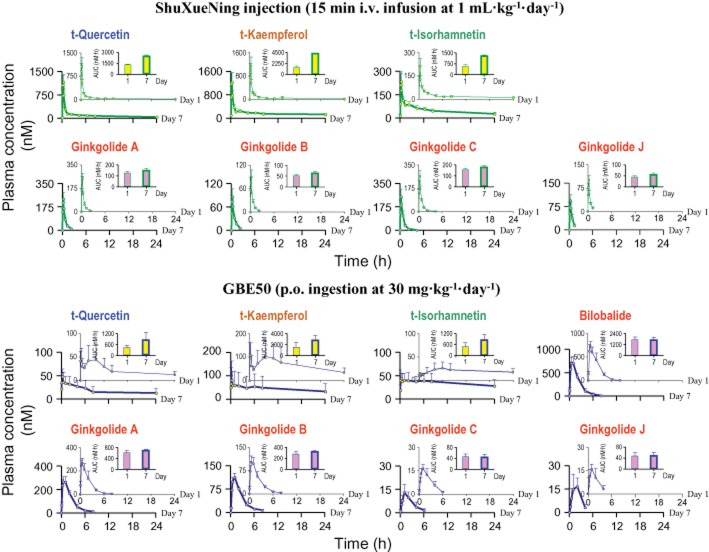
Total flavonol levels in HCl-hydrolysed rat plasma samples were measured for 24 h after a 15 min i.v. infusion dose of ShuXueNing injection (Figure 2). The plasma concentrations of t-quercetin, t-kaempferol and t-isorhamnetin decreased in a biphasic fashion with first mean half-lives (t1/2-(1)) of 1.3–3.1 h and second half-lives (t1/2-(2)) of 11.6–30.2 h (Table 2). Significant accumulation of the total flavonols in plasma was observed in the rats that received the subchronic treatment with ShuXueNing injection (1 mL·kg−1·day−1; Figure 3). This indicated that the AUC0-24h values of day 7 were 1.9–3.1 times greater than those of day 1. The mean CLtot,p values were 0.2–0.5 L·h−1·kg−1. Plasma t-quercetin was eliminated ∼2-times more rapidly than plasma t-isorhamnetin because, at least in part, of methylation of quercetin compounds into the associated isorhamnetin counterparts (Li et al., 2012). The VSS values were 4- to 14-fold greater than the volume of rat total body water (Davies and Morris, 1993).
Figure 2.
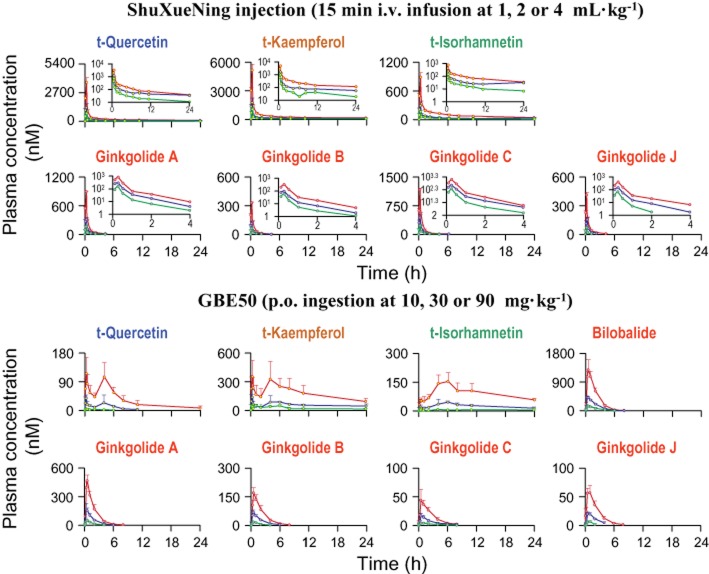
Plasma concentrations of total flavonols (circles filled in yellow) and terpene lactones (circles filled in pink) over time after a 15 min i.v. infusion dose of ShuXueNing injection (upper panel) or a p.o. dose of GBE50 (lower panel) in rats. The test dose levels of ShuXueNing injection included 1 (green curves), 2 (blue curves) and 4 mL·kg−1 (red curves), and those of GBE50 were 10 (green curves), 30 (blue curves) and 90 mg·kg−1 (red curves). The inserts are the associated semilogarithmic plots of the plasma concentrations of ginkgo compounds over time after administration of ShuXueNing injection.
Table 2.
Plasma PK parameters of total flavonols and terpene lactones after an i.v. infusion dose of ShuXueNing injection in rats
| PK parameter | t-Quercetin | t-Kaempferol | t-Isorhamnetin | Ginkgolide A | Ginkgolide B | Ginkgolide C | Ginkgolide J |
|---|---|---|---|---|---|---|---|
| ShuXueNing injection (15 min i.v. infusion at 1 mL·kg−1) | |||||||
| C15min (nM) | 732 ± 107 | 1022 ± 186 | 163 ± 23 | 161 ± 25 | 64.5 ± 6.6 | 223 ± 29 | 68.4 ± 6.5 |
| AUC0-t (h·nM) | 687 ± 125 | 1116 ± 192 | 379 ± 57 | 72.8 ± 10.9 | 31.7 ± 4.6 | 114 ± 11 | 28.1 ± 4.4 |
| MRT (h) | 14.3 ± 4.4 | 15.0 ± 9.8 | 22.3 ± 5.2 | 0.46 ± 0.20 | 0.63 ± 0.08 | 0.67 ± 0.09 | 0.23 ± 0.05 |
| t1/2-(1) (h) | 1.86 ± 0.19 | 1.34 ± 0.11 | 2.91 ± 0.46 | 0.62 ± 0.21 | 0.77 ± 0.06 | 0.92 ± 0.07 | 0.31 ± 0.04 |
| t1/2-(2) (h) | 14.3 ± 3.8 | 21.5 ± 19.7 | 18.5 ± 4.2 | N.A. | N.A. | N.A. | N.A. |
| CLtot,p (L·h−1·kg−1) | 0.46 ± 0.08 | 0.45 ± 0.24 | 0.23 ± 0.04 | 3.85 ± 0.61 | 2.96 ± 0.42 | 1.63 ± 0.13 | 3.40 ± 0.54 |
| VSS (L·kg−1) | 6.55 ± 1.68 | 8.42 ± 3.75 | 4.99 ± 1.20 | 2.20 ± 0.72 | 2.22 ± 0.27 | 1.30 ± 0.17 | 1.19 ± 0.15 |
| ShuXueNing injection (15 min i.v. infusion at 2 mL·kg−1) | |||||||
| C15min (nM) | 1326 ± 275 | 1785 ± 420 | 293 ± 73 | 331 ± 28 | 120 ± 16 | 438 ± 72 | 154 ± 25 |
| AUC0-t (h·nM) | 1660 ± 266 | 2400 ± 401 | 927 ± 77 | 185 ± 17 | 72.5 ± 7.4 | 298 ± 16 | 87.3 ± 11.1 |
| MRT (h) | 23.9 ± 11.0 | 32.6 ± 4.0 | 25.4 ± 4.0 | 0.54 ± 0.04 | 0.62 ± 0.01 | 0.71 ± 0.10 | 0.41 ± 0.03 |
| t1/2-(1) (h) | 1.62 ± 0.19 | 1.86 ± 0.31 | 2.88 ± 0.45 | 0.73 ± 0.05 | 0.69 ± 0.01 | 0.88 ± 0.07 | 0.47 ± 0.09 |
| t1/2-(2) (h) | 20.8 ± 10.3 | 27.4 ± 4.2 | 18.0 ± 2.6 | N.A. | N.A. | N.A. | N.A. |
| CLtot,p (L·h−1·kg−1) | 0.32 ± 0.08 | 0.31 ± 0.08 | 0.29 ± 0.02 | 3.05 ± 0.20 | 2.68 ± 0.10 | 1.21 ± 0.08 | 2.09 ± 0.33 |
| VSS (L·kg−1) | 7.33 ± 2.04 | 10.1 ± 1.4 | 2.78 ± 0.21 | 2.01 ± 0.03 | 2.00 ± 0.09 | 1.01 ± 0.15 | 1.11 ± 0.12 |
| ShuXueNing injection (15 min i.v. infusion at 4 mL·kg−1) | |||||||
| C15min (nM) | 3607 ± 426 | 5113 ± 589 | 881 ± 86 | 884 ± 11 | 330 ± 8 | 1159 ± 40 | 407 ± 27 |
| AUC0-t (h·nM) | 3274 ± 482 | 6275 ± 330 | 2087 ± 240 | 429 ± 38 | 174 ± 20 | 622 ± 106 | 211 ± 19 |
| MRT (h) | 10.5 ± 4.2 | 34.7 ± 17.3 | 19.3 ± 4.6 | 0.57 ± 0.09 | 0.60 ± 0.08 | 0.54 ± 0.03 | 0.56 ± 0.06 |
| t1/2-(1) (h) | 1.91 ± 0.26 | 1.80 ± 0.13 | 3.13 ± 0.60 | 0.77 ± 0.09 | 0.66 ± 0.05 | 0.78 ± 0.07 | 0.69 ± 0.03 |
| t1/2-(2) (h) | 11.6 ± 3.3 | 30.2 ± 14.6 | 16.2 ± 3.7 | N.A. | N.A. | N.A. | N.A. |
| CLtot,p (L·h−1·kg−1) | 0.44 ± 0.13 | 0.25 ± 0.08 | 0.19 ± 0.04 | 2.75 ± 0.17 | 2.32 ± 0.19 | 1.24 ± 0.17 | 1.78 ± 0.12 |
| VSS (L·kg−1) | 4.26 ± 0.75 | 7.84 ± 2.73 | 3.56 ± 0.44 | 1.92 ± 0.30 | 1.68 ± 0.28 | 0.83 ± 0.07 | 1.22 ± 0.11 |
Because of its low content level in ShuXueNing injection (Table 1), a 15 min i.v. infusion dose of bilobalide at 1 mg·kg−1 was given to FAC rats to obtain the plasma PK parameters of the compound. The C15min, AUC0-t, MRT, t1/2, CLtot,p, and VSS value of bilobalide were 2696 ± 154 nM, 1619 ± 35 h·nM, 0.80 ± 0.05 h, 0.87 ± 0.06 h, 1.89 ± 0.04 L·h−1·kg−1 and 1.51 ± 0.11 L·kg−1 respectively.
