Abstract
Selenoprotein expression in Escherichia coli redefines specific single UGA codons from translational termination to selenocysteine (Sec) insertion. This process requires the presence of a Sec Insertion Sequence (SECIS) in the mRNA, which forms a secondary structure that binds a unique Sec-specific elongation factor that catalyzes Sec insertion at the predefined UGA instead of release factor 2-mediated termination. During overproduction of recombinant selenoproteins, this process nonetheless typically results in expression of UGA-truncated products together with the production of recombinant selenoproteins. Here, we found that premature termination can be fully avoided through a SECIS-dependent Sec-mediated suppression of UGG, thereby yielding either tryptophan or Sec insertion without detectable premature truncation. The yield of recombinant selenoprotein produced with this method approached that obtained with a classical UGA codon for Sec insertion. Sec-mediated suppression of UGG thus provides a novel method for selenoprotein production, as here demonstrated with rat thioredoxin reductase. The results also reveal that the E. coli selenoprotein synthesis machinery has the inherent capability to promote wobble decoding.
INTRODUCTION
Selenocysteine (Sec) is the 21st amino acid and defining entity for selenoproteins (1). It is a sulfur-to-selenium substituted analogue of cysteine (Cys), with higher chemical reactivity and unique properties compared with Cys (2,3). The high reactivity of Sec likely explains why Sec-specific selenoprotein synthesis machineries tightly control Sec synthesis and its translational incorporation into selenoproteins. During selenoprotein expression, the genetic code is redefined in a unique process whereby specific Sec-encoding UGA codons are diverted from their natural roles of serving as translational termination signals (4). This redefining process requires a cis-acting secondary structure in the selenoprotein mRNA—a so-called Sec Insertion Sequence (SECIS) element (5)—that is recognized by a specialized Sec insertion machinery (6). This includes the unique Sec-specific SelB elongation factor binding the SECIS element and catalyzing Sec insertion at the UGA; several properties of Sec insertion machineries however differ between different domains of life (4,7,8). In Escherichia coli, the machinery requires the products of the selA, selB, selC and selD genes (1,4) (Scheme 1) that compete in their UGA decoding processes with translational termination through release factor 2 (RF2) (9) and, under certain conditions, tryptophan (Trp)-mediated UGA suppression (10,11). Recombinant selenoprotein production in E. coli therefore typically results in mixtures of products with either Sec insertion or RF2-mediated truncation products (12,13), and in some cases Trp insertion (11). In this study, we asked whether the Sec insertion machinery itself could support wobble decoding, which has not been investigated to date, although it is a common phenomenon with classical amino acids (14). Indeed, we found Sec-mediated wobble decoding to be possible, and, when using overproduction of the selA, selB and selC genes together with UGG decoding, it allowed for recombinant selenoprotein production in E. coli.
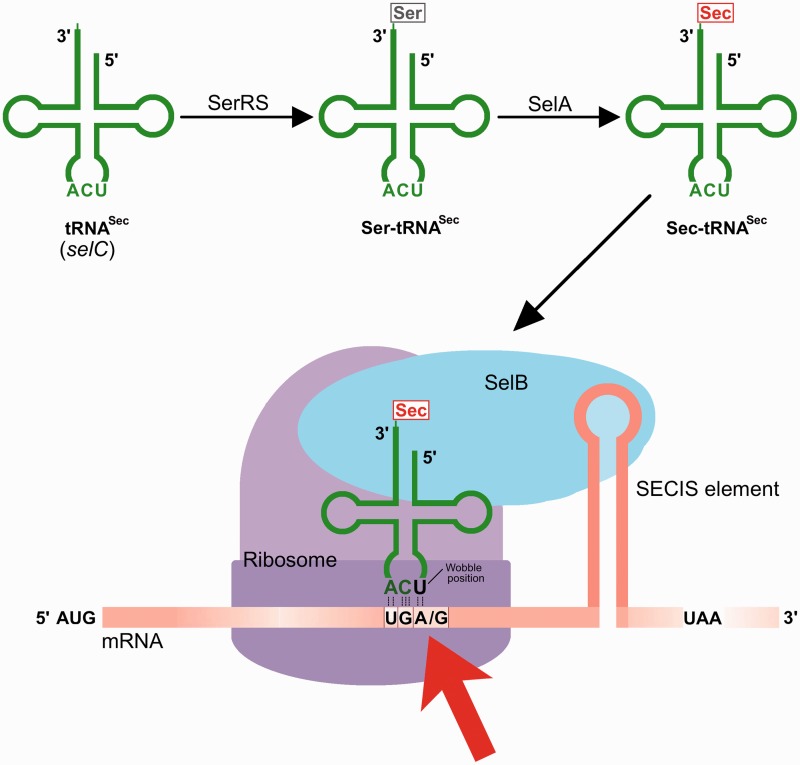
Scheme 1.
Selenoprotein expression in E. coli redefines a single predefined UGA codon from translational termination to Sec insertion. The Sec-encoding UGA is identified by a Sec-specific elongation factor (SelB) through interaction with an mRNA secondary structure formed by a SECIS. During expression of recombinant selenoproteins, this process typically requires Sec-tRNASec (the selC gene product) that is amino acylated by seryl-tRNA-synthetase (SerRS) with the seryl moiety converted to selenocysteinyl by Sec synthase (the selA gene product). Here, we demonstrate high-yield SECIS-dependent wobble decoding with Sec-mediated suppression of a UGG codon (red arrow) using this synthesis machinery.
Two non-SECIS-dependent methods for recombinant production of selenoproteins in E. coli were recently reported, using either mutations in 16S rRNA (15) or synthetic tRNA species compatible with the classical elongation factor EF-Tu (16). Those methods show promise for production of selenoproteins having internal Sec residues, where the presence of a SECIS element is a complicating factor. However, when a Sec residue is located close to the C-terminal end of the protein, such as in isoenzymes of thioredoxin reductase (TrxR) (12,17,18) or Sel-tagged proteins (19–21), high-yield production is enabled by the engineering of a synthetic SECIS element that is not itself translated but still compatible with the bacterial Sec insertion machinery for support of Sec insertion close to the C-terminus (22). This technique however results in a mixture of Sec-insertion and RF2-mediated UGA truncation (23,24). Here, we analyzed whether truncation can be avoided through Sec-mediated wobble decoding and used production of rat TrxR1 because its Sec contents at position 498 is easily probed through analyses of its enzymatic activity (13).
MATERIALS AND METHODS
TrxR1 expression plasmids
Wild-type rat TrxR1 was expressed as previously described, using a recombinant system enabling incorporation of the active site Sec with the used plasmid pET-TRSTER containing a bacterial-type SECIS (12). The truncated TrxR1 variant missing both Sec498 and Gly499 was generated as described previously (25,26) with the bacterial-type SECIS element in the plasmid (5′-ggttgcaggtctgcacc-3′; 17 nt) and furthermore replaced with a non-SECIS sequence (5′-agaatcactagtgcggc-3′; 17 nt) to yield a pET-TRSTER (-SECIS) plasmid. Either pET-TRSTER or pET-TRSTER (-SECIS) was used as PCR templates to construct the different mutants of rat TrxR1 used in this study. The primers used are shown in Table 1, and molecular cloning operations were performed as described (27,28). DNA sequencing (GATC Biotech, Konstanz, Germany) verified the mutations. For recombinant expression, the plasmids were transformed into either E. coli BL21 (DE3) gor−cells (Tet+) with or without accessory pSUABC plasmid (12) (Chl+) or its derivatives, as indicated.
Table 1.
