Summary
The Ten-Eleven-Translocation 2 (TET2) gene, which oxidates 5-methylcytosine in DNA to 5-hydroxylmethylcytosine (5hmC), is a key tumor suppressor frequently mutated in hematopoietic malignancies. However, the molecular regulation of TET2 expression is poorly understood. We show that TET2 is under extensive microRNA (miRNA) regulation and such TET2-targeting is an important pathogenic mechanism in hematopoietic malignancies. Using a high-throughput 3′UTR activity screen, we identify >30 miRNAs that inhibit TET2 expression and cellular 5hmC. Forced expression of TET2-targeting miRNAs in vivo disrupts normal hematopoiesis, leading to hematopoietic expansion and/or myeloid differentiation bias, whereas co-expression of TET2 corrects these phenotypes. Importantly, several TET2-targeting miRNAs, including miR-125b, miR-29b, miR-29c, miR-101, and miR-7, are preferentially overexpressed in TET2-wildtype acute myeloid leukemia. Our results demonstrate the extensive roles of miRNAs in functionally regulating TET2 and cellular 5hmC, and reveal miRNAs with previously unrecognized oncogenic potential. Our work suggests that TET2-targeting miRNAs might be exploited in cancer diagnosis.
Introduction
The recently discovered TET genes are key players in epigenetic regulation, with important roles in development and cancer. All three TET proteins, including TET1, TET2 and TET3, are enzymes that catalyze the conversion of 5-methylcytosine (5mC) in genomic DNA to 5hmC and its oxidative derivatives (Ito et al., 2010; Tahiliani et al., 2009; Ito et al., 2011; He et al., 2011). These enzymatic activities are involved in both active and passive DNA demethylation (reviewed in (Wu and Zhang, 2011; Cimmino et al., 2011; Shih et al., 2012)), the tight regulation of which is essential in defining and safeguarding cellular identities. Accordingly, TET gene expression and 5hmC levels are often downregulated in a wide spectrum of cancers (Lian et al., 2012; Hsu et al., 2012; Yang et al., 2013). In particular, haploinsufficient loss-of-function mutations in TET2 are frequently found in patients of a variety of hematopoietic malignancies, including acute myeloid leukemia (AML), myeloproliferative neoplasms, myelodysplastic syndromes, chronic myelomonocytic leukemia (CMML), and lymphoid malignancies (Cimmino et al., 2011; Shih et al., 2012). In mouse models, homozygous or heterozygous loss of TET2 results in enhanced hematopoietic stem cell activity and CMML-like malignant progression (Moran-Crusio et al., 2011; Quivoron et al., 2011a; Li et al., 2011).
Increasing efforts are underway to incorporate TET2 mutational status in routine clinical diagnostics to inform molecular pathogenesis and therapeutic outcomes. However, genetic TET2 mutation analysis is not sufficient to completely capture TET2 functional deregulation. For example, it was found that a substantial fraction of AML patients with wildtype TET2 shows similarly decreased 5hmC levels as TET2-mutant AMLs (Ko et al., 2010). Hence, it raises the question whether, in addition to genetic mutations and inhibition of enzyme activity (Mardis et al., 2009; Shih et al., 2012), molecular pathways regulating TET2 expression can serve as an important alternative mechanism in hematopoietic malignancies, and should be considered in diagnosis.
Despite the importance of TET gene dosage control, much less is known about the mechanisms that regulate TET gene expression (Kallin et al., 2012; Wu et al., 2012; Song et al., 2013b, 2013a; Zhang et al., 2013). miRNAs are small non-coding RNAs that downregulate target gene expression by inhibiting target messenger RNA stability and translatability (Bartel, 2009). Target downregulation by miRNAs is primarily achieved through cognitive sites in the 3′-untranslated regions (3′UTRs), with miRNA binding sites in other regions of target transcript generally contributing much less to functional regulation (Bartel, 2009). However, despite increasing understandings of how miRNAs regulate their targets, faithful identification of miRNA-mediated functional targeting still presents a major challenge.