Figure 3.
Plasma concentrations of total flavonols (circles filled in yellow) and terpene lactones (circles filled in pink) over time on day 1 (thin green and blue curves) and day 7 (thick green and blue curves) in rats given subchronic treatment with ShuXueNing injection (1 mL·kg−1·day−1; 15 min i.v. infusion; green curves) or GBE50 (30 mg·kg−1·day−1; p.o. ingestion; blue curves). The upper and smaller inserts for the ginkgo compounds show the comparative AUC0-t values for day1 and day 7.
Plasma ginkgolides A, B, C and J were monitored up to 4 h after dosing (Figure 2), while bilobalide was detected at very low plasma levels because of its low concentration in the injection. The rat plasma concentrations of the ginkgolides decreased rapidly (mean t1/2 values, 0.3–0.9 h). No accumulation of plasma ginkgolides was observed in rats during the subchronic treatment with ShuXueNing injection (Figure 3). The mean CLtot,p values of ginkgolides (1.2–3.8 L·h−1·kg−1) were greater than those of the total flavonols (0.2–0.5 L·h−1·kg−1). The VSS values of the terpene lactones (0.8–2.2 L·kg−1; smaller than those of the flavonols) were within the range of the total body water by volume, suggesting that the compounds were roughly evenly distributed throughout the rat blood and tissues. The plasma AUC0-24h of the total flavonols and the AUC0-4h of the ginkgolides increased in a ShuXueNing injection dose-dependent manner, and the results of the assessment of the dose–exposure relationship are summarized in Table 3.
Table 3.
Summary of results from dose proportionality assessment
| Compound | r | P | Slope (90% CI) | Conclusion |
|---|---|---|---|---|
| AUC0-24h for the total flavonols and AUC0-6h for the terpene lactones | ||||
| Dose range of ShuXueNing injection (15 min i.v. infusion), 1–4 mL·kg−1 | ||||
| t-Quercetin | 0.976 | 3.04 × 10−7 | 1.105 (0.954–1.257) | Inconclusive |
| t-Kaempferol | 0.981 | 9.60 × 10−8 | 1.232 (1.084–1.380) | Inconclusive |
| t-Isorhamnetin | 0.987 | 1.81 × 10−8 | 1.167 (1.051–1.282) | Inconclusive |
| Ginkgolide A | 0.990 | 4.97 × 10−9 | 1.236 (1.130–1.341) | Inconclusive |
| Ginkgolide B | 0.984 | 1.14 × 10−9 | 1.171 (1.086–1.256) | Inconclusive |
| Ginkgolide C | 0.988 | 1.09 × 10−8 | 1.201 (1.089–1.314) | Inconclusive |
| Ginkgolide J | 0.986 | 2.24 × 10−8 | 1.450 (1.302–1.597) | Nonlinear |
| AUC0-24h for the total flavonols and AUC0-8h for the terpene lactones | ||||
| Dose range of GBE50 (p.o.), 10–90 mg·kg−1 | ||||
| t-Quercetin | 0.973 | 4.64 × 10−7 | 1.668 (1.428–1.908) | Nonlinear |
| t-Kaempferol | 0.985 | 1.96 × 10−7 | 1.153 (1.022–1.284) | Inconclusive |
| t-Isorhamnetin | 0.988 | 1.48 × 10−8 | 1.582 (1.429–1.735) | Nonlinear |
| Bilobalide | 0.988 | 1.14 × 10−12 | 0.978 (0.945–1.011) | Linear |
| Ginkgolide A | 0.990 | 6.52 × 10−9 | 1.028 (0.938–1.119) | Inconclusive |
| Ginkgolide B | 0.986 | 2.58 × 10−8 | 1.037 (0.930–1.144) | Inconclusive |
| Ginkgolide C | 0.973 | 5.04 × 10−7 | 0.796 (0.680–0.911) | Inconclusive |
| Ginkgolide J | 0.974 | 4.11 × 10−7 | 1.376 (1.181–1.572) | Nonlinear |
Critical intervals were 0.839–1.161 for the plasma data with ShuXueNing injection and 0.898–1.102 for those with GBE50. The term ‘r’ denotes the correlation coefficient. Correlations were statistically significant with a ‘P’ value of <0.05. The term ‘linear’ was concluded if the 90% confidence interval (90% CI) for slope was contained completely within the critical interval; ‘inconclusive’ was concluded if the 90% CI lay partly within the critical interval; ‘nonlinear’ was concluded if the 90% CI was entirely outside the critical interval.
Supplementary assessments were made to foster understanding the metabolic profiles of total flavonols in rats and the distribution of the ginkgo compounds in the blood. The quercetin glycosides 10, 11, 12 and 13, accounting for t-quercetin, were measured predominantly in a pooled rat plasma sample taken 15 min after administration of ShuXueNing injection (Figure 4). The glucuronidated quercetin M16G-4 and the quercetin sulfate M16S-3 were detected in a pooled plasma sample taken 6 h after dosing. t-Kaempferol occurred mainly as glycosides 19, 20, 21 and 22, and t-isorhamnetin occurred mainly as glycosides 27, 29 and 30 in the 15 min plasma sample. The kaempferol glucuronides M26G-1 and M26G-2, the isorhamnetin glucuronide M32G-3 and the isorhamnetin sulfate M32S-2 were measured in the 6 h plasma sample. The B/P ratios of these flavonol glycosides and conjugated metabolites were around 0.5, suggesting that these compounds were confined to the plasma. The B/P ratios of ginkgolides C, J, B and A and bilobalide were 0.72, 0.74, 0.93, 1.12 and 1.26 respectively, which were compound membrane permeability dependent. The data suggested that these ginkgo compounds distributed into both the plasma and the erythrocytes. In addition, 36–71% of the flavonol glycosides and aglycone conjugates were bound to rat plasma protein, and the bound terpene lactones in plasma accounted for 67–83%.
Figure 4.
