Abstract
People with intellectual disability (ID) are living longer than ever before, raising concerns about old-age associated disorders. Dementia is among the most serious of these disorders, and theories relating cognitive reserve to risk predict that older adults with ID should be particularly vulnerable. Previous estimates of relative risk for dementia associated with ID have been inconsistent, and the present analyses examined the possible influence of variation in diagnostic criteria on findings. As expected, relaxation in the stringency of case definition for adults with ID increased relative risk, underscoring the importance of developing valid criteria for defining mild cognitive impairment, early dementia, and distinguishing between the two in adults with ID. Once available, these standards will contribute to more effective evidence-based planning.
Keywords: dementia, incidence, intellectual disability, mild cognitive impairment, relative risk
INTRODUCTION
People with intellectually disability (ID) are living longer and more fulfilling lives than ever before, and for the vast majority of individuals their life expectancy approaches that of the general population (e.g., Strauss & Eyman, 1996). Nevertheless, lifespan development for adults with ID (and other neurodevelopmental disorders) is atypical by definition, and their primary etiology of ID, as well as the effects of their lifelong experiences, may influence vulnerability to old-age associated impairments.
Dementia is among the most serious concerns faced by elderly adults, potentially having devastating impacts on independence and quality of life. Dementia has many causes, Alzheimer’s disease being the most prevalent (see Jicha & Carr, 2010), and increased risk associated with Down syndrome is well documented (see Zigman & Lott, 2007). However, the relationship between dementia risk and the presence/absence of ID, per se, is unclear and is the focus of the present set of analyses.
Dementia is defined in DSM IV-TR (American Psychiatric Association, 2000) as: “disorders…characterized by the development of multiple cognitive deficits (including memory impairment) that are due to the direct physiological effects of a general medical condition, to the persisting effects of a substance, or to multiple etiologies.” Diagnosis is determined by the presence of impaired cognition sufficient in severity to have consequences for occupational or social functioning. In DSM V (2013), “dementia” has been replaced by “major neurocognitive disorder”, but key features are largely unchanged in the revised definition, requiring “evidence of significant cognitive decline…in one or more cognitive domains…based on: (1) concern of the individual, a knowledgeable informant, or the clinician…and (2) a substantial impairment if cognitive performance, preferably documented by standardized neuropsychological testing, or, in its absence, another quantified clinical assessment,” While decline from a previously higher level of capability is a key feature that distinguishes dementia from ID, it is nevertheless the case that pre-existing cognitive impairments complicate diagnosis. In fact, assessment methods and objective diagnostic criteria suitable for the general population are typically uninformative for adults with ID, and the need for consensus regarding evaluation methods and diagnostic criteria is now well recognized within the developmental disabilities field (e.g., Burt & Aylward, 1999; Janicki & Dalton, 2000; Silverman, Zigman, Kim, et al., 1998).
Risk for aging-associated dementia within the typically developing population rarely occurs prior to 60 years of age and increases thereafter, with approximately 30% of the population over 80 affected (see Zaccai, Ince & Brayne, 2006). Obviously, there is substantial heterogeneity in individual risk and considerable progress has been made in determining the genetic as well as environmental factors that may contribute to this variability (e.g., Barnes & Yafee, 2011; McMurtray, Clark, Christine & Mendez, 2006). A thorough review of this topic is beyond the scope of this paper, but one factor that may contribute to increased risk associated with ID, per se, has been termed “cognitive reserve” (Stern, 2009). This is a hypothetical construct presumed to mitigate the effects of old-age associated brain pathology caused by various underlying diseases (e.g., Alzheimer’s, Parkinson’s and cerebrovascular disease). Stern (2009) argued persuasively that higher reserves, associated with high levels of cognitive capabilities, educational attainment and literacy, should be protective and delay onset of dementia while lower levels should increase vulnerability. If this is in fact the case, then ID should increase risk for old-age associated dementia due to the lifelong presence of substantial cognitive and functional limitations that defines this diagnosis.