Primers used for UGA-to-UGG substitutions
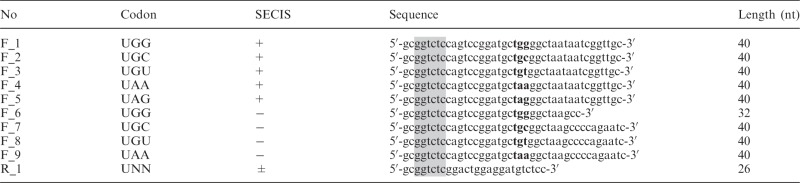 |
This table lists the primers used for introduction of mutations at the codon corresponding to Sec within the open reading frame of rat TrxR1, with or without a downstream SECIS element (see scheme in Figure 1). The nucleotides in bold denote the codon change, whereas the shaded nucleotides introduce restriction cleavage sites for cloning. The R_1 primer was used as a reverse primer in all PCR reactions.
N, represents G, A, U or C.
Variant SelC and SelABC expression plasmids
Plasmid pCDF-selC-UCA (2505 bp) was constructed by subcloning an Xba I/Acc65 I fragment (397 bp, from pSUABC) containing selC gene (91 bp) into Xba I/Acc65 I linearized pCDF-1b (Novagen, USA). The lac I region and T7 promotor region of pCDF-1b were removed by digestion with Xba I and Acc65 I. Then, plasmid pCDF-selC-UCA was used as a PCR template for mutating selC gene at the anticodon of the Sec-specific tRNASec (UCA) (WT) from UCA to either CCA or CUA, and finally resulted in pCDF-selC-CUA and pCDF-selC-CCA. The primers used are given in Table 2. The additional pSUABC-derived plasmids pSUABC-CCA, pSUABC-UCA and pSUABC-CUA were subsequently constructed by removing the standard selC cassette from the standard pSUABC using Xba I and Sac I and replacing it with the Xba I/Sac I selC fragment from the corresponding pCDF-selC plasmids. Two new primers (Table 2) were synthesized for DNA sequencing (GATC Biotech, Konstanz, Germany), which verified the mutations on pCDF-selC. For recombinant expression, the plasmids were transformed into either E. coli BL21 (DE3) gor− cells (Tet+) with or without accessory pSUABC plasmid (Chl+) or pCDF-selC plasmid (Sm+) as indicated in the text.
Table 2.
Primers used for selC (tRNASec) mutations
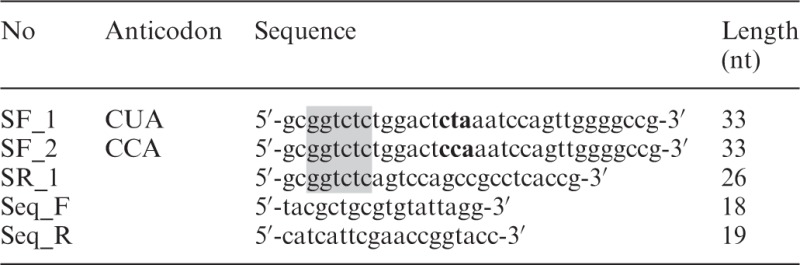 |
This table lists the primers used for introduction of mutations at the anticodon of tRNASec (the selC gene product), using the pCDF-selC-UCA plasmid as template. The nucleotides in bold denote the anticodon change, whereas the shaded nucleotides introduce restriction cleavage sites for cloning. The SR_1 primer was used as the reverse primer in both PCR reactions, whereas the two Seq primers were used for DNA sequencing verification of the resulting products.
Protein expression and purification
All rat TrxR1 variants were recombinantly expressed in E. coli BL21 (DE3) gor− host strains, with or without co-transformation with the pSUABC plasmid, according to previously described methods (12). The ‘2.4/24/24’ protocol was used to facilitate production of wild-type rat TrxR1 as previously described (23) with all the variants produced under the same conditions for comparison reasons. After 2′,5′-ADP SepharoseTM (GE Healthcare Life Sciences, Uppsala, Sweden) column purification, samples were concentrated over 30 kDa cutoff Centrifugal Filters (UltracelTM YM-30, Millipore, MA, USA) and thereupon sterile-filtered over 0.20 μm autoclaved filter membranes. For each protein, 5 ml of concentrated samples were subsequently loaded onto a gel filtration column SuperdexTM 200 GL 10/300 (120 ml, GE Healthcare Life Sciences, Uppsala, Sweden) pre-equilibrated with 50 mM Tris–HCl, 2 mM EDTA (pH 7.5) (TE) buffer. The dimeric enzyme species were collected and pooled analyses and activity assays. The >99% pure enzymes were desalted after the gel filtration column and stored in TE buffer at 4°C. All protein purifications were performed at 4°C using an ÄKTA Explorer 100 workstation (GE Healthcare Life Sciences, Uppsala, Sweden) with absorbance tracking at 280 nm (protein absorbance) and 463 nm (flavin adenine dinucleotide [FAD] detection). Flavoprotein concentrations of purified enzymes were determined using the FAD absorbance at 463 nm (with the extinction coefficient of 11 300 M−1 cm−1), and protein concentration was estimated using a Bradford assay Kit (Bio-Rad, USA) with bovine serum albumin as standard. For purity analyses, the TrxR1 samples were separated on NuPAGETM 4–12% Bis-Tris SDS–PAGE gels (Life Technologies, USA) on heating at 70°C in SDS Loading buffer (Life Technologies, USA) containing 20 mM dithiothreitol for 10 min. The protein bands were visualized by staining with Coomassie Brilliant Blue R250 (GE Healthcare Life Sciences, Uppsala, Sweden) and documented using a Bio-Rad ChemiDoc XRS scanner (Bio-Rad, USA).
75Se autoradiography
Bacteria were grown at 37°C for 12 h until an OD600nm = 2.4 whereupon temperature was lowered to 24°C. For radiolabeling, 100 μg ml−1 of l-Cys (to avoid non-specific 75Se incorporation into sulfur metabolism), 5 μM cold sodium selenite and 1 μCi [75Se]-selenite (Missouri Research Reactor, USA) were added into the culture medium. Thereupon, 0.5 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to induce protein expression, which was allowed for another 24 h of growth at 24°C with constant aeration in rotating culture flasks. Thereafter, cells were collected and lyzed with 1 mg ml−1 lysozyme and ultrasonication whereupon samples clarified supernatants on centrifugation at 16 000 g for 30 min were used for analyses with reducing SDS–PAGE gel and autoradiography with documentation using a GE TyphoonTM FLA 7000 Biomolecular Imagers and ImageQuantTM TL software version 7.0 (GE Healthcare Life Sciences, Uppsala, Sweden).
TrxR activity assays
Enzymatic activities of the TrxR1 variants were determined using three separate assays: the standard 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) reduction assay (29), an insulin-coupled Trx reduction assay (29), or a phenanthrenequinone reduction assay (30). The experimental details in 96-well microtiter plate format are as follows: (i) DTNB reduction assay. The standard reaction mixture (200 μl) contained 2.5 mM DTNB, 17 nM TrxR variant enzyme and 300 μM NADPH in 50 mM TE buffer, pH 7.5. DTNB reduction was evaluated by the formation of TNB− following the absorbance at 412 nm for 10 min, with the extinction coefficient of 13 600 M−1 cm−1. (ii) Insulin-coupled Trx reduction assay. The standard reaction mixture (200 μl) contained 20 μM hTrx1, 160 μM insulin, 170 nM TrxR variant enzyme and 300 μM NADPH in 50 mM TE buffer (pH7.5). Activity assay was performed following the NADPH consumption as decrease of A340 nm using an extinction coefficient of 6200 M−1 cm−1. (iii) Phenanthrenequinone reduction assay. The standard reaction mixture (200 μl) in this assay contained 50 μM phenanthrenequinone, 17 nM enzyme and 200 μM NADPH in 50 mM TE buffer (pH 7.5), and activity was measured by following NADPH consumption at 340 nm. All the activity assays were performed with 10 s time interval reads at 25°C using a VersaMax microplate reader (Molecular Devices, USA), with the reaction mixtures without enzyme serving as reference. Activity measurements were performed in at least duplicate and analyzed with the Prism 5 software (GraphPad, USA).