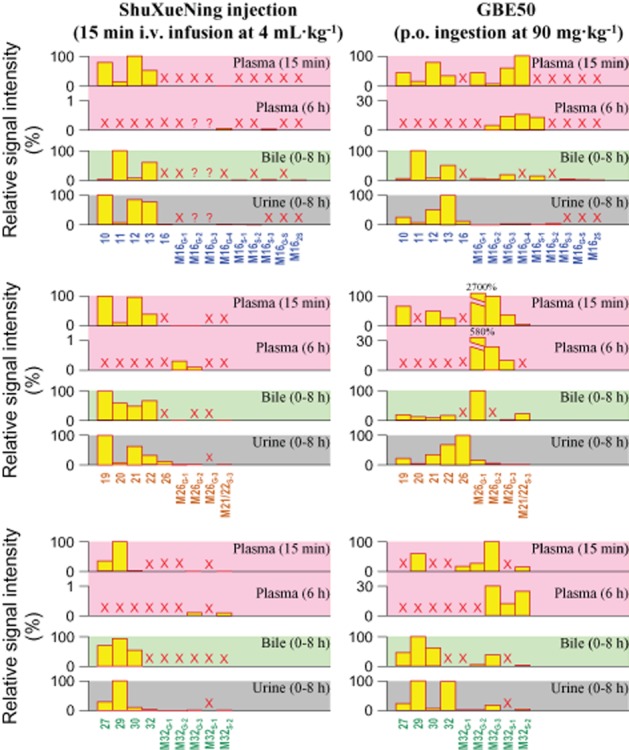
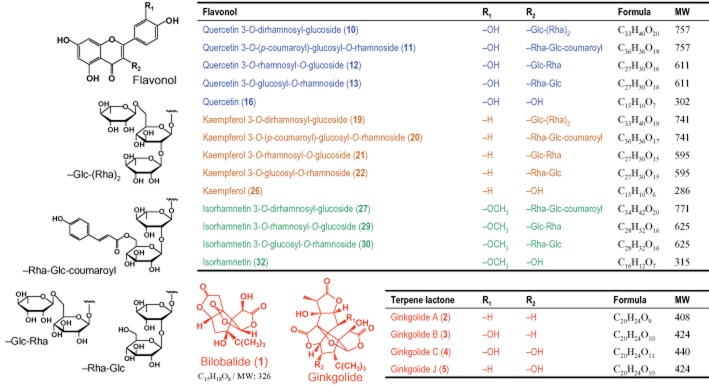
Detection of flavonol glycosides and their metabolites in plasma (pink plots), bile (light green plots), and urine (gray plots) after a 15 min i.v. infusion dose of ShuXueNing injection (4 mL·kg−1) or a p.o. dose of GBE50 (90 mg·kg−1) in rats. The names of the flavonol glycosides 10–13, 19–22, 27, 29 and 30 and of the flavonol aglycones 16, 26 and 32, are given in Table 1. The conjugated metabolites M16G-1–M16G-4, M16S-1–M16S-3, M16G-S, M162S, M26G-1–M26G-3, M21/22S-3, M32G-1–M32G-3 and M32S-1/M32S-2 were quercetin glucuronide, quercetin sulfate, glucuronidated quercetin sulfate, quercetin disulfate, kaempferol glucuronide, kaempferol 3-O-rhamnosyl-O-glucoside/kaempferol 3-O-glucosyl-O-rhamnoside sulfate, isorhamnetin glucuronide and isorhamnetin sulfate respectively. M16G-1 and M26G-1 are quercetin-3-O-glucuronide and kaempferol-3-O-glucuronide respectively. The symbol ‘×’ (in red) indicates that no substance was detected in the sample. The symbol ‘?’ (in red) indicates that the substance was only detected once.
Plasma pharmacokinetics of ginkgo flavonols and terpene lactones after acute or subchronic daily ingestion of GBE50
The total ginkgo flavonols were monitored in all the rat plasma samples for 24 h after a p.o. dose of GBE50. The plasma concentration-time curves of total flavonols were bimodal with the first peak concentrations (Cmax-(1)) occurring 15 min and the second ones (Cmax-(2)) appearing 4–6 h after dosing (Figure 2). Oral F values of the total flavonols were low, 0.5–2%, 7–9% and 3–9% for t-quercetin, t-kaempferol and t-isorhamnetin respectively. As with subchronic treatment with ShuXueNing injection, accumulation of the total flavonols in plasma was observed in rats that received subchronic treatment with oral GBE50 (30 mg·kg−1·day−1). This was indicative of the AUC0-24h values of day 7 being 1.8–1.9-times greater than those of day 1 (Figure 3).
The terpene lactones were also monitored in the rat plasma samples up to 8 h after dosing (Figure 2). Bilobalide had higher plasma concentrations than the other terpene lactones. Table 4 summarizes the plasma PK data of ginkgo compounds after p.o. administration of GBE50 at 10–90 mg·kg−1. The plasma terpene lactones reached their Cmax within 0.5–1 h of administration. The mean oral F of all terpene lactones except for ginkgolide C ranged from 33 to 64%. That of ginkgolide C was 5 to 10%. The plasma AUC values of total flavonols and terpene lactones increased with the increased dose of GBE50 (10–90 mg·kg−1). The results of dose–exposure relationship assessment for dosing with GBE50 are summarized in Table 3.
Table 4.
Plasma PK parameters of total flavonols and terpene lactones after a p.o. dose of GBE50 in rats
| PK parameter | t-Quercetin | t-Kaempferol | t-Isorhamnetin | Bilobalide | Ginkgolide A | Ginkgolide B | Ginkgolide C | Ginkgolide J |
|---|---|---|---|---|---|---|---|---|
| GBE50 (p.o., 10 mg·kg−1) | ||||||||
| Tpeak-(1) (h) | 0.25 | 1.00 | 0.50 | 0.25–0.50 | 0.25–0.50 | 0.50 | 0.50–1.00 | 0.50–1.00 |
| Cmax-(1) (nM) | 5.24 ± 1.34 | 49.2 ± 4.1 | 5.47 ± 1.48 | 153 ± 10 | 53.9 ± 5.3 | 20.1 ± 3.2 | 6.25 ± 0.85 | 7.40 ± 1.27 |
| Tpeak-(2) (h) | 2.00 | 6.00 | 4.00 | N.A. | N.A. | N.A. | N.A. | N.A. |
| Cmax-(2) (nM) | 3.76 ± 1.25 | 46.6 ± 9.3 | 9.51 ± 1.94 | N.A. | N.A. | N.A. | N.A. | N.A. |
| AUC0-t (h·nM) | 19.4 ± 3.4 | 321 ± 39 | 68.0 ± 10.0 | 318 ± 26 | 88.5 ± 15.6 | 38.7 ± 9.2 | 20.3 ± 5.0 | 9.17 ± 1.10 |
| t1/2 (h) | – | – | – | 0.90 ± 0.11 | 1.40 ± 0.31 | 0.93 ± 0.12 | 2.97 ± 0.32 | 1.42 ± 0.85 |
| MRT (h) | 12.8 ± 7.7 | 12.3 ± 8.3 | 8.14 ± 4.32 | 1.84 ± 0.12 | 1.86 ± 0.24 | 1.76 ± 0.07 | 4.24 ± 0.20 | 2.35 ± 1.22 |
| F (%)a | 0.48 | 6.58 | 2.62 | 63.5 | 51.5 | 32.9 | 9.91 | 32.9 |
| GBE50 (p.o., 30 mg·kg−1) | ||||||||
| Tpeak-(1) (h) | 0.25 | 0.25 | 0.25 | 0.25–0.50 | 0.25–0.50 | 0.50 | 0.50–1.00 | 0.50–1.00 |
| Cmax-(1) (nM) | 39.2 ± 11.2 | 136 ± 82 | 20.0 ± 9.8 | 427 ± 34 | 171 ± 43 | 69.4 ± 11.7 | 15.1 ± 3.6 | 21.0 ± 3.0 |
| Tpeak-(2) (h) | 4.00 | 6.00 | 6.00 | N.A. | N.A. | N.A. | N.A. | N.A. |
| Cmax-(2) (nM) | 24.5 ± 25.2 | 83.4 ± 8.8 | 45.6 ± 4.7 | N.A. | N.A. | N.A. | N.A. | N.A. |
| AUC0-t (h·nM) | 211 ± 103 | 1286 ± 116 | 584 ± 113 | 936 ± 20 | 304 ± 49 | 140 ± 19 | 46.2 ± 5.1 | 43.5 ± 5.6 |
| t1/2 (h) | – | – | – | 1.00 ± 0.07 | 1.23 ± 0.05 | 1.17 ± 0.33 | 2.84 ± 1.17 | 1.37 ± 0.39 |
| MRT (h) | 9.78 ± 4.89 | 20.4 ± 7.3 | 22.2 ± 7.8 | 1.91 ± 0.25 | 1.89 ± 0.31 | 2.05 ± 0.38 | 4.15 ± 1.26 | 2.41 ± 0.52 |
| F (%) | 1.75 | 8.66 | 7.49 | 62.3 | 58.2 | 39.7 | 6.72 | 34.7 |
| GBE50 (p.o., 90 mg·kg−1) | ||||||||
| Tpeak-(1) (h) | 0.25 | 0.25 | 0.25 | 0.50–1.00 | 0.50 | 0.50–1.00 | 0.50–2.00 | 0.25–0.50 |
| Cmax-(1) (nM) | 119 ± 50 | 354 ± 173 | 54.3 ± 14.6 | 1356 ± 235 | 474 ± 63 | 176 ± 23 | 47.6 ± 16.0 | 64.9 ± 6.9 |
| Tpeak-(2) (h) | 4.00 | 6.00 | 6.00 | N.A. | N.A. | N.A. | N.A. | N.A. |
| Cmax-(2) (nM) | 107 ± 46 | 251 ± 87 | 156 ± 46 | N.A. | N.A. | N.A. | N.A. | N.A. |
| AUC0-t (h·nM) | 768 ± 205 | 4195 ± 1295 | 2212 ± 427 | 2724 ± 135 | 840 ± 78 | 371 ± 39 | 115 ± 24 | 148 ± 17 |
| t1/2 (h) | – | – | – | 1.05 ± 0.16 | 1.31 ± 0.22 | 1.20 ± 0.18 | 1.63 ± 0.71 | 1.25 ± 0.15 |
| MRT (h) | 10.6 ± 2.8 | 20.8 ± 6.5 | 22.8 ± 4.7 | 1.76 ± 0.09 | 1.74 ± 0.07 | 2.07 ± 0.12 | 2.65 ± 0.43 | 2.20 ± 0.11 |
| F (%)a | 2.12 | 9.46 | 9.42 | 60.4 | 53.4 | 35.2 | 5.12 | 34.1 |
The oral F was calculated using the AUC data of p.o. ingested GBE50 and the associated AUC data of i.v. infused ShuXueNing injection at 2 mL·kg−1.