Only two large studies to date have examined relative risk for dementia in adults with ID 65 years of age and older (Strydom et al., 2009: Zigman et al., 2004), both of which omitted adults with Down syndrome, given the high risk for Alzheimer’s disease within this specific subpopulation. Zigman et al. relied primarily on analyses of cumulative incidence and found no evidence of increased risk compared with findings for the general population (Saunders et al., 1993). In contrast, Strydom et al. focused on prevalence and found an increased standardized morbidity ratio of 2.8 associated with presence of ID.
Strydom et al. considered several possible explanations for this divergence in findings, including differences in sample size (Zigman et al. included 126 individuals with ID over 65 while Strydom et al. had 142), bias in sampling method (enrollment did not consider dementia status in either study), and differences in inclusion criteria (suggesting that Zigman et al. limited dementia cases to Alzheimer’s disease, which was not actually the case). The two studies also employed different approaches to analyses of findings, were conducted in different countries (U.S. versus U.K.), and recruited participants through two very different networks of services. Nevertheless, these factors seem unlikely explanations for such a remarkable divergence in observed findings, and the present analysis focused on another possibility.
Strydom et al. and Zigman et al. employed different methods to evaluate and classify cases, and this seemed to be an intuitively likely source of the differences in study outcomes. Although convergence in case classifications should be expected, given that both studies relied on the judgments of highly experienced professionals, both groups were working without an established consensus regarding evaluation methods and, especially, criteria for defining dementia objectively. Strydom et al. based their case classifications on a single assessment of capabilities and combined cases with “mild”, “moderate” and “severe” dementia for analyses. Zigman et al. employed a substantially different method and based case classifications on individual profiles of change over a period of up to three years. They also made a clear distinction between adults with dementia and their peers showing milder declines, comparable to mild cognitive impairment (MCI) within the general population (Petersen, Smith, Waring et al, 1999), while Strydom et al. made no mention of such a distinction. This suggests that the difference in outcomes between Strydom et al. and Zigman et al. may have been caused by differences in distinguishing adults with mild dementia from those with MCI, a distinction that would be particularly difficult to make for adults with ID, especially without consideration of objective longitudinal findings. In fact, Strydom, Chan, Fenton, Livingston and Hassiotis (2012) recently reported a re-examination of the Stydom et al. (2009) cases approximately 3 years later. Using classification methods similar to their earlier study, they found that 33% of the original dementia cases who survived were no longer demented and 48% of the original group redefined operationally as having MCI improved in status at follow-up. Both findings imply imprecision in classifications, as did the reported inter-rater reliability of κ = 0.68.
Conceptually, MCI is defined clearly enough as a state intermediate between “normal cognition” and dementia. Thus, adults with MCI have experienced noticeable declines in cognition, but not of sufficient severity to meet diagnostic criteria for dementia (Winblad, Palmer, Kivipalto et al., 2004). While explicit operationalization of this definition remains in flux, even for older adults with typical lifespan development (e.g., Ganguli, Snitz, Sexton, et al., 2011, Gauthier, Reisberg, Zaudig, Petersen et al, 2006, Peterson et al., 1999, Winblad et al., 2004), there is clear consensus that this condition will often precede old-age associated dementia and can persist for an extended period of time. With an estimated prevalence of between 8 and 42% for adults 65 years of age and older (20.6% median prevalence in nine population-based studies using various definitions of MCI; Ward, Arrighi, Michels & Cedarbaum, 2012), approximately three times the number of elderly adults have MCI compared to frank dementia, the latter condition having an estimated prevalence of 6.1% (Wimo, Winblad, Aquero-Torres & von Strauss, 2003).