Electrospray Ionization mass spectrometry
For analyses with mass spectrometry, buffer exchange of the enzyme samples to 10 mM ammonium bicarbonate (NH4HCO3) buffer (pH7.7) was performed using NAP-25TM column (GE Healthcare Life Sciences, Uppsala, Sweden) and further concentrated to final concentrations of 40 μM using 30 kDa cut-off Centrifugal Filter devices (UltracelTM 30K, Millipore, MA, USA). Aliquots of 2 μl of the cleaned proteins were diluted in 20 μl of 50% ACN/H2O and 20 μl of 0.5% HCOOH. Data were acquired on a QTOF Premier API mass spectrometer (Waters, Milford, US) equipped with a Z-spray source, operated in the positive ion mode under the control of MassLynx 4.1. Samples were introduced via a nanoflow electrospray interface from metal-coated borosilicate glass capillary needles (EconoTips™, New Objective), and scans were acquired between m/z 500 and 2000 with a rate of 1 scan/s and an interscan interval of 0.1 s. The source temperature was set to 80°C, capillary voltage to 3.3 kV, cone and RF lens energies to 40 and 50 V, respectively. Cone gas 85 was set to and Nano gas to 0.22. The instrument was operated in single-reflector mode at a resolution of 10 000 (full width at half-maximum definition). The mass scale was calibrated against [Glu1]-fibrinopeptide B. Mass spectra were analyzed using Waters MassLynx software and MaxEnt1 for deconvolution of the data.
RESULTS
IPTG-induced production of Sec-containing TrxR1 using alternative Sec-encoding codons
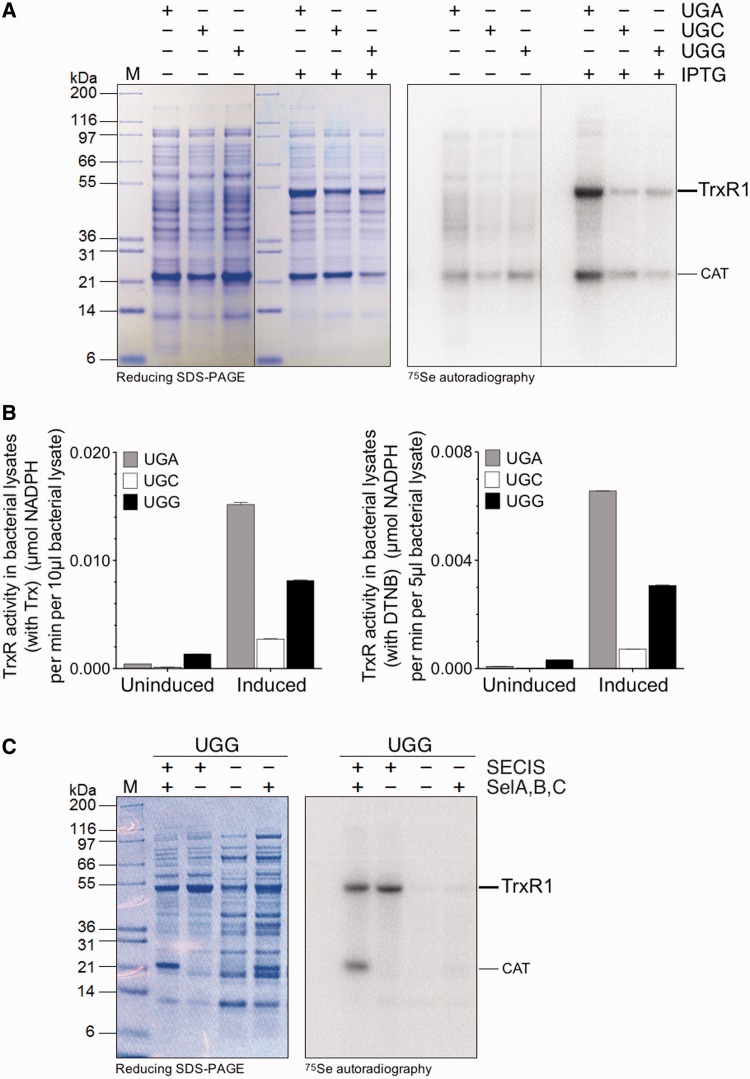
Because a full TrxR1 activity is dependent on the presence of a Sec residue in its C-terminal active site (12,13), we first assessed whether active recombinant TrxR1 could be expressed in E. coli utilizing constructs having either UGA, UGC or UGG codons at the position corresponding to Sec (see Figure 1 for a scheme of the different constructs used in this study). We found a notable IPTG-inducible 75Se-labeled protein expression and TrxR1 activity not only using a UGA codon (Sec/Stop) but also with UGG (Trp) or, to a lesser extent, UGC (Cys) (Figure 2A and B). The 75Se incorporation with UGG still required the presence of a SECIS element in the construct, thus implying selenoprotein synthesis machinery-dependent Sec-mediated wobble decoding at the single UGG codon located directly upstream of the SECIS element (Figure 2C).
Figure 1.
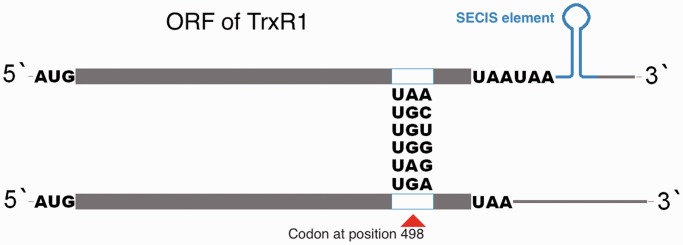
Scheme of the herein used constructs. The plasmid pET-TRSTER, designed with a UGA codon at the position 498 corresponding to Sec and a SECIS element at its 3′-UTR for production of recombinant TrxR as a selenoprotein 1, was used as a PCR template for site-directed mutagenesis (for primers, see Table 1). Five codons including UGG (encoding Trp), UGC or UGU (both encoding Cys), UAG (amber stop codon), or UAA (ochre stop codon), were made to replace the UGA codon. The stem-loop structure in blue indicates the functional SECIS element, recognized by SelB as required to catalyze Sec insertion. Regarding constructs made to encode truncated enzyme or other variants lacking a SECIS element (DNA sequence being 5′-ggttgcaggtctgcacc-3′), this was replaced with a non-SECIS encoding sequence (5′-agaatcactagtgcggc-3′), and an additional UAA codon was introduced at position 500 (as indicated in the lower cartoon of the scheme).
Figure 2.