In this study, we systematically surveyed miRNA-mediated regulation of TET2 expression, and the roles of TET2-targeting miRNAs in abnormal hematopoiesis. Using a high-throughput screen, we identified a large network of miRNAs capable of inhibiting TET2 expression. Among the TET2-targeting miRNAs were those that induce traits associated with malignant hematopoiesis in vivo, and were preferentially expressed in TET2-wiltype AMLs. This study reveals a group of miRNAs with previously unrecognized oncogenic potentials in malignant hematopoiesis. Given the limited expression range of TET2 itself, our data suggest that for cancers with wildtype TET2 status, in addition to screening IDH1/2 (Shih et al., 2012), TET2-targeting miRNAs could be useful diagnostic biomarkers and potential therapeutics.
Results
A High-throughput Reporter Screen Identifies a Large Network of miRNAs that Inhibit TET2 3′UTR
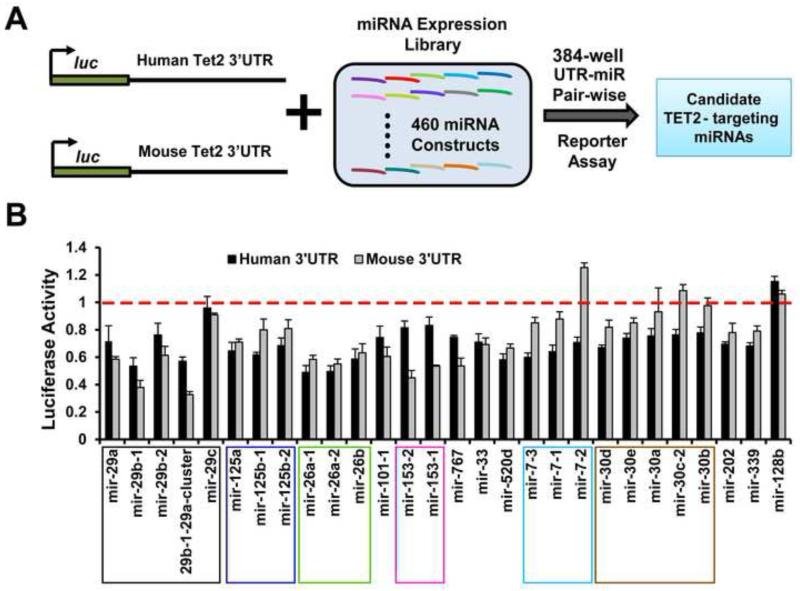
To identify TET2-targeting miRNAs, we undertook an unbiased high throughput screen to identify miRNA-mediated regulation of TET2 3′UTR (Fig 1A). Unlike biochemical identification of miRNA binding regions on target mRNA (Lipchina et al., 2011; Chi et al., 2009; Hafner et al., 2010), this approach produced functional miRNA-target relationships rather than just binding relationship. We first cloned 3′UTR luciferase reporters of human and mouse TET2 from the corresponding full length isoforms. Although several splicing variants of TET2 have been reported (Langemeijer et al., 2009; Moran-Crusio et al., 2011), only the full length isoforms encode the catalytic domain in the C-terminus, the importance of which was confirmed in a murine knockout study (Quivoron et al., 2011b). We next successfully miniaturized a cell-based reporter assay system, with which we quantified the effects of ~460 individual miRNA constructs (expressing a single miRNA or miRNA cluster) one-by-one with human or mouse TET2 3′UTR reporters in quadruplicates in 384-well plates. For the vast majority of the assayed miRNA-3′UTR pairs, the miRNAs had either no or weak effect on the corresponding TET2 3′UTR (Fig S1A, S1B, Table S1). In contrast, 48 miRNA-3′UTR pairs (see Experimental Procedures) led to a >25% repressive effect. (Fig 1B, Fig S1B, Table S1). Compared to two popular computational target prediction algorithms TargetScan and mirSVR (Grimson et al., 2007; Betel et al., 2010), these inhibitory miRNA-3′UTR relations include only 13% (32 out of 246) of predicted relations by both algorithms, or 9% (44 out of 491) of those predicted by either algorithm, suggesting that the majority of the algorithm-predicted miRNA-3′UTR pairs had weak or no effect (Fig S1C). In addition, 4 (8.3% of all) inhibitory miRNA-3′UTR relations were not predicted, and 12 (25%) were only predicted by one of the two algorithms, suggesting a significant level of false-negatives by these computational predictions. These data support the importance of defining TET2-targeting miRNAs through experimental approaches.
Figure 1. A 3′UTR reporter screen identifies candidate TET2-targeting miRNAs.