Unfortunately, standard practices for diagnosing MCI in adults experiencing typical lifespan development are ill-suited for situations where substantial pre-existing cognitive impairments are present, as is the case for adults with ID. In fact, very few studies have focused on MCI among adults with ID and none have proposed explicit diagnostic criteria applicable to this population, although several reports have emphasized the significance of this condition (Ball et al., 2006; 2008; Holland et al., 2000; Krinsky-McHale et al., 2002; 2008; Nelson et al., 2001; Urv et al., 2008). With adults with ID who develop old-age associated dementia very likely to experience a similar progression of underlying disease as their typically developing peers, the degree to which cases with ID and MCI are distinguished from those with dementia would affect estimates of relative risk. If prevalence for these two distinct conditions is unrelated to presence of ID, the expected relative risk for dementia associated with ID would be overestimated to the extent that those actually having MCI are misclassified as having mild dementia. On the other hand, relative risk would be underestimated to the extent that individuals with ID and mild dementia are misclassified as having MCI.
The current study addressed this issue by re-examining the dementia classifications from the original Zigman et al. sample, employing operational definitions of dementia that varied in stringency and comparing estimates of cumulative incidence with those for a sample representative of the general population in one New York State county matched for age and duration of surveillance. Importantly, these analyses were not intended to determine the validity of any of the specific operational definitions of dementia or MCI under examination. While the present results may have implications for the eventual development of best-practice definitions of dementia and MCI for adults with ID, the goals of these analyses are far more modest and are limited to showing how varying the stringency of criteria defining dementia can impact estimates for age-specific incidence and, therefore, relative risk.
Method
All procedures involved re-examination of findings for the sample described in Zigman et al. (2004), and readers are referred there for details of the evaluation procedures. In brief, 149 adults with ID without Down syndrome 65 years of age or older were enrolled in a longitudinal study of aging and dementia.1 They were assessed at baseline and then on up to two subsequent occasions approximately 18 months apart. The present sample included only the 101 individuals who were evaluated on all three occasions (to provide a surveillance period clearly sufficient to observe objective indications of declining status), who were younger than 85.5 years of age at baseline (too few cases were over 85 years of age or older at baseline to provide stable estimates of population incidence), who’s lifelong impairments were not of such severity as to preclude objective evaluation of decline (the case for three individuals), and who did not experience a significant health-related concern clearly unrelated to an underlying neuropathology that could account for functional decline (the case for four individuals).
Assessments included informant interviews focused on adaptive and maladaptive behaviors as well as neuropsychiatric problems, comprehensive reviews of clinical charts, and approximately two hours of cognitive assessments focused on both memory and non-memory processes. Following each cycle of assessment, all findings were examined during individual consensus case conferences and the original dementia status for the cases included in these analyses was determined as: (a) not declining (other than as expected with aging, per se), (b) questionable/MCI, corresponding to presence of declines larger than expected with aging, per se, but of insufficient breadth or severity to be considered dementia, (c) possible dementia, corresponding to the presence of substantial decline in multiple domains of cognition and functioning, or (d) definite dementia, where even more substantial declines are observed or with clear progression in decline.
For the current study, those original dementia classifications following the third assessment cycle were re-determined, expanding upon the distinction between MCI and dementia to address the possibility that the Zigman et al. (2004) classification decisions may have characterized some cases truly having mild dementia as MCI, thereby underestimating dementia risk. Each case was now rated independently by three investigators (S.K.M., W.P.S., W.B.Z.) based on complete profiles of performance over time and blind to the original decisions, with dementia classifications accompanied by a confidence rating using a 7 point Likert scale (virtual certainty = 7, pure guess = 1). In all cases where certainty was less than “6”, the investigator provided a second choice dementia classification to indicate if uncertainty reflected greater or lesser severity of decline.
In cases where there was perfect agreement among raters, dementia status was determined based on the three independent ratings. In cases where there was any disagreement, a full consensus conference including no fewer than five members of the research team was conducted during which dementia status was determined based on detailed consideration of all available data (see Zigman et al., 2004). These ratings of dementia status generated seven first/second choice categories for analyses: (a) Not declining with high confidence (N = 73), (b) Not declining but possibly MCI (N = 7), (c) MCI but possibly Not declining (N = 6), (d) MCI with high confidence (N = 7), (e) MCI but possibly Demented (N = 1), (f) Demented but possibly MCI (N = 3), and (g) Demented with high confidence (N = 4).
