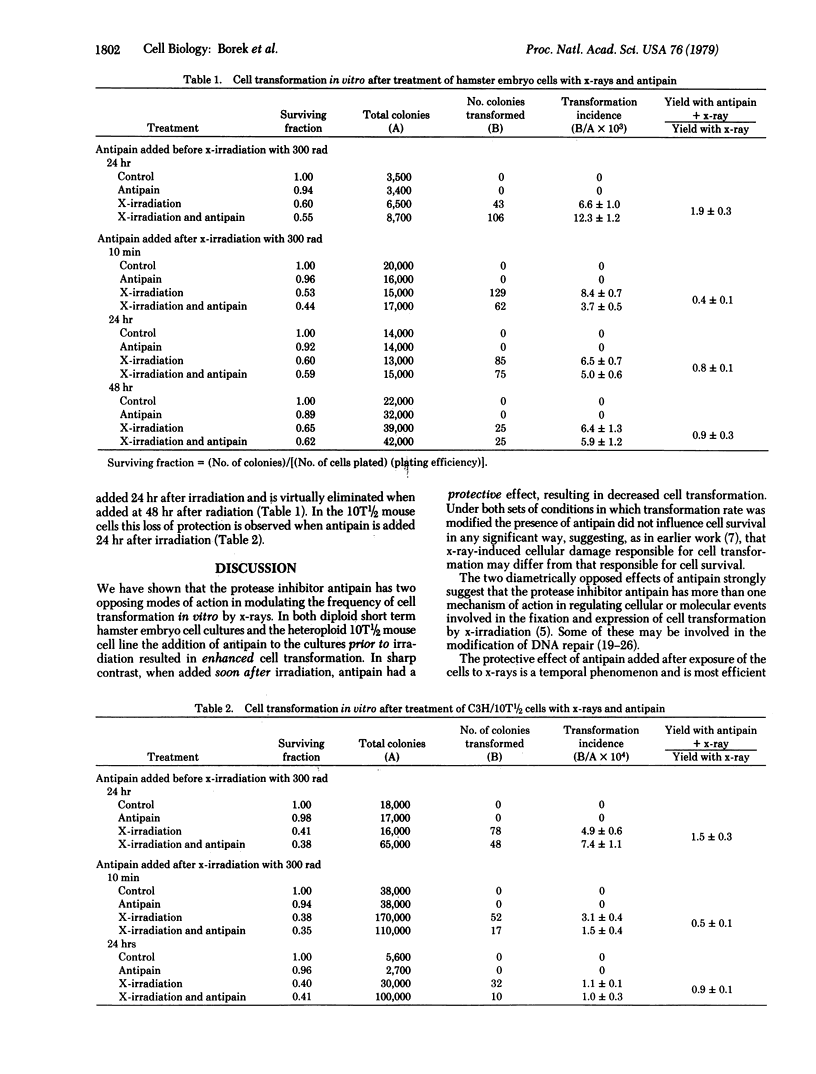
Abstract
Using cultured normal hamster embryo cells and the heterploid mouse C3H cell line 10T1/2, clone 8, we have studied the effect of the protease inhibitor antipain on x-ray-induced neoplastic transformation. We found in both cell systems that, while there was no effect on cell survival as compared to irradiated controls, the addition of antipain at a concentration of 6 microgram/ml to the cultures 24 hr prior to irradiation resulted in enhanced transformation as compared to the frequency in cultures exposed to radiation alone. Yet the addition of antipain to cultures 10 min after irradiation resulted in a decreased transformation rate. This decrease was not found when antipain was added to the mouse cells 24 hr after irradiation or to the hamster cells 48 hr after irradiation. These results suggest that the protease inhibitor antipain has more than one mechanism of action in modulating the fixation and expression of transformation by x-irradiation, possibly by the modification of DNA repair.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berwald Y., Sachs L. In vitro transformation of normal cells to tumor cells by carcinogenic hydrocarbons. J Natl Cancer Inst. 1965 Oct;35(4):641–661. [PubMed] [Google Scholar]
- Borek C., Hall E. J. Effect of split doses of x rays on neoplastic transformation of single cells. Nature. 1974 Dec 6;252(5483):499–501. doi: 10.1038/252499a0. [DOI] [PubMed] [Google Scholar]
- Borek C., Hall E. J., Rossi H. H. Malignant transformation in cultured hamster embryo cells produced by X-rays, 460-keV monoenergetic neutrons, and heavy ions. Cancer Res. 1978 Sep;38(9):2997–3005. [PubMed] [Google Scholar]
- Borek C., Hall E. J. Transformation of mammalian cells in vitro by low doses of X-rays. Nature. 1973 Jun 22;243(5408):450–453. doi: 10.1038/243450a0. [DOI] [PubMed] [Google Scholar]
- Borek C., Pain C., Mason H. Neoplastic transformation of hamster embryo cells irradiated in utero and assayed in vitro. Nature. 1977 Mar 31;266(5601):452–454. doi: 10.1038/266452a0. [DOI] [PubMed] [Google Scholar]
- Borek C., Sachs L. Cell susceptibility to transformation by x-irradiation and fixation of the transformed state. Proc Natl Acad Sci U S A. 1967 May;57(5):1522–1527. doi: 10.1073/pnas.57.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C., Sachs L. In vitro cell transformation by x-irradiation. Nature. 1966 Apr 16;210(5033):276–278. doi: 10.1038/210276a0. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A., Nelson R. L., Donovan P. J. In vitro transformation of Syrian hamster embryo cells by diverse chemical carcinogens. Nature. 1972 Feb 4;235(5336):278–280. doi: 10.1038/235278a0. [DOI] [PubMed] [Google Scholar]
- Dugle D. L., Gillespie C. J., Chapman J. D. DNA strand breaks, repair, and survival in x-irradiated mammalian cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):809–812. doi: 10.1073/pnas.73.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind M. M., Kamper C. Two forms of repair of DNA in mammalian cells following irradiation. Biophys J. 1970 Mar;10(3):237–245. doi: 10.1016/S0006-3495(70)86296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A., Elkind M. M. Transformation of mouse C3H/10T1/2 cells by single and fractionated doses of X-rays and fission-spectrum neutrons. Cancer Res. 1979 Jan;39(1):123–130. [PubMed] [Google Scholar]