Sec-mediated suppression of UGG in E. coli using the bacterial Sec insertion machinery. Detection of selenoprotein production by 75Se radiolabeling and SDS–PAGE analysis as well as TrxR1 activity assays on cell lysates were used to screen for selenoprotein production, using conditions indicated in the figure: with (‘Induced’) or without (‘Uninduced’) IPTG induction of recombinant TrxR1production, with either UGA, UGC or UGG codon at position 498 corresponding to Sec in the native selenoprotein. All expression conditions were made using a bacterial-type SECIS element in the construct and overproduction of the selA, selB and selC genes. (A) A Coomassie-stained SDS–PAGE analysis with the corresponding autoradiography is shown in the left panel, with the position of the TrxR1 subunit size indicated. (B) TrxR1-specific activities in crude cell lysates using either a Trx-linked assay or direct DTNB reduction. (C) Production of TrxR1 using constructs with a UGG codon at the position corresponding to Sec in the enzyme was performed with or without a SECIS element or overexpression of the sel genes and visualized using 75Se radiolabeling, as indicated. The diffuse radioactive band seen just above ≈21 kDa on contransformation with pSUABC, together with expression of 75Se-labeled TrxR1, may be due to unspecific labeling of the pSUABC plasmid resistance gene, chloramphenicol acetyl transferase (indicated in the figure as ‘CAT’).
Enzymatically active recombinant TrxR1 can be produced using several different Sec-defining codons, with UGG being the best alternative to UGA
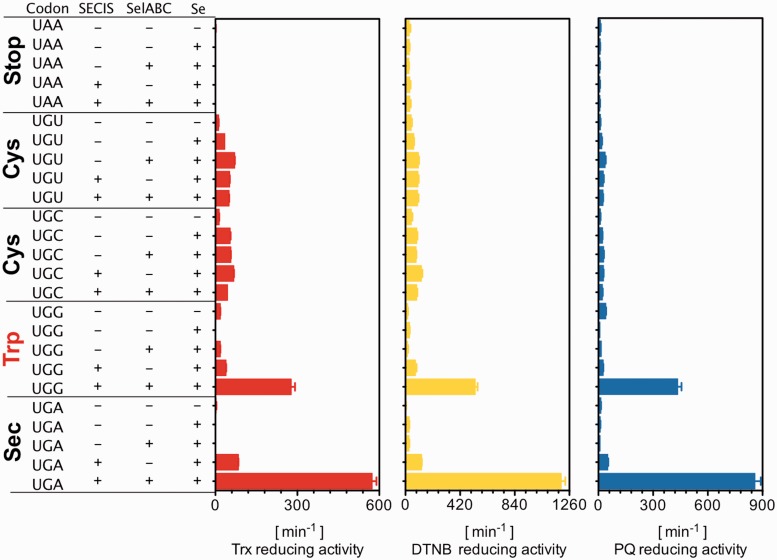
To further analyze the properties of these recombinant TrxR1 variants, we expressed and purified to apparent homogeneity the proteins made from constructs using either of four codons at the position corresponding to Sec (UAA, UGG, UGU, UGA or UGG), with or without the presence of a SECIS element. The protein variants were furthermore expressed under conditions of either selenium supplementation in the medium (5 μM sodium selenite) and/or overexpression of the bacterial selA, selB and selC genes. The latter was previously shown to increase yield in expression of recombinant TrxR1 as a selenoprotein when UGA is used as the Sec-encoding codon (17,18). Using three distinct well-defined assays for measurement of TrxR1 activity, i.e. Trx-coupled insulin reduction (22), direct DTNB reduction (22) or direct phenanthrene quinone (PQ) reduction (30), we found that the TrxR1 protein expressed with a UGG codon at the position of Sec, in the presence of a SECIS element and together with overexpression of the sel genes, produced an enzyme having approximately half the specific activity as compared with that using a native UGA codon, when expressed and purified under identical conditions (Figure 3).
Figure 3.
Identification of Sec-mediated UGG-encoded TrxR1 products in E. coli. Expression of TrxR1 from constructs having either UAA (Stop), UGU (Cys), UGC (Cys), UGG (Trp) or UGA (Stop/Sec) at the position of Sec in the wild-type enzyme were performed in E. coli either with or without a SECIS element in the construct, overexpression of the sel genes and supplementation of the medium with selenite, as indicated in the figure. The 25 enzyme variants were purified to apparent homogeneity (Figure 6) whereupon turnover in three separate TrxR1-specific assays was determined (Trx1-coupled insulin reduction, direct DTNB reduction or PQ reduction), as indicated in the figure and further described in the text.
Complementary anticodon of tRNASec does not necessarily improve yield
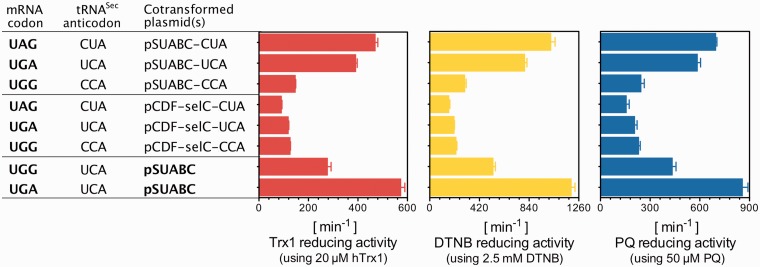
We next asked whether the specific activity of TrxR1, and thus its Sec contents, when produced using a UGG codon could be further improved by mutation of the anticodon of the selC-encoded tRNASec from UCA to CCA, to obtain an exact match to the UGG. Interestingly, this change of the tRNASec lowered the specific activity of the produced enzyme, probably because of less efficient usage of this non-natural tRNA in one or several steps of the selenoprotein synthesis cascade (16,29). However, it was possible to instead use UAG amber codon suppression in place of UGA, thereby allowing selenoprotein synthesis to compete with RF1 instead of RF2, especially when used in a context of a perfect codon–anticodon pair. That strategy resulted in production of TrxR1 having about the same specific activity as when using native UGA-directed Sec insertion (Figure 4).
Figure 4.
Effect of the Sec-specific tRNASec variants and codon combinations on TrxR1 production in E. coli. By changing the natural anticodon UCA of tRNASec to either CCA or CUA, we analyzed its support of Sec insertion using expression of TrxR1 from constructs having either UAG (Stop), UGA (Stop/Sec) or UGG (Trp) at the position of Sec. Expression was performed in conjunction with a SECIS element in the construct, overexpression of the sel genes (pSUABC) or only the selC gene (pCDF-selC), and supplementation of the medium with selenite, as indicated in the figure. As for details of expression yields using the cotransformed plasmid(s), please see Table 3. The eight enzyme variants were purified to apparent homogeneity (Figures 6 and 7) whereupon turnover in three TrxR1-specific assays was determined (Trx1-coupled insulin reduction, direct DTNB reduction or PQ reduction), as indicated in the figure and further described in the text.