To allow a comparison with the general population, data were obtained from the Washington Heights and Inwood Columbia Aging Project (WHICAP), which included a multiracial representative sample of adults 65 years of age and older living in northern Manhattan, New York (Tang, Cross, Andrews, Jacobs, et al. 2001). Dementia status for these individuals was rated as either “present” or “absent” based on a consensus process that considered physical and neurological evaluations by physicians and performance on a battery of standardized neuropsychological assessments, consistent with DSM criteria (Stern, Andrews, Pittman, et al., 1992).
Individuals within the WHICAP sample (N=940) were screened based on age at enrollment and duration of surveillance in order to match the sample with ID. A subgroup was excluded because they were either followed for less than 1.6 years (N=125) or longer than 4.0 years (N=128). A final excluded subgroup consisted of individuals over 85.5 years of age at baseline (N=78), yielding a final sample of 609 individuals. The general characteristics of the ID and WHICAP samples are summarized in Table 1.
Table 1.
Characteristics of Participants with (ID) and without (WHICAP) Intellectual Disabilities
| ID (N = 101) | WHICAP (N = 609) | |
|---|---|---|
|
|
||
| Age (Years) | ||
| Mean | 73.2 | 75.2 |
| S.D. | 5.1 | 4.9 |
| Range | 64.9 – 85.4 | 65.1 – 85.4 |
| Sex | ||
| Males | 44% | 33% |
| Females | 56% | 67% |
| IQ | ||
| Mean | 41 | - |
| S.D. | 12.5 | - |
| Range | <20 – 71 | - |
| Duration of Follow-up (Years) | ||
| Mean | 3.0 | 2.7 |
| S.D. | 0.38 | 0.72 |
| Range | 1.9 – 4.6 | 1.6 – 4.5 |
Analytic Methods
Cumulative incidence was estimated employing life-table analyses with individuals considered to be at risk from birth until their age at dementia onset (the point midway between age at assessment when “dementia” was first detected and the immediately preceding assessment) or, if unaffected, age at their last assessment. Here, as distinct from Zigman et al., cumulative incidence of dementia was compared for groups with and without ID in a series of analyses, the only difference being the operational case definition for adults with ID. An initial analysis classified “dementia” conservatively as any case classified as “Dementia with high confidence”. Subsequent analyses relaxed that criterion stepwise, such that the dementia group was expanded to include cases as follows: (a) Dementia but possible MCI, (b) MCI but possible Dementia, (c) definite MCI, (d) MCI but possibly not declining, and (e) Not declining but possible MCI. Of course, as more cases were added the estimated risk for adults with ID increased relative to the risk in the general population, and a goal of these analyses was to determine how relaxed the criterion for “dementia” had to become within the Zigman et al. (2004) sample in order to replicate Strydom et al. (2009).
Results
Hazard ratios (HRs) for five of the operationalized dementia criteria for adults with ID are provided in Table 2. As can be seen in Table 2, there was no significant indication of increased risk of dementia associated with ID when only cases with a primary classification of dementia were considered, HRs = 1.1 – 1.6. When cases classified as MCI but possibly demented were added, relative risk increased further (HR = 1.9), but as indicated by the confidence interval, the group with ID was still not significantly different from the general population reference. Once cases with ID classified as MCI without suspicion of dementia were added, however, the two samples differed significantly, HRs ≥ 3.2.
Table 2.