- Howard A., Cowie F. G. Induced resistance in Closterium: indirect evidence for the induction of repair enzyme. Radiat Res. 1978 Sep;75(3):607–616. [PubMed] [Google Scholar]
- Huberman E., Sachs L. Cell susceptibility to transformation and cytotoxicity by the carcinogenic hydrocarbon benzo[a]pyrene. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1123–1129. doi: 10.1073/pnas.56.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Selkirk J. K., Heidelberger C. Metabolism of polycyclic aromatic hydrocarbons in cell cultuires. Cancer Res. 1971 Dec;31(12):2161–2167. [PubMed] [Google Scholar]
- Meyn M. S., Rossman T., Troll W. A protease inhibitor blocks SOS functions in Escherichia coli: antipain prevents lambda repressor inactivation, ultraviolet mutagenesis, and filamentous growth. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1152–1156. doi: 10.1073/pnas.74.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R., Hall E. J. X-ray dose fractionation and oncogenic transformations in cultured mouse embryo cells. Nature. 1978 Mar 2;272(5648):58–60. doi: 10.1038/272058a0. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Repair replication in mammalian cells after x-irradiation. Mutat Res. 1972 Feb;14(2):225–235. doi: 10.1016/0027-5107(72)90049-8. [DOI] [PubMed] [Google Scholar]
- Poste G. Production of a serine-protease with macrophage migration-inhibitory factor activity by virus-transformed cells and human tumor cell lines. Cancer Res. 1975 Sep;35(9):2558–2566. [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Setlow R. B., Faulcon F. M., Regan J. D. Defective repair of gamma-ray induced DNA damage in xeroderma pigmentosum cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1976 Feb;29(2):125–136. doi: 10.1080/09553007614550141. [DOI] [PubMed] [Google Scholar]
- Terzaghi M., Little J. B. X-radiation-induced transformation in a C3H mouse embryo-derived cell line. Cancer Res. 1976 Apr;36(4):1367–1374. [PubMed] [Google Scholar]
- Troll W., Klassen A., Janoff A. Tumorigenesis in mouse skin: inhibition by synthetic inhibitors of proteases. Science. 1970 Sep 18;169(3951):1211–1213. doi: 10.1126/science.169.3951.1211. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Structures and activities of protease inhibitors of microbial origin. Methods Enzymol. 1976;45:678–695. doi: 10.1016/s0076-6879(76)45058-9. [DOI] [PubMed] [Google Scholar]
- Wigler M., Weinstein I. B. Tumour promotor induces plasminogen activator. Nature. 1976 Jan 22;259(5540):232–233. doi: 10.1038/259232a0. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M., Quigley J. P., Ashe B., Dorn C., Goldfarb R., Troll W. Direct fluorescent assay of urokinase and plasminogen activators of normal and malignant cells: kinetics and inhibitor profiles. Proc Natl Acad Sci U S A. 1978 Feb;75(2):750–753. doi: 10.1073/pnas.75.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]