The metabolic profiling analysis of rat plasma samples revealed that the t-quercetin in a pooled plasma sample taken 15 min after administration of GBE50 consisted of the quercetin glucuronides M16G-1, M16G-2, M16G-3 and M16G-4 and the absorbed quercetin glycosides 10, 11, 12 and 13 (Figure 4). The quercetin glucuronides were most likely derived from the GBE50-containing aglycone quercetin that was absorbed from the small intestine and conjugated in the intestinal epithelia, hepatocytes or both. M16G-2, M16G-3, M16G-4 and M16S-1 accounted for the t-quercetin in a pooled plasma sample taken 6 h after dosing. These conjugated metabolites were most likely formed via the colonic microflora-induced deglycosylation of the unabsorbed quercetin glycosides and the subsequent enterohepatic conjugation of the resulting quercetin aglycone. The kaempferol glucuronide M26G-1 was detected in the 15 min plasma sample. This metabolite accounted for the vast majority of t-kaempferol. Levels of the kaempferol glycosides 19, 21 and 22 and the kaempferol glucuronides M26G-2 and M26G-3 were also measured. The kaempferol glucuronides M26G-1, M26G-2 and M26G-3 were observed in the 6 h plasma sample. Levels of the isorhamnetin glycoside 29 and the isorhamnetin conjugates M32G-1, M32G-2, M32G-3 and M32S-2 were measured in the 15 min plasma sample, and the 6 h plasma sample was found to contain M32G-3, M32S-1 and M32S-2.
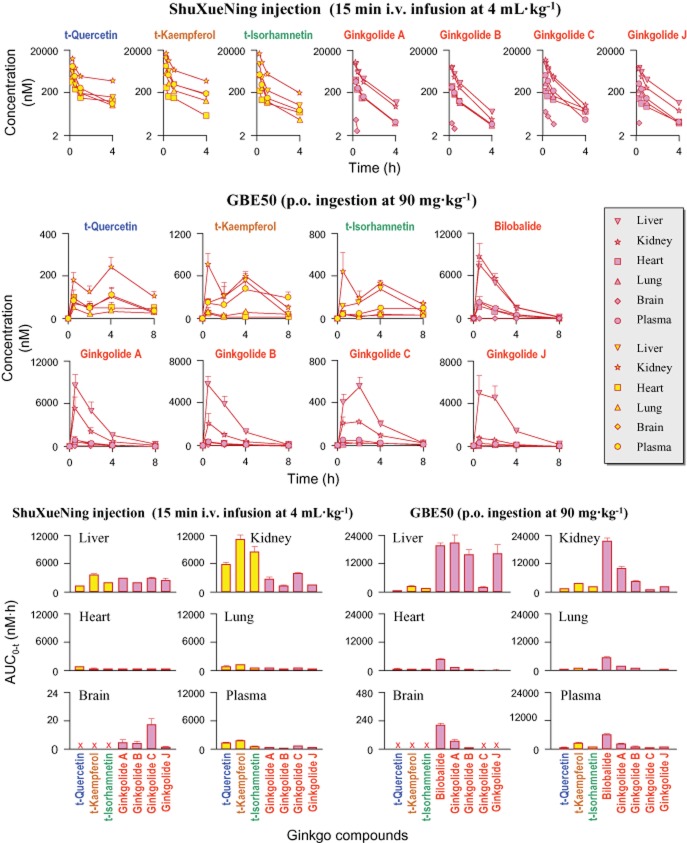
Exposure of brain tissue and other types of tissue to ginkgo flavonols and terpene lactones after administration of ShuXueNing injection or GBE50
After i.v. infusion of ShuXueNing injection (4 mL·kg−1) or p.o. administration of GBE50 (90 mg·kg−1), levels of the terpene lactone bilobalide (p.o. only), ginkgolides A, B and C (i.v. only), and ginkgolide J (i.v. only) were measured in rat bECF (Figure 5), but the ginkgo flavonols were absent. The bECF concentrations of terpene lactones were significantly lower than the associated unbound plasma concentrations; these ginkgo compounds had average Kp values of 0.12–0.29. The results suggested that their ability to enter brain tissue was not very good. The unbound plasma concentrations of terpene lactones and their associated bECF concentrations concomitantly increased up to a maximum and then decreased. Correlations between the unbound plasma concentrations and the associated bECF concentrations were significant for the ginkgo compounds.
Figure 5.
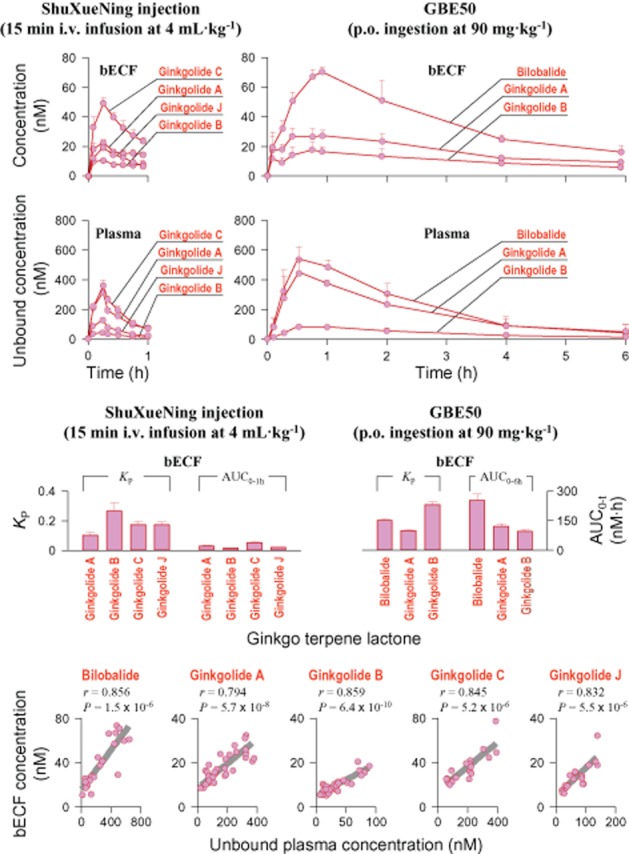
bECF concentrations and unbound plasma concentrations of terpene lactones over time (upper panel), comparative KP values and comparative bECF AUC values of the ginkgo compounds (middle panel) and correlations between the unbound plasma concentrations and the bECF concentrations (lower panel) after a 15 min i.v. infusion dose of ShuXueNing injection (4 mL·kg−1; red curves) or a p.o. dose of GBE50 (90 mg·kg−1; red curves) in rats. The letter ‘r’ represents the correlation coefficient. Correlations were statistically significant, with a P-value of <0.05.
After i.v. infusion of ShuXueNing injection (4 mL·kg−1), the total flavonols had the highest concentration in the kidneys, followed by the liver, lungs, plasma and heart, but they were not detected in the brain (Figure 6). The liver and kidneys showed significantly higher levels of ginkgolide exposure than the plasma, and the levels of exposure to the heart and lungs were comparable with or lower than those of the plasma. Although ginkgolides A, B, C and J were also detected in the brain homogenate samples, the concentrations were low.
Figure 6.
Tissue distribution of total flavonols (markers filled in yellow) and terpene lactones (markers filled in pink) after a 15 min i.v. infusion dose of ShuXueNing injection (4 mL·kg−1; red curve; upper panel) or a p.o. dose of GBE50 (90 mg·kg−1; red curve; middle panel) in rats. Tissue AUC values and the associated plasma AUC values are shown in the lower panel.