Validation of recombinant TrxR1 variants using mass spectrometry for identification of the penultimate amino acid
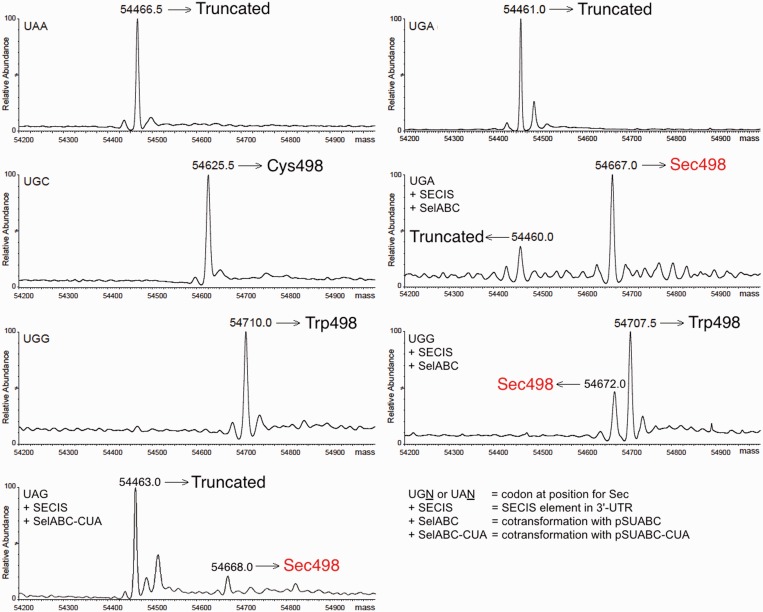
To further verify the identities of the TrxR1 species indirectly probed for Sec contents through enzyme activity measurements, we performed ESI-Q-TOF mass spectrometry analyses of all protein species within the range of 54–55 Da (zero-charged average mass) as found in the enzyme preparations (Figure 5). These analyses confirmed that the nature of the purified enzyme species were as we had presumed. This included enzyme produced with UAA at the position corresponding to Sec (solely truncated protein, theoretical mass 54 464.39 Da), with UGC in the absence of a SECIS element (solely Cys-containing protein, theoretical mass 54 624.50 Da), with UGG in a construct without a SECIS element (Trp-containing variant, theoretical mass 54 707.70 Da) or with UGA but without a SECIS element (again solely truncated enzyme). It was also clear, as expected, that the enzyme expressed from a construct containing a SECIS element together with selgene overexpression and selenite supplementation, having a UGA at the position of Sec, gave a major peak of 54 667 Da corresponding to the theoretical size of wild-type TrxR1 (54 671.48 kDa) mixed with a peak of 54 460 Da corresponding to UGA-truncated enzyme. The analyses, finally, confirmed that the UGG codon variant expressed in the presence of a SECIS element with sel gene overexpression and selenite supplementation, resulted in a product containing a mixture of Sec-to-Trp substituted enzyme and wild-type Sec-containing protein (54 672 Da, indicated in red in Figure 5), without any trace of a truncation product. The enzyme preparation produced using UAG in place of UGA at the Sec-encoding position together with a tRNASec mutated to a complementary anticodon also clearly contained the full-length Sec-containing TrxR1 enzyme, in addition to truncated enzyme (Figure 5, lower left panel). These results showed that the identity of the TrxR1 variants agreed well with the expected results. As a final analysis, we next wished to evaluate all of the production strategies for TrxR1 as studied here, with regards to final yields and specific activities when produced and purified under identical conditions.
Figure 5.
ESI-Q-TOF mass spectrometry of variant TrxR1 forms produced in E. coli. The purified TrxR1 variants expressed from constructs having either UAA, UGC, UGG or UGA at the position corresponding to Sec were analyzed with Q-TOF-MS, as described in the ‘Materials and Methods’ section. Deconvoluted zero-charged ions at average mass are shown (expected accuracy ±0.01% ≈±5.5 Da for TrxR1). When the UGG codon variant was expressed in conjunction with a SECIS element, sel gene overexpression and selenite supplementation, the product contained a mixture of the Sec-to-Trp substituted enzyme (Trp498, 54 707 Da) and wild-type Sec-containing protein (54 672.0 Da, indicated in red), without any trace of the truncation product seen for enzyme produced with UGA or UAA at the position of Sec (54 461 Da).
High specific activities and total yields of recombinant selenoprotein TrxR1 can be achieved using either UGA, UAG or UGG as the Sec-defining codon
For the final analyses of efficiency in Sec insertion and versatility for production of TrxR1 as a selenoprotein, we produced all of the variants of TrxR1 studied herein using 1 l of bacterial cultures, with identical production and purification procedures, with determination of specific activities and final yields. All the variants could be purified with rather high yields with regards to protein amount and at high apparent purity, when using either alternative codons for Sec with or without the SECIS and the pSUABC plasmid (Figure 6), or in combinations with novel variants of tRNASec (Figure 7). Determining the specific activities and total yields of these TrxR variants, we found that, under the conditions used in the present study, production of TrxR1 using either native UGA at position 498 together with the native tRNASec, or the UAG amber codon together with a complementary anticodon in the tRNASec, yielded specific activities of TrxR1 at 10–15 U/mg, with total yields of 308 to 448 U from 1 l of bacterial culture. Using UGG together with the native selenoprotein synthesis machinery resulted in a specific activity of 6.3–6.8 U/mg and yields of 191–195 U/l culture. None of the other production strategies used herein, with regards to choice of Sec-defining codon, presence of SECIS element or tRNASec variant usage, gave any specific activities or total yields that could approach these values (Table 3).
Figure 6.
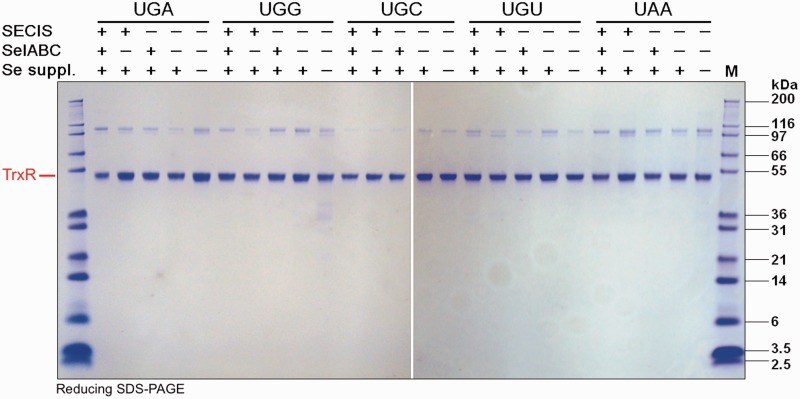
Purity analysis of purified TrxR variants. The TrxR1 variants produced using the indicated codons at position 498 were recombinantly expressed in BL21 (DE3) gor− host strains and further spurified over 2′,5′-ADP SepharoseTM affinity chromatography followed by SuperdexTM G-200 gel filtration chromatography (GE Healthcare Life Sciences, Uppsala, Sweden). The purified protein samples were here analyzed on reducing SDS–PAGE gels. ‘M’ stands for M12 protein standards (Life Technologies, USA), with size (kDa) indicated in the figure. The strong ≈55 kDa protein bands represent the resolved TrxR1 subunits, whereas the weak ≈110 kDa bands are traces of covalently linked TrxR1 dimers stable in reducing SDS–PAGE, typically seen in analyses of this enzyme.
Figure 7.