(A) A schematic of the high-throughput screen, in which ~460 human miRNA expression constructs were assayed one by one with human or mouse TET2 3′UTR reporters. (B) Data for a subset of candidate TET2-targeting miRNAs identified through the screen, as well as the non-targeting miR-128b, are shown. Normalized luciferase activities are plotted with the red line and with 1 representing averaged luciferase activities of controls. miRNAs that belong to the same family are boxed. Error bars represent standard deviation. N=4. See also Figure S1, S2 and Tables S1 and S6.
The 48 miRNA-3′UTR pairs with >25% repression consist of 32 unique miRNAs that repress either human or mouse TET2 3′UTRs. Among such TET2-targeting miRNAs, different constructs from the same miRNA family (i.e. sharing the same seed sequence) often led to similar effects, such as miR-29, miR-125 and miR-26 families (Fig 1B). We also noticed that while many candidate miRNAs had similar levels of repression on both human and mouse TET2 3′UTRs, some show species-specificity in functional targeting. For example miR-7 family had a much weaker repression of mouse 3′UTR compared to human. To validate the screen result, we repeated the luciferase reporter assays on the candidate miRNAs and obtained highly consistent data (Fig S1D). In addition, by mutagenesis, we confirmed that miR-29b, miR-125a, miR-101 and miR-26a regulate TET2 3′UTR through specific binding sites, supporting direct regulation (Fig S2). The data above show that our high-throughput reporter assay approach can reproducibly and systematically reveal miRNA-mediated regulation of TET2 3′UTR by an extensive network of miRNAs.
These candidate TET2-targeting miRNAs contain mostly two classes of miRNAs: (1) miRNAs that are not known to be involved in hematopoietic malignancies; (2) oncogenic miRNAs without fully understood downstream mechanisms. For example, forced expression of miR-29a induces malignant hematopoiesis, yet the relevant miR-29a target for this biology is unknown (Han et al., 2010). On the other hand, miR-29b and miR-29c are not known to be myeloid oncogenes (Han et al., 2010), and miR-29b was instead reported as a tumor suppressor in myeloid leukemia (Garzon et al., 2009a, 2009b; Huang et al., 2013). In contrast to these reports, our data (Fig 1B) suggest that miR-29b and miR-29c could regulate TET2 and function as hematopoietic oncogenes under certain circumstances. Another example is the miR-125a/b family, which is known to induce a CMML-like disease (reviewed in (Shaham et al., 2012), (Guo et al., 2012)) via incompletely characterized mechanisms. Our data also suggest that additional TET2-targeting miRNAs, such as miR-101, miR-7 and miR-26a/b, may play oncogenic roles in hematopoietic malignancies, which have not been previously recognized. We thus went on to further characterize the effects of TET2-targeting miRNAs in vitro and in vivo.
TET2-targeting miRNAs Downregulate TET2 Protein and 5hmC Levels in Hematopoietic Cells
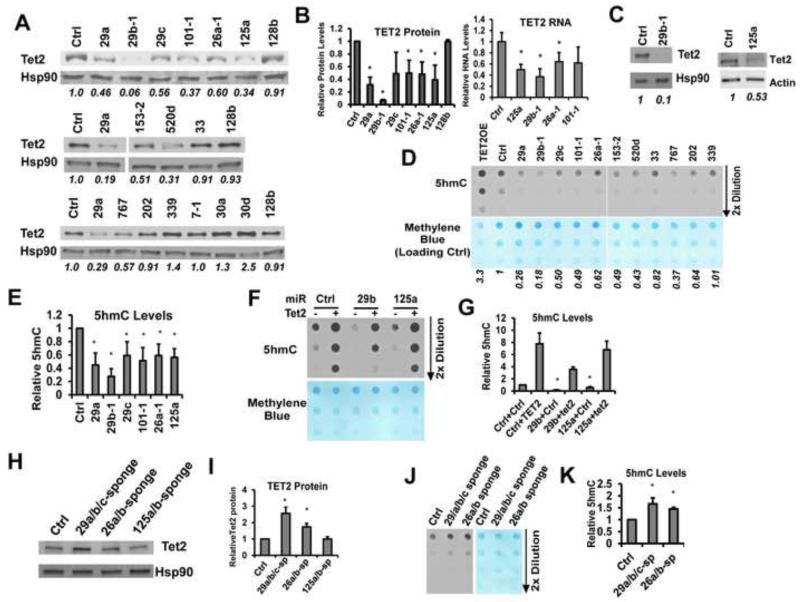