After p.o. ingestion of GBE50 (90 mg·kg−1), most tissue concentration-time profiles of the total flavonols were bimodal. The levels of exposure of t-quercetin, t-kaempferol and t-isorhamnetin were ranked as follows: kidneys > liver ≈ plasma > lungs ≈ heart. The flavonols were not measured in the brain homogenate samples. Bilobalide had higher tissue exposure levels than the other terpene lactones. Ginkgolide C had the lowest tissue concentration. The highest tissue exposure levels of terpene lactones from the p.o. ingested GBE50 were observed in the liver and kidneys, followed by the concentrations in heart and lungs. The brain concentrations of bilobalide and ginkgolides A and B were low.
Excretion of ginkgo flavonols and terpene lactones after administration of ShuXueNing injection or GBE50
After i.v. infusion of ShuXueNing injection (4 mL·kg−1), substantial amount of total flavonols was excreted into rat bile, and t-quercetin, t-kaempferol and t-isorhamnetin had fe-B values of 73, 67, and 321% respectively (Table 5). The renal excretion of total flavonols was relatively low and the fe-U values were 9, 12 and 26% respectively. The recovery levels of total flavonols in faeces were notably lower than those in bile. Unlike the situation of biliary excretion (CLB, 0.20–1.41 L·h−1·kg−1), the urinary excretion of total flavonols was slow (CLR, 0.04–0.06 L·h−1·kg−1). Metabolic profiling revealed that the biliary total flavonols were largely present in the form of the flavonol glycosides, that is 11 and 13 accounting for t-quercetin, 19, 20, 21 and 22 accounting for t-kaempferol, and 27, 29 and 30 accounting for t-isorhamnetin (Figure 4). The glycosides 10, 12 and 13 accounted for urinary t-quercetin; 19, 21 and 22 for urinary t-kaempferol; and 29 for urinary t-isorhamnetin. Although the flavonol aglycones quercetin (16), kaempferol (26) and isorhamnetin (32) were not detected in the plasma and bile samples, they were observed in urine despite their low levels. Some of the flavonol aglycone glucuronides and sulfates were also detected in the urine, but they were present at low levels (Figure 4). The urinary and biliary recovery levels of intact ginkgolides were 14–34% and 13–29% respectively, after i.v. infusion of ShuXueNing injection. Although the biliary and urinary excretions are two major elimination pathways, the elimination of ginkgolides may also involve additional major metabolic reactions mediated by an unknown enzyme or enzymes. The biliary excretion of flavonols and ginkgolides described above appeared to involve active transport mechanisms. This is indicative of high AUCB/AUCP ratios ranging from 31 to 289. The CLR/(fu·GFR) data implied active tubular secretion in the renal excretion of ginkgolides rather than that of flavonols (Table 5).
Table 5.
Excretory data of total flavonols and terpene lactones after an i.v. infusion dose of ShuXueNing injection or a p.o. dose of GBE50 in rats
| Biliary data | Faecal data | Urinary data | |||||
|---|---|---|---|---|---|---|---|
| Compound | fe-B (%) | CLB (L·h−1·kg−1) | AUCB/AUCP | fe-F (%) | fe-U (%) | CLR (L·h−1·kg−1) | CLR/(fu·GFR) |
| ShuXueNing injection (15 min i.v. infusion, 4 mL·kg−1) | |||||||
| t-Quercetin | 73.2 ± 8.3 | 0.36 ± 0.03 | 70.7 ± 8.9 | 12.5 ± 4.9 | 8.68 ± 2.1 | 0.04 ± 0.01 | <1 |
| t-Kaempferol | 66.7 ± 16.8 | 0.20 ± 0.04 | 41.0 ± 8.3 | 5.20 ± 1.57 | 11.8 ± 3.2 | 0.05 ± 0.01 | <1 |
| t-Isorhamnetin | 321 ± 54 | 1.41 ± 0.27 | 289 ± 58 | 12.6 ± 4.2 | 25.6 ± 8.5 | 0.06 ± 0.02 | <1 |
| Ginkgolide A | 17.5 ± 6.2 | 0.20 ± 0.07 | 36.8 ± 5.8 | 4.46 ± 0.77 | 28.9 ± 4.0 | 0.78 ± 0.14 | 7.46 ± 1.34 |
| Ginkgolide B | 34.2 ± 10.6 | 0.34 ± 0.12 | 63.4 ± 16.4 | 17.7 ± 3.1 | 28.0 ± 9.2 | 0.65 ± 0.24 | 10.7 ± 4.0 |
| Ginkgolide C | 17.2 ± 7.6 | 0.17 ± 0.07 | 35.6 ± 13.3 | 12.4 ± 2.0 | 22.9 ± 3.1 | 0.27 ± 0.04 | 3.51 ± 0.52 |
| Ginkgolide J | 13.9 ± 6.7 | 0.17 ± 0.08 | 31.4 ± 13.4 | 15.9 ± 3.9 | 13.2 ± 2.0 | 0.23 ± 0.04 | 4.29 ± 0.75 |
| GBE50 (p.o., 90 mg·kg−1) | |||||||
| t-Quercetin | 1.1 ± 0.2 | 0.20 ± 0.05 | 46.1 ± 14.1 | 31.0 ± 6.9 | 0.28 ± 0.14 | 0.05 ± 0.01 | <1 |
| t-Kaempferol | 1.7 ± 0.3 | 0.14 ± 0.07 | 30.0 ± 12.8 | 17.7 ± 3.5 | 0.75 ± 0.21 | 0.04 ± 0.01 | <1 |
| t-Isorhamnetin | 7.6 ± 1.7 | 0.44 ± 0.14 | 90.9 ± 19.1 | 3.22 ± 0.91 | 1.34 ± 0.33 | 0.05 ± 0.02 | <1 |
| Bilobalide | 1.9 ± 0.4 | 0.04 ± 0.00 | 8.4 ± 1.4 | 5.35 ± 0.42 | 30.1 ± 10.1 | 0.96 ± 0.33 | 12.5 ± 4.30 |
| Ginkgolide A | 14.7 ± 2.5 | 0.42 ± 0.11 | 76.8 ± 24.8 | 22.4 ± 3.9 | 39.9 ± 3.2 | 2.04 ± 0.88 | 19.5 ± 8.4 |
| Ginkgolide B | 23.9 ± 3.7 | 0.84 ± 0.24 | 155 ± 46 | 61.4 ± 9.2 | 27.7 ± 0.9 | 1.92 ± 0.71 | 31.7 ± 11.6 |
| Ginkgolide C | 6.5 ± 2.7 | 0.37 ± 0.11 | 66.3 ± 24.8 | 84.6 ± 7.8 | 4.07 ± 1.09 | 1.01 ± 0.36 | 13.1 ± 4.7 |
| Ginkgolide J | 22.2 ± 10.1 | 0.35 ± 0.16 | 69.9 ± 23.9 | 112 ± 10 | 23.3 ± 2.3 | 0.21 ± 0.04 | 3.92 ± 0.75 |
After p.o. administration of GBE50 (90 mg·kg−1), substantial amounts of the flavonol glycosides 11, 13, 19, 20, 21, 22, 27, 29 and 30, the flavonol aglycone conjugates M16G-1, M16G-2, M16G-3, M16S-1, M16S-3, M16G-S, M162S, M26G-1 and M32G-3 and the sulfated kaempferol glycoside M21/22S-3 were recovered in rat bile. The flavonol glycosides 10, 12, 13, 19, 21, 22, 27 and 29 and the metabolites M26G-1, M26G-2 and M32G-3 were recovered in urine. The flavonol aglycones 16, 26 and 32 were also detected in urine but absent from plasma and bile (Figure 4). Despite the poor intestinal absorption of flavonol glycosides (Li et al., 2012), the faecal recoveries of total flavonols were found to be very low. Around 30% of unchanged bilobalide was excreted via urine, but its biliary excretion was poor (fe-B, 2%). The renal excretion of bilobalide appeared to involve active tubular secretion (Table 5).
Discussion and conclusions
Understanding of the body's exposure to and PK profiles of medicinal ingredients can be extremely beneficial to assessment of the drug responses of a botanical product.