Numbers of Cases of Dementia among Adults with Intellectual Disability Related to Stringency of Case Definition, along with Hazard Ratios Indicating Relative Risk Compared to the Typically Developing Population
| Criterion to be Exceeded | # Cases | Hazard Ratio | 5% Confidence |
|---|---|---|---|
|
|
|
||
| Demented | 4 | 1.1 | 0.4 – 2.8 |
| Demented but Possibly MCI | 7 | 1.6 | 0.7 – 3.5 |
| MCI but Possibly Dementia | 8 | 1.9 | 0.9 – 3.9 |
| MCI | 15 | 3.2 | 1.7 – 6.0 |
| MCI but Possibly Not Demented | 21 | 4.3 | 2.4 – 7.8 |
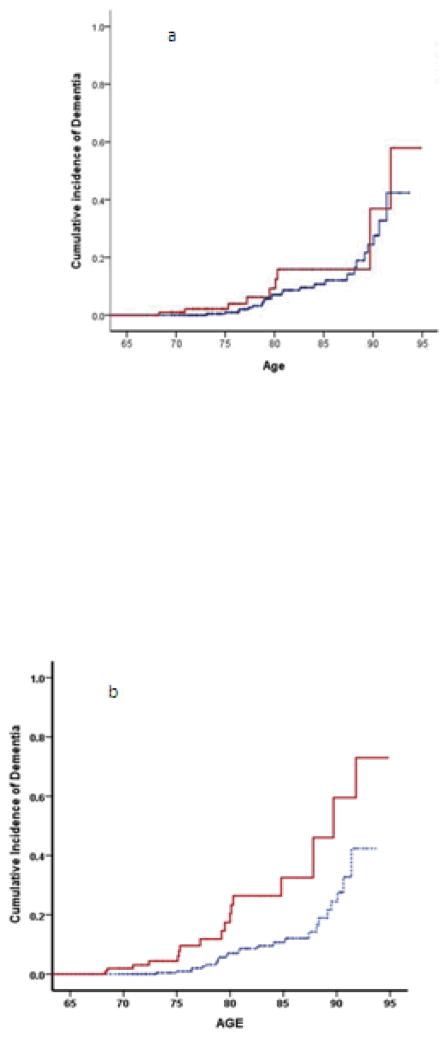
Figure 1a illustrates two cumulative incidence curves, one for the WHICAP sample (controls) and a second for adults with ID when those cases were limited to individuals with consensus classifications indicating dementia as the “primary” classification (“Dementia with high confidence” or “Dementia but possibly MCI”). Figure 1b compares cumulative incidence between the WHICAP and ID samples when cases in the latter group also included individuals having a primary consensus rating of MCI (“MCI but possibly demented” or “MCI with high confidence” or “MCI but possibly not declining”). Figure 1a essentially replicates Zigman et al. (2004) while Figure 1b essentially replicates Strydom et al. (2009) (although omitting MCI cases with some doubt in the direction of “Not Declining” is an even closer approximation – see Table 2).
Figure 1.
Cumulative incidence of dementia for adults with intellectual disability (higher curves in each panel) compared to general population controls (lower curves). Positive cases with ID were restricted to individuals with a primary consensus classification of “Dementia” in Figure 1a, while Figure 1b shows comparable curves when cases with ID also included individuals with mild cognitive impairment.
Discussion
The present analyses were able to reconcile the disparate findings of Zigman et al. (2004) and Strydom et al. (2009) simply by varying the operational definition of dementia. Results showed comparable risk for adults with and without ID, replicating Zigman et al., when “dementia cases” were limited to individuals classified as having possible or definite dementia during consensus conferences, even when some uncertainty existed in the direction of MCI. Results indicated increased risk associated with ID, matching Strydom et al. (2009) and even Strydom et al. (2013) when confidence intervals are taken in consideration, when the case definition was expanded to include individuals with MCI as well as those with dementia. Thus, the present results tended to support the findings of Zigman et al. more strongly than Strydom et al., and this could have practical implications for program planning as well as theoretical implications suggesting that risk for dementia may be less dependent on cognitive reserve than currently hypothesized. However, null findings always raise power concerns, and reasonable people could argue that even when the hazard ratio itself was consistent with Zigman et al., the confidence interval also overlapped with the findings of Strydom et al. (2009). A slight increase in the number of cases classified with dementia was observed compared to the original Zigman et al. results (seven versus five, respectively), and this also increases uncertainty regarding a null conclusion. Whatever that uncertainty may be, though, the present results clearly illustrate that subtle differences in case definition can have substantial impacts of estimates of incidence/prevalence.