To systematically examine the functions of TET2-targeting miRNAs, we first determined whether these candidate miRNAs can regulate endogenous TET2 expression and function in hematopoietic cells. We initially examined hematopoietic cell lines BaF3 (murine) and K562 (human), which express endogenous TET2 protein at detectible levels (Fig S3A, S3B). We individually expressed ~16 candidate miRNAs in BaF3 and K562 cells, as well as a negative control miR-128b. We focused on those miRNAs that target both human and mouse 3′UTRs, as well as a few that preferentially target the human 3′UTR. Many of the miRNAs, including those from miR-29, miR-125, and miR-26 families, miR-101, and miR-520d, significantly suppressed endogenous TET2 protein expression in both murine and human cells (Fig 2A, S3C). The miRNA-mediated downregulation of TET2 was also detected on the RNA level (Fig 2B). To confirm the regulation of TET2 in primary cells, we expressed miR-29b and miR-125a in primary murine bone marrow cells, which similarly decreased TET2 protein levels (Fig 2C). Consistent with the luciferase reporter screen data, miR-7 only reduced TET2 protein level in human but not in murine cells (Fig 2A, S3C). The decreases in endogenous TET2 protein levels were also accompanied by decreases in total cellular 5hmC levels (Fig 2D-2G, S3D), supporting that the function of TET protein(s) was compromised. Importantly, the downregulation of cellular 5hmC by miR-29b or miR-125a could be rescued by expression of a TET2 cDNA without 3′UTR (Fig 2F, 2G), further supporting the role of a miRNA-TET pathway in the control of cellular 5hmC levels.
Figure 2. TET2-targeting miRNAs regulate TET2 expression and cellular 5hmC levels.
(A) BaF3 cells were transduced with control vector (ctrl) or indicated miRNAs, including miR-128b which does not target TET2. TET2 and HSP90 protein levels were determined by western blot. Representative data are shown out of two to three repeats. Quantification of TET2 protein level is indicated, after normalization to HSP90. (B) Quantification of the effect of indicted miRNAs on TET2 protein or RNA levels in BaF3 cells. Data were quantified from three repeats. (C) Primary mouse bone marrow cells were transduced with indicated miRNAs. Representative western blots are shown out of two (miR-125a) and three (miR-29b) experiments. (D) Genomic DNA from BaF3 cells transduced with ctrl or indicated miRNAs or a TET2 overexpression vector (TET2OE) was analyzed for 5hmC levels using dot blot assay. Blot was stained with methylene blue to control for loading. Normalized 5hmC levels are indicated. Representative blot is shown out of two to three repeats. Note that the separated 5hmC images were from the same exposure of the same blot, and same for methylene blue. (E) Quantification of 5hmC data, N=3. (F) TET2 cDNA or ctrl was co-expressed with indicated miRNAs. Cellular 5hmC was assayed with dot blot. Representative data are shown out of three repeats. (G) Quantification of data in (F). N=3. Indicated statistical significance (by *) was evaluated in comparison to Ctrl+Ctrl. (H) BaF3 cells were transduced with a control vector, or sponges that inhibit miR-29 family, miR-26 family, or miR-125 family. TET2 and HSP90 protein levels were determined by western blot. Representative data are shown from three repeats. (I) Quantification of western data in (H), N=3. (J) Cellular 5hmC levels from BaF3 cells transduced with ctrl or indicated miRNA sponges were determined by dot blot analysis. Representative data are shown from three repeats. (K) Quantification of 5hmC levels for (J), after normalizing with methylene blue. N=3. For all figures: error bars represent standard deviation. * p<0.05. See also Figures S3, S4 and Table 2.