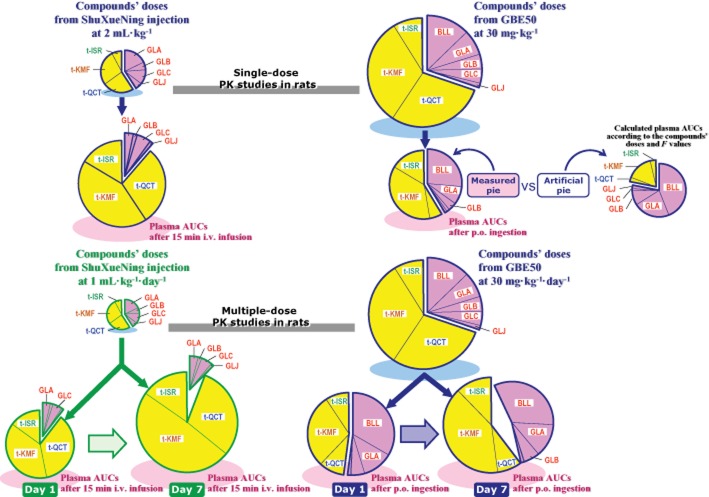
In the current study, after an i.v. dose of ShuXueNing injection, the plasma concentrations of t-quercetin, t-kaempferol and t-isorhamnetin at first decreased rapidly (t1/2-(1), ∼1.9 h) and then entered a slow terminal elimination phase (t1/2-(2), ∼15 h). The concentrations of plasma ginkgolides A, B, C and J decreased more rapidly (t1/2, ∼0.7 h). As depicted in Figure 7, the difference in elimination kinetics led to the mean flavonol-to-terpene lactone ratio in plasma AUC0-t (7.8; dose, 2 mL·kg−1), which was significantly larger than the associated flavonol-to-terpene lactone ratio in concentration in ShuXueNing injection (1.4). In addition, the plasma total flavonols, rather than the plasma terpene lactones, showed significant accumulation in rats that received multiple doses of ShuXueNing injection (daily dose, 1 mL·kg−1) and the mean flavonol-to-terpene lactone ratio in AUC0–t increased from 8.4 (day 1) to 16.4 (day 7). The mean flavonol-to-terpene lactone ratio in plasma AUC0–t (1.4; dose, 30 mg·kg−1) was over one half the flavonol-to-terpene lactone ratio in concentration in GBE50 (2.3), even though the oral F values of total flavonols (0.5%–9.5%) were significantly lower than those of terpene lactones (32.9–63.5%; excluding ginkgolide C). These data can be explained by the slow terminal elimination features of the flavonols, which counterbalanced their poor oral bioavailability. This resulted in significant accumulation of flavonols during the subchronic treatment with GBE50 (daily dose, 30 mg·kg−1).
Figure 7.
Levels of systemic exposure to total flavonols and terpene lactones after i.v. administration of ShuXueNing injection or p.o. administration of GBE50 in rats and concentrations of these ginkgo compounds in the administered botanical products. The yellow area (ginkgo flavonols) is here compared with the pink area (ginkgo terpene lactones) in the pie chart. The sizes of pies on light blue shadows and the associated sizes of individual sectors for the ginkgo compounds present in ShuXueNing injection or GBE50 were calculated using the content level data shown in Table 1. The sizes of pies on light pink shadows and the associated sizes of the individual sectors for the ginkgo compounds in plasma after administration of ShuXueNing injection or GBE50 were calculated using the AUC data shown in Tables 2 and 4. For comparison purposes, calculations were also made to generate an artificial pie chart for p.o. ingestion of GBE50 at 30 mg·kg−1 by considering only the differences in the compound's dose and in compound's oral bioavailability. The differences between the measured pie (on light pink shadow) and the artificial pie (on no shadow) suggested that the slow terminal elimination features of the flavonols counterbalanced the influence of poor oral bioavailability on their levels of systemic exposure. The abbreviations t-QCT, t-KMF, t-ISR, BLL, GLA, GLB, GLC and GLJ denote t-quercetin, t-kaempferol, t-isorhamnetin, bilobalide and ginkgolides A, B, C and J respectively.
This difference between the flavonols and the terpene lactones in elimination kinetics likely resulted from enterohepatic circulation of the flavonols. The terpene lactones did not undergo this type of circulation. Enterohepatic circulation of drugs often occurs by biliary excretion and intestinal reabsorption, which also can involve enterohepatic conjugation and colonic deconjugation. The circulation often leads to prolonged apparent elimination half-lives (Roberts et al., 2002). Most of the major ginkgo flavonol glycosides in ShuXueNing injection had substantial biliary excretion followed by deglycosylation by glycosidases of the colonic microflora. After the resulting aglycones were absorbed, they were immediately conjugated into glucuronides or sulfates. Although the flavonol aglycone conjugates M16G-2, M16G-3, M26G-1 and M32G-3 were excreted into bile, they could re-enter into the systemic circulation by the colonic deconjugation and the intestinal reabsorption and reconjugation, which caused enterohepatic circulation. The aglycone conjugates M16G-4 and M26G-2 were poorly excreted in bile and almost all of them appeared to enter the systemic circulation. Because they were also slowly eliminated via renal excretion, these flavonol aglycone conjugates became long-circulating ginkgo metabolites. Although the terpene lactones showed significant biliary excretion and favourable intestinal absorption, their enterohepatic circulation was terminated by substantial elimination from the systemic circulation via the urinary excretion and unknown metabolic reactions (Table 5).
Routes of administration were found to have significant influence on the levels of systemic exposure to the ginkgo flavonols and terpene lactones. In rats given i.v. doses of ShuXueNing injection, the dosed flavonol glycosides were the major forms of flavonols circulating in the bloodstream. In rats given GBE50 by p.o. administration, many flavonol aglycone conjugates were found to be major circulating forms (Figure 4). The total flavonols from p.o. administered GBE50 exhibited bimodal plasma concentration-time profiles (Figure 2). The first concentration peaks were produced by absorption-conjugation of the GBE50-containing aglycones in the small intestine, and the second greater peaks were produced by the colonic deglycosylation-absorption-conjugation of the unabsorbed flavonol glycosides. Unlike that associated with ShuXueNing injection, bilobalide from the p.o. administered GBE50 represented the most abundant terpene lactone measured in plasma. Ginkgolide C from i.v. administered ShuXueNing injection had a higher dose-corrected level of systemic exposure than the other terpene lactones did, but ginkgolide C from p.o. ingestion of GBE50 had a lower dose-corrected level of systemic exposure than the other terpene lactones did.
Drug therapy can fail for PK reasons if too little or too much concentration occurs at the site of action for too short or too long a time. Terpene lactones were found to be eliminated from the body very rapidly (Figure 2 and Tables 2 and 4). It remains open to question whether the reported pharmacological effects of these ginkgo compounds are more closely related to the duration or magnitude of the in vivo concentrations produced. The majority of pharmacological assessments have been performed on the flavonol aglycones (Perez-Vizcaino and Duarte, 2010), rather than those ginkgo flavonol glycosides and aglycone conjugates that substantially detected in plasma after dosing with ginkgo extracts. The flavonol aglycone quercetin, kaempferol and isorhamnetin were not detected in rat plasma after administration of ShuXueNing injection or GBE50. Several recent studies have evaluated the anti-inflammatory and anti-atherogenic activities of quercetin-3-O-glucuronide and quercetin-3′-O-sulfate from p.o. ingested quercetin or onions (Tribolo et al., 2008; Lodi et al., 2009; Suri et al., 2010; Ishizawa et al., 2011). However, pharmacological evaluations of ginkgo flavonol glycosides and kaempferol and isorhamnetin conjugates have been scarce. Accurate and complete information regarding the pharmacological activity of individual ginkgo flavonol glycosides and aglycone conjugates substantially circulating in the bloodstream after dosing may enhance understanding of the therapeutic effects of ShuXueNing injection and GBE50 and of the differences between them.
The delivery of therapeutic substances to the brain is often limited by the blood–brain barrier, which has often impeded the translation of many promising findings from the laboratory to the clinic (Cecchelli et al., 2007). In the current study, brain exposure to the flavonols and terpene lactones was measured in rats. This is relevant to assessment of the ginkgo's reported neuroprotective properties. Cerebral exposure was found to be negligible for the flavonols and low for the terpene lactones (Figures 5 and 6). Brain penetration of drugs across the blood–brain barrier requires high membrane permeability (Fagerholm, 2008). The flavonol glycosides and aglycone conjugates measured in plasma have poor membrane permeability, and the membrane permeability of terpene lactones, which is intermediate (Li et al., 2012), is probably still too low for brain penetration. Reported concentrations of terpene lactones exerting in vitro neuroprotective activities range from 10 to 80 μM (Luo et al., 2002; Ivic et al., 2003; Tchantchou et al., 2009; Shi et al., 2010), which is 2 or 3 orders of magnitude higher than the concentrations measured in the extracellular fluid of the brain in the current study. Given this information, caution should be exercised when translation of the in vitro experimental findings of ginkgo compounds’ neuroprotective properties to clinical settings. Chronic vascular insufficiency, hypoperfusion, and blood–brain barrier damage are involved in the aetiology of neurodegenerative disorders and vascular alterations may take place at a presymptomatic stage. Insights into cerebrovascular disorders have opened perspectives for new treatment of neurodegenerative diseases by targeting vessels or using angiogenic factors (Storkebaum et al., 2011). Unlike cerebral exposure, the levels of which were very low, systemic exposure to ginkgo flavonols and terpene lactones was significant. Instead of symptomatic treatment of existing dementia, ginkgo extracts might be more effective in preventing of neurodegenerative disorders by slowing down vascular alterations. The nervous, endocrine and immune systems are intimately linked and interdependent. Age-related changes in the neuro-endocrine-immune axis influence both the aging progress and the related diseases, such as neurodegenerative disorders (Vida and De la Fuente, 2013). A link has been drawn between diabetes mellitus and Alzheimer's disease. Drug therapies that increase peripheral insulin sensitivity are showing benefits in Alzheimer's disease patients (Glass et al., 2010). In addition, somatostatin receptors may be pharmacological-target candidates for prevention and treatment of Alzheimer's disease. Intravenous administration of octreotide, which has shown poor brain penetration, can improve memory in Alzheimer's disease patients (Craft et al., 1999; Watson et al., 2009). Enhancement of a patient's innate immunity may represent a novel approach to Alzheimer's disease therapy (Cashman et al., 2008). The naturally occurring compounds curcuminoids selectively enhance Aβ phagocytosis and gene transcription in blood cells of Alzheimer's disease patients (Darvesh et al., 2012). Ginkgo extract has been found to prevent insulin resistance (Zhou et al., 2011). It is unclear whether the ginkgo flavonols and terpene lactones that have poor brain penetration have effects on the neuro-endocrine-immune axis.