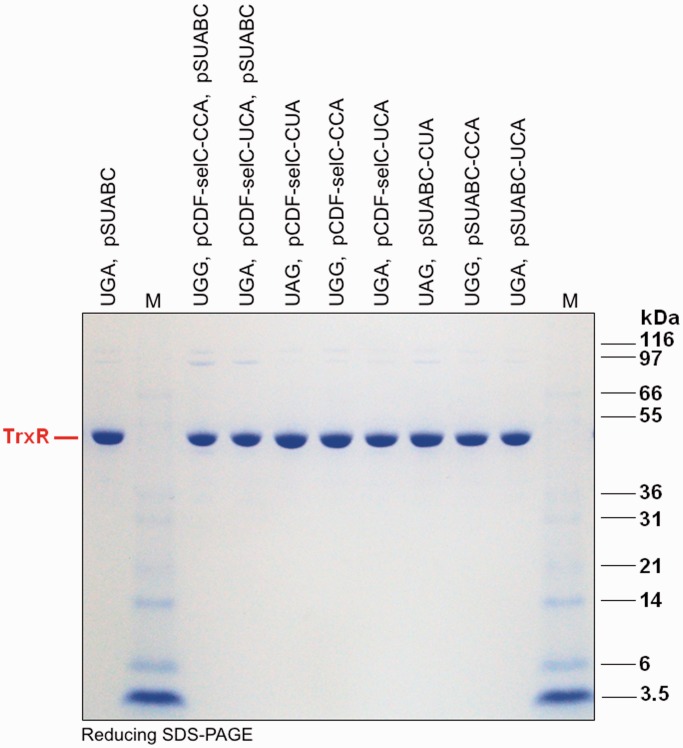
Purity analysis of additional TrxR variants produced using alternative tRNASec variants. Using co-transformations with the pCDF-selC plasmid and/or pSUABC together with the pET-TRSTER TrxR1 expression plasmid in E. coli BL21 (DE3) gor− host strains, these TrxR1 were recombinantly expressed and further purified over 2′,5′-ADP SepharoseTM affinity chromatography followed by SuperdexTM G-200 gel filtration chromatography (GE Healthcare Life Sciences, Uppsala, Sweden). Sodium selenite was supplemented at 5 μM in the bacterial medium for all productions. The purified protein samples were here analyzed on reducing SDS–PAGE gels. ‘M’ stands for M12 protein standards (Life Technologies, USA), with size (kDa) indicated in the figure. The strong ≈ 55 kDa protein bands represents the resolved TrxR1 subunits, whereas the weak ≈110 kDa bands are traces of covalent TrxR1 dimers stable in reducing SDS–PAGE, as typically seen in analyses of this enzyme.
Table 3.
Specific activities and total yields of TrxR1 variants purified from 1 l cultures
| Enzyme variants and production parameters |
TrxR variants Yield and specific activitya |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variants at position 498 of TrxR1 | mRNA codonb | tRNASec anticodonc | SECISd | Selenitee | Contransformed plasmid(s)f | mg | U mg−1,g | U l−1 culture |
| Stop | UAA | UCA | − | − | − | 17.2 | 0.36 | 6.28 |
| UAA | UCA | − | + | − | 27.8 | 0.38 | 10.5 | |
| UAA | UCA | − | + | pSUABC | 20.6 | 0.33 | 6.82 | |
| UAA | UCA | + | + | − | 23.7 | 0.42 | 9.94 | |
| UAA | UCA | + | + | pSUABC | 24.2 | 0.42 | 10.2 | |
| Cys | UGU | UCA | − | − | − | 27.0 | 0.55 | 14.8 |
| UGU | UCA | − | + | − | 28.3 | 0.81 | 22.9 | |
| UGU | UCA | − | + | pSUABC | 19.4 | 1.3 | 24.5 | |
| UGU | UCA | + | + | − | 16.7 | 1.2 | 20.5 | |
| UGU | UCA | + | + | pSUABC | 15.6 | 1.2 | 18.5 | |
| Cys | UGC | UCA | − | − | − | 34.8 | 0.58 | 20.2 |
| UGC | UCA | − | + | − | 33.5 | 1.1 | 37.1 | |
| UGC | UCA | − | + | pSUABC | 34.5 | 1.0 | 35.5 | |
| UGC | UCA | + | + | − | 20.5 | 1.6 | 31.8 | |
| UGC | UCA | + | + | pSUABC | 26.9 | 1.1 | 29.3 | |
| Trp | UGG | UCA | − | − | − | 7.84 | 0.22 | 1.72 |
| UGG | UCA | − | + | − | 17.6 | 0.39 | 6.85 | |
| UGG | UCA | − | + | pSUABC | 16.1 | 0.20 | 3.21 | |
| UGG | UCA | + | + | − | 18.3 | 0.98 | 17.9 | |
| UGG | UCA | + | + | pSUABC | 28.3 | 6.8 | 191 | |
| Sec/Stop | UGA | UCA | − | − | − | 19.0 | 0.03 | 0.57 |
| UGA | UCA | − | + | − | 27.9 | 0.36 | 9.98 | |
| UGA | UCA | − | + | pSUABC | 23.8 | 0.35 | 8.35 | |
| UGA | UCA | + | + | − | 16.8 | 1.6 | 26.1 | |
| UGA | UCA | + | + | pSUABC | 22.5 | 15 | 339 | |
| Stop | UAG | CUA | + | + | pSUABC-CUA | 34.6 | 13 | 448 |
| Sec/Stop | UGA | UCA | + | + | pSUABC-UCA | 30.4 | 10 | 308 |
| Trp | UGG | CCA | + | + | pSUABC-CCA | 30.9 | 3.8 | 116 |
| Stop | UAG | CUA | + | + | pCDF-selC-CUA | 30.3 | 2.1 | 62.1 |
| Sec/Stop | UGA | UCA | + | + | pCDF-selC-UCA | 17.3 | 2.6 | 45.5 |
| Trp | UGG | CCA | + | + | pCDF-selC-CCA | 33.7 | 2.8 | 95.1 |
| Sec/Stop | UGA | UCA+UCA | + | + | pSUABC, pCDF-selC-UCA | 27.0 | 7.6 | 205 |
| Trp | UGG | UCA+CCA | + | + | pSUABC, pCDF-selC-CCA | 30.9 | 6.3 | 195 |
All TrxR1 protein variants studied here were expressed under identical conditions in 1 l of cultures and subsequently purified to apparent near-homogeneity (see Figures 6 and 7 for Coomassie-stained SDS–PAGE analyses of the final purifications). This table summarizes the final yields and specific activities for these TrxR1 variants.
aFinal yields and activities of purified dimeric TrxR variants as purified from 1 l of bacterial culture.
bIn-frame codon corresponding to position 498 of TrxR1 (natural variant encoding Sec/Stop is UGA).
cThe identity of the anticodon of the Sec-specific tRNASec selC gene product (natural variant is UCA).
dPresence (+) or absence (−) of a bacterial-type SECIS element in the construct.
ePresence (+) or absence (−) of sodium selenite (5 µM) supplementation to the bacterial growth medium.
fPlasmids cotransformed with the TrxR1-encoding pET plasmid.
gSpecific activity as determined in the standard DTNB reduction assay (29).
DISCUSSION
In this study, we found that a single predefined UGG codon can be redirected for Sec incorporation into a recombinantly expressed selenoprotein, using efficient wobble decoding mediated by the E. coli selenoprotein synthesis machinery. We furthermore found that premature termination at the Sec-defining codon is thereby avoided; instead, yielding a mixture of Trp and Sec insertion in contrast to the UGA-mediated truncation typically seen in overproduction of selenoproteins. These findings reveal a hitherto unknown flexibility in the E. coli selenoprotein synthesis machinery that may be of importance for both a full understanding of the bacterial Sec insertion mechanisms, as well as for future use in biotechnological applications based on production of recombinant selenoproteins.