For individuals with typical lifespan development, MCI is associated with measurable declines in cognition and was originally thought to leave functional abilities largely unaffected, providing a qualitative standard for ruling out dementia. However, subsequent research has shown that declines in instrumental activities of daily living (IADLs) can be affected (see Winblad, et al, 2004). Thus, the distinction between mild dementia and MCI has shifted somewhat over time toward one of degree of impairment rather than the presence of decline in any specific domain, broadly considered. Paralleling the declines in IADLs accompanying MCI in the general population, it seems reasonable to suppose that some of the relatively more cognitively demanding functional skills assessed by adaptive behavior scales could be affected in adults with ID as MCI develops.
Currently, there is no consensus within the ID field regarding methods to assess dementia or objective criteria for diagnosis (cf. Krinsky-McHale & Silverman, in press). In particular, the distinction between MCI and early/mild dementia has attracted very little attention despite the dramatic increase in interest in MCI within the broader fields of geriatric medicine and aging research. Objectively differentiating between MCI and dementia for older adults with ID is clearly complicated by the presence of pre-existing impairments and even more so by the substantial variability in the severity of these impairments characteristic of this population. As the present findings show, that distinction is critically important for determining if ID, in and of itself, is associated with increased risk for dementia. Because the prevalence of MCI within the general population over 65 is approximately 20% and that of dementia is approximately 6%, misclassifying adults with ID and MCI as demented could easily show the approximately threefold increase in risk reported by Strydom et al. (2009) when no such effect in fact exists. The fact that Strydom et al. (2012) found considerable divergence from their original classifications of dementia status at follow-up some three years later reinforces this point. On the other hand, relative risk will be underestimated to the extent that individuals with mild dementia are misclassified as having MCI. While the present findings seem more supportive of the former alternative, they also suggest that the original Zigman et al. (2004) criterion for dementia may have leaned toward the conservative side. Given these distinct possibilities, the most prudent conclusion to reach at this point is that the true relative risk for dementia associated with ID remains uncertain.
Three strategies seem promising for resolving this uncertainty. The first is to develop evidence-based methods for making the distinction between MCI and mild dementia, specifying empirically those features most useful for differentiating between these conceptually distinct diagnoses. That can be an immediate focus of research, but a cautionary note seems appropriate. Considerable time has elapsed since Burt and Aylward (1997) published recommendations for developing a consensus regarding diagnosis of dementia, yet the operationalization of those recommendations has not yet been realized. Given the complications inherent in the task of differentiating between MCI and dementia for adults with ID, it seems unrealistic to expect our field to develop and validate appropriate methods and standards within the near future.
A second potential strategy might capitalize on current efforts to identify biomarkers that can inform diagnosis of MCI or dementia within the general population. To date, these efforts have focused primarily on neuroimaging findings and assays of blood and cerebrospinal fluid, but no definitive biomarker has been found. Nevertheless, this is a very active area of present research and promising directions for current and future work are being identified (cf. Hampel, Lista and Khachaturian, 2012). In addition to physical biomarkers, behavioral changes distinct from declining performance, such as the emergence of problem behaviors or neuropsychiatric concerns, might indicate progression of underlying progressive neuropathology, although findings to date suggest that only a minority of affected individuals would be expected to experience these changes (e.g., Urv et al, 2010).
A third strategy seems more promising for determining if ID is associated with increased dementia risk. Rather than struggling with the task of distinguishing between MCI and mild dementia, the combined incidence/prevalence of MCI and dementia can be compared across populations. Technically, this would entail its own set of challenges, requiring case definitions of “substantial decline in cognition” for adults with ID comparable to the standard of practice for MCI within the general population. Obviously, the primary focus of such case definitions would need to be on declines in performance or abilities referenced to an individual’s established baseline. While this seems like it should be achievable in the near future, the types and magnitude of declines sufficient for defining “substantial declines” over and above those expected with aging, per se, would still need to be determined empirically, and this goal needs to be added to the research priorities within the field. Once that is accomplished, we will be in a better position to determine whether ID, in and of itself, increases dementia risk and to anticipate more accurately the levels of supports that will be needed by the increasing numbers adults with ID reaching ages at risk for the many causes of neuropathology underlying old-age associated dementia.