PK studies aim to bridge the gaps between phytochemistry and pharmacology and between pharmacology and botanical therapeutics (Li, 2012). Body exposure to the bioactive ingredients of a botanical medicine is a crucial determinant of its drug response and therefore the efficacy and safety. In earlier studies (Lu et al., 2008; Liu et al., 2009; Hu et al., 2013), the compounds that had significant and dose-dependent levels of systemic exposure after dosing with herbal medicines were identified from groups of the known pharmacologically active constituents present in the administered medicines. In the current study, the systemic exposure levels of total flavonols and terpene lactones were found to increase in a dose-dependent manner in rats given ShuXueNing injection or GBE50 (Table 3). These plasma ginkgo compounds could be suitable for use as dose-dependent PK markers to indicate rat exposure to the botanical products. Unlike the plasma terpene lactones, which were detected as single chemical entities, the plasma total flavonols were detected on the basis of the HCl-hydrolysis of multiple flavonol glycosides and aglycone conjugates. The formation of the flavonol aglycone conjugates was affected by the action of the colonic microflora. Because the human colon is significantly more heavily bacterially colonized than the rat colon (Sousa et al., 2008), whether plasma total flavonols are still dose-dependent PK markers in humans remains to be assessed.
In summary, the ginkgo flavonols and terpene lactones have a noted difference in elimination kinetics. The slow terminal elimination features of the flavonols counterbalance the influence of poor oral bioavailability on their levels of systemic exposure. This causes significant accumulation in the bloodstream in response to subchronic treatment with ShuXueNing injection or GBE50. Unlike the flavonols, terpene lactones showed rapid renal excretion and may have undergone unknown metabolic processes, which terminated their enterohepatic circulation. The routes of administration showed significant influences on the systemic exposure to the ginkgo compounds. The flavonol glycosides were detected as major forms in plasma after ShuXueNing injection, but plasma flavonol aglycone conjugates were predominant after dosing with GBE50. Although the levels of systemic exposure to flavonols and terpene lactones were significant and increased in a ShuXueNing injection or GBE50 dose-dependent manner, the levels of cerebral exposure to these ginkgo compounds were very low. Further PK studies are planned to assess the pharmacokinetics and metabolism of the ginkgo compounds in humans and the influence of the colonic microflora on the compounds’ exposure in the body.
Acknowledgments
This work was funded by grants from the National Natural Science Fund of China for Distinguished Young Scholars (30925044), the Shanghai Science and Technology Major Project (07DZ19703), the National Science & Technology Major Project ‘Key New Drug Creation and Manufacturing Program’ (2009ZX09304-002) and the National Natural Science Fund of China (30772772 and 30873120). This work was presented in part as a poster presentation at the CPSA, Shanghai, China, April 13–16, 2011: Chen F et al. Systemic and cerebral exposure to and pharmacokinetics of terpene lactones and flavonols after dosing standardized extracts of Ginkgo biloba leaves to rats via different routes of administration.
Glossary
- AUC
area under concentration-time curve
- B/P ratio
blood to plasma ratio
- bECF
brain extracellular fluid
- CLR
renal clearance
- CLtot,p
total plasma clearance
- F
bioavailability
- fu
unbound fraction in plasma
- i.p
intraperitoneally
- i.v
intravenously
- Kp
AUC ratio of unbound drug in bECF to that in plasma
- MRT
mean residence time
- p.o
peroral
- PK
pharmacokinetic
- VSS
apparent volume of distribution at steady state
Conflict of interest
There are no competing interests to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Appendix S1 Supplemental Methods.
References
- Bhattaram VA, Graefe U, Kohlert C, Veit M, Derendorf H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine. 2002;9(Suppl. 3):1–33. doi: 10.1078/1433-187x-00210. [DOI] [PubMed] [Google Scholar]
- Biber A. Pharmacokinetics of Ginkgo biloba extracts. Pharmacopsychiatry. 2003;36(Suppl. 1):S32–S37. doi: 10.1055/s-2003-40446. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Ghirmai S, Abel KJ, Fiala M. Immune defects in Alzheimer's disease: new medications development. BMC Neurosci. 2008;9(Suppl. 2):S13. doi: 10.1186/1471-2202-9-S2-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck M-P, et al. Modeling of the blood–brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6:650–661. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- Chen JS, Huang PH, Wang CH, Lin FY, Tsai HY, Wu TC, et al. Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis. 2011;214:301–309. doi: 10.1016/j.atherosclerosis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Chen JW, Chen YH, Lin FY, Chen YL, Lin SJ. Ginkgo biloba extract inhibits tumor necrosis factor-induced reactive oxygen species generation, transcription factor activation, and cell adhesion molecule expression in human aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:1559–1566. doi: 10.1161/01.ATV.0000089012.73180.63. [DOI] [PubMed] [Google Scholar]
- Chen Z-P, Sun J, Chen H-X, Xiao Y-Y, Liu D, Chen J, et al. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia. 2010;81:1045–1052. doi: 10.1016/j.fitote.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Carroll RT, Bishayee A, Novotny NA, Geldenhuys WJ, Van de Schyf CJ. Curcumin and neurodegenerative diseases: a perspective. Expert Opin Investig Drugs. 2012;21:1123–1140. doi: 10.1517/13543784.2012.693479. [DOI] [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- DeFeudis FV, Drieu K. In vitro studies of the pharmacological and biochemical activities of Ginkgo biloba extract (EGb761) and its constituents. In: van Beek TA, editor. Ginkgo Biloba. Amsterdam: Harwood Academic Publishers; 2000. pp. 279–301. [Google Scholar]
- Drieu K, DeFeudis FV. In vivo studies of the pharmacological and biochemical activities of Ginkgo biloba Extract (EGb761) and its constituents. In: van Beek TA, editor. Ginkgo Biloba. Amsterdam: Harwood Academic Publishers; 2000. pp. 303–329. [Google Scholar]
- Fagerholm U. The role of permeability in drug ADME/PK, interactions and toxicity – presentation of a permeability-based classification system (PCS) for prediction of ADME/PK in humans. Pharm Res. 2008;25:625–638. doi: 10.1007/s11095-007-9397-y. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Li C, Wang G-J, Chen L-S. Rapid and direct measurement of free concentrations of highly protein-bound fluoxetine and its metabolite norfluoxetine in plasma. Rapid Commun Mass Spectrom. 2006;20:39–47. doi: 10.1002/rcm.2265. [DOI] [PubMed] [Google Scholar]
- Hu Z-Y, Yang J-L, Cheng C, Huang Y-H, Du F-F, Wang F-Q, et al. Combinatorial metabolism notably affects human systemic exposure to ginsenosides from orally administered extract of Panax notoginseng roots (Sanqi) Drug Metab Dispos. 2013;41:1457–1469. doi: 10.1124/dmd.113.051391. [DOI] [PubMed] [Google Scholar]
- Huang SY, Jeng C, Kao SC, Yu JJH, Liu DZ. Improved haemorrheological properties by ginkgo biloba extract (Egb761) in type 2 diabetes mellitus complicated with retinopathy. Clin Nutr. 2004;23:615–621. doi: 10.1016/j.clnu.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Yoshizumi M, Kawai Y, Terao J, Kihira Y, Ikeda Y, et al. Pharmacology in health food: metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J Pharmacol Sci. 2011;115:466–470. doi: 10.1254/jphs.10r38fm. [DOI] [PubMed] [Google Scholar]
- Ivic L, Sands TT, Fishkin N, Nakanishi K, Kriegstein AR, StrØmgaard K. Terpene Trilactones from Ginkgo biloba are antagonists of cortical glycine and GABAA receptors. J Biol Chem. 2003;278:49279–49285. doi: 10.1074/jbc.M304034200. [DOI] [PubMed] [Google Scholar]
- Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136–1139. doi: 10.1016/0140-6736(92)93158-j. [DOI] [PubMed] [Google Scholar]
- Koltermann A, Hartkorn A, Koch E, Furst R, Vollmar AM, Zahler S. Ginkgo biloba extract EGb761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715–1722. doi: 10.1007/s00018-007-7085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Ude C, Wurglics M, Schubert-Zsilavecz M, Klein J. Brain permeability of bilobalide as probed by microdialysis before and after middle cerebral artery occlusion in mice. J Pharm Pharm Sci. 2010;13:607–614. doi: 10.18433/j31c7q. [DOI] [PubMed] [Google Scholar]
- Li C. Absorption, disposition, and pharmacokinetics of herbal medicines: what and how? Curr Drug Metab. 2012;13:491–493. doi: 10.2174/1389200211209050491. [DOI] [PubMed] [Google Scholar]
- Li L, Zhao Y-S, Du F-F, Yang J-L, Xu F, Niu W, et al. Intestinal absorption and presystemic elimination of various chemical constituents present in GBE50 extract, a standardized extract of Ginkgo biloba leaves. Curr Drug Metab. 2012;13:494–509. doi: 10.2174/1389200211209050494. [DOI] [PubMed] [Google Scholar]
- Liu H-F, Yang J-L, Du F-F, Gao X-M, Ma X-T, Huang Y-H, et al. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- Lodi F, Jimenez R, Moreno L, Kroon PA, Needs PW, Hughes DA, et al. Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis. 2009;204:34–39. doi: 10.1016/j.atherosclerosis.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Longpré F, Garneau P, Christen Y, Ramassamy C. Protection by Egb761 against β-amyloid-induced neurotoxicity : involvement of NF-κB, SIRT1, and MAPKs pathways and inhibiton of amyloid fibril formation. Free Radic Biol Med. 2006;41:1781–1794. doi: 10.1016/j.freeradbiomed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Lu T, Yang J-L, Gao X-M, Chen P, Du F-F, Sun Y, et al. Plasma and urinary tanshinol from Salvia miltiorrhiza (Danshen), can be used as pharmacokinetic markers for cardiotonic pills, a cardiovascular herbal medicine. Drug Metab Dispos. 2008;36:1578–1586. doi: 10.1124/dmd.108.021592. [DOI] [PubMed] [Google Scholar]
- Luo Y, Smith JV, Paramasivam V, Burdick A, Curry KJ, Buford JP, et al. Inhibition of amyoid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc Natl Acad Sci U S A. 2002;99:12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekh A, Pham DL, Yousem DM, Dizon M, Barker PB, Lin DDM. Effects of Ginkgo biloba on cerebral blood flow assessed by quantitative MR perfusion imaging: a pilot study. Neuroradiology. 2011;53:185–191. doi: 10.1007/s00234-010-0790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Lee WJ, Lee IT, Chiu TH, Tsai KL, Lin CY, et al. Ginkgo biloba extract attenuates oxLDL-induced oxidative functional damages in endothelial cells. J Appl Physiol. 2009;106:1674–1685. doi: 10.1152/japplphysiol.91415.2008. [DOI] [PubMed] [Google Scholar]
- Perez-Vizcaino F, Duarte J. Flavonols and cardiovascular disease. Mol Aspects Med. 2010;31:478–494. doi: 10.1016/j.mam.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Rangel-Ordóñez L, Nöldner M, Schubert-Zsilavecz M, Wurglics M. Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba extract EGb761. Planta Med. 2010;76:1683–1690. doi: 10.1055/s-0030-1249962. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41:751–790. doi: 10.2165/00003088-200241100-00005. [DOI] [PubMed] [Google Scholar]
- Rossi R, Basillco F, Rossoni G, Riva A, Morazzoni P, Mauri PL. Liquid chromagraphy/atmospheric pressure chemical ionization ion trap mass spectrometry of bilobalide in plasma and brain of rats after oral administration of its phospholipidic complex. J Pharm Biomed Anal. 2009;50:224–227. doi: 10.1016/j.jpba.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Saleem S, Zhuang H, Biswal S, Christen Y, Doré S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39:3389–3396. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Wu F, Yew DT, Xu J, Zhu Y. Bilobalide prevents apoptosis through activation of the PI3K/Akt pathway in SH-SY5Y cells. Apoptosis. 2010;15:715–727. doi: 10.1007/s10495-010-0492-x. [DOI] [PubMed] [Google Scholar]
- Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17:1278–1283. doi: 10.1023/a:1026451721686. [DOI] [PubMed] [Google Scholar]
- Sousa T, Paterson R, Moore V, Carlsson A, Abrahamsson B, Basit AW. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008;363:1–25. doi: 10.1016/j.ijpharm.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Quaegebeur A, Vikkula M, Carmeliet P. Cerebrovascular disorders: molecular insights and therapeutic opportunities. Nat Neurosci. 2011;14:1390–1397. doi: 10.1038/nn.2947. [DOI] [PubMed] [Google Scholar]
- Sun Y, Dai J-Y, Hu Z-Y, Du F-F, Niu W, Wang F-Q, et al. Oral bioavailability and brain penetration of (−)-stepholidine, a tetrahydroprotoberberine agonist at dopamine D1 and antagonist at D2 receptors, in rats. Br J Pharmacol. 2009;158:1302–1312. doi: 10.1111/j.1476-5381.2009.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri S, Liu XH, Rayment S, Hughes DA, Kroon PA, Needs PW, et al. Quercetin and its major metabolites selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Br J Pharmacol. 2010;159:566–575. doi: 10.1111/j.1476-5381.2009.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchantchou F, Xu Y, Wu YJ, Christen Y, Luo Y. EGb761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer's disease. FASEB J. 2007;21:2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- Tchantchou F, Lacor PN, Cao Z, Lao L, Hou Y, Cui C, et al. Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J Alzheimers Dis. 2009;18:787–798. doi: 10.3233/JAD-2009-1189. [DOI] [PubMed] [Google Scholar]
- Tribolo S, Lodi F, Connor C, Suri S, Wilson VG, Taylor MA, et al. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis. 2008;197:50–56. doi: 10.1016/j.atherosclerosis.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Tsai JY, Su KH, Shyue SK, Kou YR, Yu YB, Hsiao SH, et al. EGb761 ameliorates the formation of foam cells by regulating the expression of SR-A and ABCA1: role of haem oxygenase-1. Cardiovasc Res. 2010;88:415–423. doi: 10.1093/cvr/cvq226. [DOI] [PubMed] [Google Scholar]
- Vida C, De la Fuente M. Stress-related Behavioural Responses, Immunity and Ageing in Animal Models. In: Bosch JA, Phillips AC, Lord JM, editors. Immunosenescence: Psychosocial and Behavioral Determinans. New York: Springer; 2013. pp. 125–144. Chapter 8. [Google Scholar]
- Watson GS, Baker LD, Cholerton BA, Rhoads KW, Merriam GR, Schellenberg GD, et al. Effects of insulin and octreotide on memory and growth hormone in Alzheimer's disease. J Alzheimers Dis. 2009;18:595–602. doi: 10.3233/JAD-2009-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-Z, Li S-Q, Cui W, Zu X-G, Wang F-F, Du J. Ginkgo biloba extract improves coronary blood flow in patients with coronary artery disease: role of endothelial-dependent vasodilation. Planta Med. 2007;73:624–628. doi: 10.1055/s-2007-981536. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Yoshitake S, Kehr J. The Ginkgo biloba extract EGb761 and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br J Pharmacol. 2010;159:659–668. doi: 10.1111/j.1476-5381.2009.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun Y, Li C. Simultaneous determination of ginkgo flavonoids and terpenoids in plasma: ammonium formate in LC mobile phase enhancing electrospray ionization efficiency and capacity. J Am Soc Mass Spectrom. 2008;19:445–449. doi: 10.1016/j.jasms.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Zhou L, Meng Q-J, Qian T, Yang Z-Q. Ginkgo biloba extract enhances glucose tolerance in hyperinsulinism-induced hepatic cells. J Nat Med. 2011;65:50–56. doi: 10.1007/s11418-010-0456-z. [DOI] [PubMed] [Google Scholar]
- Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q-Z, Chen C-Y. Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovasc Drug Rev. 2004;22:309–319. doi: 10.1111/j.1527-3466.2004.tb00148.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.