We indirectly examined the efficiency of Sec insertion through the production of enzymatically active TrxR1, using several different Sec-defining codons in place of the native UGA, with or without the SECIS element or overexpression of the selA, selB and selC genes. We also analyzed the effects of anticodon mutations in tRNASec (the selC gene product). Several of the results are noteworthy, as they yield further insights into the fidelity or specificity of the bacterial Sec insertion machinery. First, it is clear that very little, if any, selenoprotein was produced in absence of a bacterial-type SECIS element, irrespective of codon choice. This result could have been expected, as it is in full agreement with the early work of Böck and coworkers first deciphering the selenoprotein synthesis machinery of E. coli (1,4,5). The finding underscores that any Sec insertion that is not mediated through SelB interacting with the SECIS element, is virtually non-existent, under the production conditions used herein. The finding that by changing the in-frame UGA codon to UAG, concurrent with mutation of the anticodon of tRNASec to a complementary CUA, yielded about the same expression of selenoprotein TrxR1 as using the native UGA/UCA combination, was also in agreement with early findings by Böck et al., having shown similar results in other reporter systems (31). The findings were also reminiscent to results of Berry et al., who showed that mutations of UGA to either UAA or UUA abolished production and enzymatic activities of recombinantly expressed Sec-dependent thyroid hormone deiodinases in mammalian cells, whereas complementary mutations of a contransfected tRNASec could, at least partially, restore their production (32). Indeed, match pairing between a codon and the anticodon of the corresponding tRNA is, naturally, important for the decoding process during translation. With tRNASec-mediated Sec insertion, more factors are however of crucial importance and may help explain some of the other results in our attempts to express Sec-containing TrxR in the present study. Importantly, the product of the selC gene, i.e. the Sec-specific tRNASec, is originally aminoacylated with serine, which is later converted to Sec by replacement of the side chain hydroxyl group with a selenol, in an intricate process catalyzed by SelA (33–36). In the recent crystal structure of a SelA/tRNASec complex, there is no evidence for interactions between SelA and the anticodon of tRNASec (34). Thus, the selenocysteinylation of tRNASec is likely to be independent of the nature of the anticodon. It is thereby plausible that some other step in the selenoprotein synthesis pathway should explain why our mutation of the anticodon of tRNASec to CCA, when used as a perfect match to UGG, ‘lowered’ Sec incorporation as compared with the wobble decoding of the UGG codon using the native tRNASec. However, this UGG wobble decoding in itself was a novel finding, as we are not aware of any prior studies having described Sec-mediated wobble decoding of a defined UGG (Trp) codon, using SECIS-guided insertion. However, we only achieved good yields when both the selenoprotein-encoding mRNA and the selA, selB and selC genes were overexpressed. Overexpression of these key mediators of selenoprotein synthesis was previously shown to increase the Sec insertion (12,37), which probably relates to the fact that intracellular stoichiometry in the mRNA/SelB/tRNASec complex needs to be maintained (38). Our results reveal that these criteria are valid also when the selenoprotein translation machinery mediates wobble decoding of UGG.
The highly intricate machinery for selenoprotein synthesis makes Sec insertion the limiting factor for production of recombinant selenoproteins at high yields, even if growth or culture conditions that lower the competing RF2-mediated translational termination can somewhat improve final yields, such as expression at late exponential phase (23). Still, the basic methodology used by us for synthesis of selenoproteins in E. coli, with an engineered SECIS element variant compatible with the bacterial machinery (12), has in some ways become a cornerstone production method for production of selenoproteins having their Sec residue located close to the C-terminal end of the protein (18,20,39). One direct approach for lowering RF2-levels clearly increases Sec incorporation specificity, but at the cost of a lower growth rate and reduced total yield, which has hampered widespread use of that strategy (40). Other researchers have developed alternative methods for selenoprotein synthesis, including synthetic production (41), use of novel tRNA species (16) or orthogonal ribosomes (15). Our production of TrxR1 using a UAG codon for Sec coupled with the pSUABC-CUA plasmid encoding tRNASec(CUA) resembled the utilization of UAG for incorporation of Sec in GPx coupled with the new tRNASer(CUA) variant recognized by EF-Tu (16), with the difference that in our study the original selC-encoded tRNASec(UCA) scaffold was used and thus required SelB-mediated insertion in concert with a SECIS element. All the currently available methods for selenoprotein production still have limitations and will typically result in either low yields or in products being mixtures of the target selenoprotein with non-Sec-containing protein species. The methodologies of our present study, using Sec-mediated wobble decoding at alternative codons to the natural UGA, with especially good yields at UGG (resulting in Sec/Trp mixtures in the final product) or at UAG (competing with translational termination by RF1 instead of RF2), provide additional production strategies. These may prove useful as alternative approaches for recombinant selenoprotein production and, furthermore, illustrate the inherent capability of the E. coli selenoprotein synthesis machinery to suppress UGG with Sec, provided that a UGG codon is positioned within a functional context of a bacterial-type SECIS element.
FUNDING
ESJA from the Swedish Cancer Society, the Swedish Research Council (Medicine) and Karolinska Institutet. Funding for open access charge: Swedish Research Council and Karolinska Institutet.
Conflict of interest statement. None declared.
REFERENCES
- 1.Böck A, Forchhammer K, Heider J, Leinfelder W, Sawers G, Veprek B, Zinoni F. Selenocysteine: the 21st amino acid. Mol. Microbiol. 1991;5:515–520. doi: 10.1111/j.1365-2958.1991.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 2.Arnér ES. Selenoproteins-what unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 2010;316:1296–1303. doi: 10.1016/j.yexcr.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Hondal RJ, Marino SM, Gladyshev VN. Selenocysteine in thiol/disulfide-like exchange reactions. Antioxid. Redox Signal. 2013;18:1675–1689. doi: 10.1089/ars.2012.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böck A, Forchhammer K, Heider J, Baron C. Selenoprotein synthesis: an expansion of the genetic code. Trends Biochem. Sci. 1991;16:463–467. doi: 10.1016/0968-0004(91)90180-4. [DOI] [PubMed] [Google Scholar]
- 5.Thanbichler M, Böck A. The function of SECIS RNA in translational control of gene expression in Escherichia coli. EMBO J. 2002;21:6925–6934. doi: 10.1093/emboj/cdf673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ringquist S, Schneider D, Gibson T, Baron C, Böck A, Gold L. Recognition of the mRNA selenocysteine insertion sequence by the specialized translational elongation factor SELB. Genes Dev. 1994;8:376–385. doi: 10.1101/gad.8.3.376. [DOI] [PubMed] [Google Scholar]