Acknowledgments
Appreciation is extended to all our participants, their families and the agencies serving the needs of individuals with intellectual and developmental disabilities for their invaluable support over many years. We acknowledge Deirdre Conlon, Lisa Kullman, Tracy Listwan, Catherine Marino, Giovanna Palma, David Swift, Anna Trzeciak, and Sheelagh Vietze for their dedication and skills in data collection and processing, and Deborah Pang, Robert Ryan and Marcia Dabbene, our project coordinators, for their many contributions to our research program. We thank Dr. Richard Mayeux and the WHICAP team of investigators for the generous sharing of their incidence data. This work was supported by NIH grants P01 HD035897, R01 AG037212, P01 AG007232, and P30 HD024061, as well as the New York State Office for People with Developmental Disabilities.
Footnotes
Except for ruling out Down syndrome based on phenotype, etiology of ID was not determined.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: Fourth edition text revision, DSM-IV-TR. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Ball S, Holland A, Hon J, Huppert F, Treppner P, Watson P. Personality and behaviour changes mark the early stages of Alzheimer’s disease in adults with Down’s syndrome: findings from a prospective population-based study. International Journal of Geriatric Psychiatry. 2006;21:661–673. doi: 10.1002/gps.1545. [DOI] [PubMed] [Google Scholar]
- Ball S, Holland A, Treppner P, Watson P, Huppert F. Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer’s disease in adults with Down syndrome and mild to moderate learning disabilities. British Journal of Clinical Psychology. 2008;47:1–29. doi: 10.1348/014466507X230967. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurology. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt DB, Aylward EH. Assessment methods for diagnosis of dementia. In: Janicki MP, Dalton AJ, editors. Dementia, Aging, and Intellectual Disabilities: A Handbook. Philadelphia: Taylor and Francis; 1999. pp. 141–153. [Google Scholar]
- Ganguli M, Snitz B, Saxton J, Chang C, Lee C, Vander Bilt J, Petersen R. Outcomes of mild cognitive impairment by definition: A population study. Archives of Neurology. 2011;68:761–767. doi: 10.1001/archneurol.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen R, Ritchie K, Brioch, Winblad B on behalf of the participants of the International Psychogeriatric Association Expert Conference On Mild Cognitive Impairment. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Hampel H, Lista S, Khachaturian Z. Development of biomarkers to chart all Alzheimer’s disease stages: The royal road to cutting the therapeutic Gordian Knot. Alzheimer’s & Dementia. 2012;8:312–336. doi: 10.1016/j.jalz.2012.05.2116. [DOI] [PubMed] [Google Scholar]
- Holland A, Hon J, Huppert F, Stevens F. Incidence and course of dementia in people with Down’s syndrome: findings from a population-based study. Journal of Intellectual Disabilities Research. 2000;44:138–146. doi: 10.1046/j.1365-2788.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- Janicki MP, Dalton AJ. Prevalence of dementia and impact on intellectual disability services. Mental Retardation. 2000;38:276–288. doi: 10.1352/0047-6765(2000)038<0276:PODAIO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Carr SA. Conceptual evolution in Alzheimer’s disease: Implication for understanding the clinical phenotype of progressive neurodegenerative disease. Journal of Alzheimer’sDisease. 2010;19:253–272. doi: 10.3233/JAD-2010-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky-McHale S, Devenny D, Silverman W. Changes in explicit memory associated with early dementia in adults with Down’s syndrome. Journal of Intellectual Disabilities Research. 2002;46:198–208. doi: 10.1046/j.1365-2788.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- Krinsky-McHale S, Devenny D, Kittler P, Silverman W. Selective attention deficits associated with mild cognitive impairment and early stage Alzheimer’s disease in adults with Down syndrome. American Journal of Mental Retardation. 2008;113:369–386. doi: 10.1352/2008.113:369-386. [DOI] [PubMed] [Google Scholar]
- Krinsky-McHale S, Silverman W. Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Developmental Disabilities Research Reviews. doi: 10.1002/ddrr.1126. (In press) [DOI] [PubMed] [Google Scholar]
- McMurtray A, Clark DG, Christine D, Mendez MF. Early-onset dementia: Frequency and causes compared to late-onset dementia. Dementia and Geriatric Cognitive Disorders. 2006;21:59–64. doi: 10.1159/000089546. [DOI] [PubMed] [Google Scholar]
- Nelson L, Orme D, Osann K, Lott I. Neurological changes and emotional functioning in adults with Down syndrome. Journal of Intellectual Disabilities Research. 2001;45:450–456. doi: 10.1046/j.1365-2788.2001.00379.x. [DOI] [PubMed] [Google Scholar]
- Petersen R, Smith G, Waring S, Ivnik R, Tangalos E, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Saunders P, Copeland J, Dewey M, Gilmore C, Larkin B, Phaterpekar H, Scott A. The prevalence of dementia, depression and neurosis in later life: the Liverpool MRC-Alpha study. International Journal of Epidemiology. 1993;22:838–847. doi: 10.1093/ije/22.5.838. [DOI] [PubMed] [Google Scholar]
- Silverman W, Zigman WB, Kim H, Krinsky-McHale S, Wisniewski HM. Aging and dementia among adults with mental retardation and Down syndrome. Topics in Geriatric Rehabilitation. 1998;13:49–64. [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, Mayeau R. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Archives of Neurology. 1992;49:453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- Strauss D, Eyman R. Mortality of people with mental retardation in California with and without Down syndrome, 1986–1991. American Journal on Mental Retardation. 1996;100:643–653. [PubMed] [Google Scholar]
- Strydom A, Chan T, Fenton C, Jamieson-Craig R, Livingston G, Hassiotis A. Validity of criteria for dementia in older people with intellectual disability. American Journal of Geriatric Psychiatry. 2013;21:279–288. doi: 10.1016/j.jagp.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Strydom A, Chan T, King M, Hassiotis A. Incidence of dementia in older adults with intellectual disabilities. Research in Developmental Disabilities. 2013;34:1881–1885. doi: 10.1016/j.ridd.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Strydom A, Hassiotis A, King M, Livingston G. The relationship of dementia prevalence in older adults with intellectual disability (ID) to age and severity of ID. Psychological Medicine. 2009;39:13–21. doi: 10.1017/S0033291708003334. [DOI] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Urv T, Zigman W, Silverman W. Psychiatric symptoms in adults with Down syndrome and Alzheimer’s disease. American Journal on Intellectual and Developmental Disabilities. 2010;115:265–276. doi: 10.1352/1944-7558-115.4.265. [DOI] [PubMed] [Google Scholar]
- Ward A, Arrighi HM, Michels S, Cedarbaum J. Mild cognitive impairment: Disparity of incidence and prevalence estimates. Alzheimer’s and Dementia. 2012;8:14–21. doi: 10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Wimo A, Winblad B, Aquero-Torres H, von Strauss E. The magnitude of dementia occurrence in the world. Alzheimer’s Disease and Related Disorders. 2003;17:67–67. doi: 10.1097/00002093-200304000-00002. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Petersen RC. Mild cognitive impairment – beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Zaccai J, Ince P, Brayne C. Population-based neuropathological studies of dementia: design, methods and areas of investigation – a systematic review. BMC Neurology. 2006;6:2. doi: 10.1186/1471-2377-6-2. Published online 2006 January 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman W, Lott I. Alzheimer’s disease in Down syndrome: Neurobiology and risk. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:237–246. doi: 10.1002/mrdd.20163. [DOI] [PubMed] [Google Scholar]
- Zigman WB, Schupf N, Devenny DA, Miezejeski C, Ryan R, Urv TK, Schubert R, Silverman W. Incidence and prevalence of dementia in elderly adults with mental retardation without Down syndrome. American Journal on Mental Retardation. 2004;109:126–141. doi: 10.1352/0895-8017(2004)109<126:IAPODI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]