- 7.Driscoll DM, Copeland PR. Mechanism and regulation of selenoprotein synthesis. Ann. Rev. Nutr. 2003;23:17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- 8.Stock T, Rother M. Selenoproteins in archaea and gram-positive bacteria. Biochim. Biophys. Acta. 2009;1790:1520–1532. doi: 10.1016/j.bbagen.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Mansell JB, Guevremont D, Poole ES, Tate WP. A dynamic competition between release factor 2 and the tRNA(Sec) decoding UGA at the recoding site of Escherichia coli formate dehydrogenase H. EMBO J. 2001;20:7284–7293. doi: 10.1093/emboj/20.24.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelberg-Kulka H. UGA suppression by normal tRNA Trp in Escherichia coli: codon context effects. Nucleic Acids Res. 1981;9:983–991. doi: 10.1093/nar/9.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandman KE, Noren CJ. The efficiency of Escherichia coli selenocysteine insertion is influenced by the immediate downstream nucleotide. Nucleic Acids Res. 2000;28:755–761. doi: 10.1093/nar/28.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnér ES, Sarioglu H, Lottspeich F, Holmgren A, Bock A. High-level expression in Escherichia coli of selenocysteine-containing rat thioredoxin reductase utilizing gene fusions with engineered bacterial-type SECIS elements and co-expression with the selA, selB and selC genes. J. Mol. Biol. 1999;292:1003–1016. doi: 10.1006/jmbi.1999.3085. [DOI] [PubMed] [Google Scholar]
- 13.Arnér ES. Focus on mammalian thioredoxin reductases—important selenoproteins with versatile functions. Biochim. Biophys. Acta. 2009;1790:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Agris PF, Vendeix FA, Graham WD. tRNA's wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 2007;366:1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 15.Thyer R, Filipovska A, Rackham O. Engineered rRNA enhances the efficiency of selenocysteine incorporation during translation. J. Am. Chem. Soc. 2013;135:2–5. doi: 10.1021/ja3069177. [DOI] [PubMed] [Google Scholar]
- 16.Aldag C, Brocker MJ, Hohn MJ, Prat L, Hammond G, Plummer A, Söll D. Rewiring translation for elongation factor Tu-dependent selenocysteine incorporation. Angew. Chem. Int. Ed. Engl. 2013;52:1441–1445. doi: 10.1002/anie.201207567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bar-Noy S, Gorlatov SN, Stadtman TC. Overexpression of wild type and SeCys/Cys mutant of human thioredoxin reductase in E. coli: the role of selenocysteine in the catalytic activity. Free Radic. Biol. Med. 2001;30:51–61. doi: 10.1016/s0891-5849(00)00448-2. [DOI] [PubMed] [Google Scholar]
- 18.Kuntz AN, Davioud-Charvet E, Sayed AA, Califf LL, Dessolin J, Arnér ES, Williams DL. Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson L, Chen C, Thorell JO, Fredriksson A, Stone-Elander S, Gafvelin G, Arnér ES. Exploiting the 21st amino acid-purifying and labeling proteins by selenolate targeting. Nat. Methods. 2004;1:61–66. doi: 10.1038/nmeth707. [DOI] [PubMed] [Google Scholar]
- 20.Wallberg H, Grafstrom J, Cheng Q, Lu L, Martinsson Ahlzen HS, Samen E, Thorell JO, Johansson K, Dunas F, Olofsson MH, et al. HER2-positive tumors imaged within 1 hour using a site-specifically 11C-labeled Sel-tagged affibody molecule. J. Nucl. Med. 2012;53:1446–1453. doi: 10.2967/jnumed.111.102194. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Q, Lu L, Grafstrom J, Olofsson MH, Thorell JO, Samen E, Johansson K, Ahlzen HS, Stone-Elander S, Linder S, et al. Combining [11C]-AnxA5 PET imaging with serum biomarkers for improved detection in live mice of modest cell death in human solid tumor xenografts. PLoS One. 2012;7:e42151. doi: 10.1371/journal.pone.0042151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnér ES. Recombinant expression of mammalian selenocysteine-containing thioredoxin reductase and other selenoproteins in Escherichia coli. Methods Enzymol. 2002;347:226–235. doi: 10.1016/s0076-6879(02)47022-x. [DOI] [PubMed] [Google Scholar]
- 23.Rengby O, Johansson L, Carlson LA, Serini E, Vlamis-Gardikas A, Karsnas P, Arnér ES. Assessment of production conditions for efficient use of Escherichia coli in high-yield heterologous recombinant selenoprotein synthesis. Appl. Environ. Microbiol. 2004;70:5159–5167. doi: 10.1128/AEM.70.9.5159-5167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rengby O, Cheng Q, Vahter M, Jornvall H, Arnér ES. Highly active dimeric and low-activity tetrameric forms of selenium-containing rat thioredoxin reductase 1. Free Radic. Biol. Med. 2009;46:893–904. doi: 10.1016/j.freeradbiomed.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Anestål K, Arnér ES. Rapid induction of cell death by selenium-compromised thioredoxin reductase 1 but not by the fully active enzyme containing selenocysteine. J. Biol. Chem. 2003;278:15966–15972. doi: 10.1074/jbc.M210733200. [DOI] [PubMed] [Google Scholar]
- 26.Anestål K, Prast-Nielsen S, Cenas N, Arnér ES. Cell death by SecTRAPs: thioredoxin reductase as a prooxidant killer of cells. PLoS One. 2008;3:e1846. doi: 10.1371/journal.pone.0001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Arnér ES. Pyrroloquinoline quinone modulates the kinetic parameters of the mammalian selenoprotein thioredoxin reductase 1 and is an inhibitor of glutathione reductase. Biochem. Pharmacol. 2012;83:815–820. doi: 10.1016/j.bcp.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Q, Sandalova T, Lindqvist Y, Arnér ES. Crystal structure and catalysis of the selenoprotein thioredoxin reductase 1. J. Biol. Chem. 2009;284:3998–4008. doi: 10.1074/jbc.M807068200. [DOI] [PubMed] [Google Scholar]
- 29.Arnér ES, Holmgren A. Measurement of thioredoxin and thioredoxin reductase. Curr. Protoc. Toxicol. 2001 doi: 10.1002/0471140856.tx0704s05. Chapter 7, Unit 7 4. [DOI] [PubMed] [Google Scholar]
- 30.Cenas N, Nivinskas H, Anusevicius Z, Sarlauskas J, Lederer F, Arnér ES. Interactions of quinones with thioredoxin reductase: a challenge to the antioxidant role of the mammalian selenoprotein. J. Biol. Chem. 2004;279:2583–2592. doi: 10.1074/jbc.M310292200. [DOI] [PubMed] [Google Scholar]
- 31.Heider J, Baron C, Böck A. Coding from a distance: dissection of the mRNA determinants required for the incorporation of selenocysteine into protein. EMBO J. 1992;11:3759–3766. doi: 10.1002/j.1460-2075.1992.tb05461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry MJ, Harney JW, Ohama T, Hatfield DL. Selenocysteine insertion or termination: factors affecting UGA codon fate and complementary anticodon: codon mutations. Nucleic Acids Res. 1994;22:3753–3759. doi: 10.1093/nar/22.18.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leinfelder W, Zehelein E, Mandrand-Berthelot MA, Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988;331:723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- 34.Itoh Y, Brocker MJ, Sekine S, Hammond G, Suetsugu S, Söll D, Yokoyama S. Decameric SelA*tRNA(Sec) ring structure reveals mechanism of bacterial selenocysteine formation. Science. 2013;340:75–78. doi: 10.1126/science.1229521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manzine LR, Serrao VH, da Rocha e Lima LM, de Souza MM, Bettini J, Portugal RV, van Heel M, Thiemann OH. Assembly stoichiometry of bacterial selenocysteine synthase and SelC (tRNAsec) FEBS Lett. 2013;587:906–911. doi: 10.1016/j.febslet.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Forchhammer K, Böck A. Selenocysteine synthase from Escherichia coli. Analysis of the reaction sequence. J. Biol. Chem. 1991;266:6324–6328. [PubMed] [Google Scholar]
- 37.Suppmann S, Persson BC, Böck A. Dynamics and efficiency in vivo of UGA-directed selenocysteine insertion at the ribosome. EMBO J. 1999;18:2284–2293. doi: 10.1093/emboj/18.8.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tormay P, Sawers A, Böck A. Role of stoichiometry between mRNA, translation factor SelB and selenocysteyl-tRNA in selenoprotein synthesis. Mol. Microbiol. 1996;21:1253–1259. doi: 10.1046/j.1365-2958.1996.881450.x. [DOI] [PubMed] [Google Scholar]
- 39.Rackham O, Shearwood AM, Thyer R, McNamara E, Davies SM, Callus BA, Miranda-Vizuete A, Berners-Price SJ, Cheng Q, Arnér ES, et al. Substrate and inhibitor specificities differ between human cytosolic and mitochondrial thioredoxin reductases: Implications for development of specific inhibitors. Free Radic. Biol. Med. 2011;50:689–699. doi: 10.1016/j.freeradbiomed.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Rengby O, Arnér ES. Titration and conditional knockdown of the prfB gene in Escherichia coli: effects on growth and overproduction of the recombinant mammalian selenoprotein thioredoxin reductase. Appl. Environ. Microbiol. 2007;73:432–441. doi: 10.1128/AEM.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hondal RJ. Using chemical approaches to study selenoproteins-focus on thioredoxin reductases. Biochim. Biophys. Acta. 2009;1790:1501–1512. doi: 10.1016/j.bbagen.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]