To determine whether the regulation by TET2-targeting miRNAs are physiologically or pathologically relevant, we first quantified the overexpression levels of miR-125a, miR-29b, miR-101 and miR-26a using quantitative RT-PCR, and compared to those seen in human AML samples (see Experimental Procedures for details). Results showed that the overexpression levels of miR-125a and miR-101were comparable to or lower than those reachable in AML samples, especially when considering the contribution of family members such as miR-125b (Fig S4C, S4D). For miR-29b and miR-26a, the levels of overexpression were ~2 to 10-fold higher than clinical AML samples (Fig S4E, S4F). We thus asked whether TET2 expression was under the control of endogenous miR-29 and miR-26 family miRNAs. We used miRNA sponges against miR-29 family or miR-26 family, which are decoy targets that inhibit miRNA function (Ebert et al., 2007). In BaF3 cells that express endogenous miR-29b and miR-26a at levels comparable to those in clinical samples (Fig S4E, S4F), both miR-29a/b/c sponge and miR-26a/b sponge led to a significant increase in endogenous TET2 protein level, as well as an increase in cellular 5hmC (Fig 2H-2K). In contrast, miR-125a/b sponge did not increase TET2 protein, since endogenous miR-125a/b expression is low in BaF3 cells (Fig S4C, Table S2) and thus serving as a negative control. Taken together, these data demonstrate that the endogenous TET2 can be regulated by an extensive network of miRNAs, the expression of which contributes to controlling the epigenetic landscape via targeting a central regulator of cellular 5hmC levels.
Multiple TET2-targeting miRNAs Also Regulate TET1 and TET3
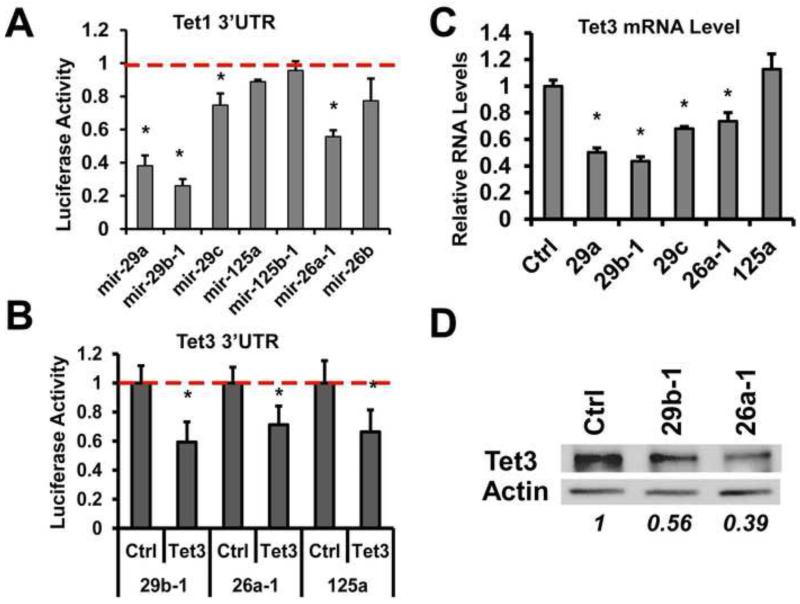
Given that there are three TET family proteins and that TET3, like TET2, is abundantly expressed in hematopoietic tissues (Ito et al., 2010), we asked whether miR-29b, miR-26a and several other TET2-targeting miRNAs can also regulate TET1 and TET3. Indeed, expression of miR-29 and miR-26 family miRNAs resulted in an inhibition of TET1 and TET3 3′UTR reporter activities (Fig 3A, 3B). These miRNAs also decreased endogenous TET3 RNA and protein levels in hematopoietic cells (Fig 3C, 3D). The endogenous TET1 RNA level was too low to be reliably quantified in these cells (data not shown). These data reveal miRNA-mediated regulation of TET1 and TET3, and suggest that these specific miRNAs can function as “master regulators” of cellular 5mC to 5hmC conversion by targeting all three TET family members.
Figure 3. Some TET2-targeting miRNAs regulate additional TET family members.
(A) Human TET1 luciferase reporter was analyzed with indicated miRNAs, with red line representing control (Ctrl) levels. N=4. (B) Mouse TET3 luciferase reporter was analyzed with indicated miRNAs, with red line representing control levels. N=4 (C) TET3 RNA levels in K562 cells transduced with indicated ctrl or miRNAs were determined by qRT-PCR. N=3. (D) TET3 protein levels were analyzed in K562 cells transduced with control or indicated miRNAs by western blot. Representative blots are shown out of two repeated experiments. All error bars represent standard deviation. * p<0.05.
Expression of TET2-targeting miRNAs Leads to Malignant Hematopoietic Traits
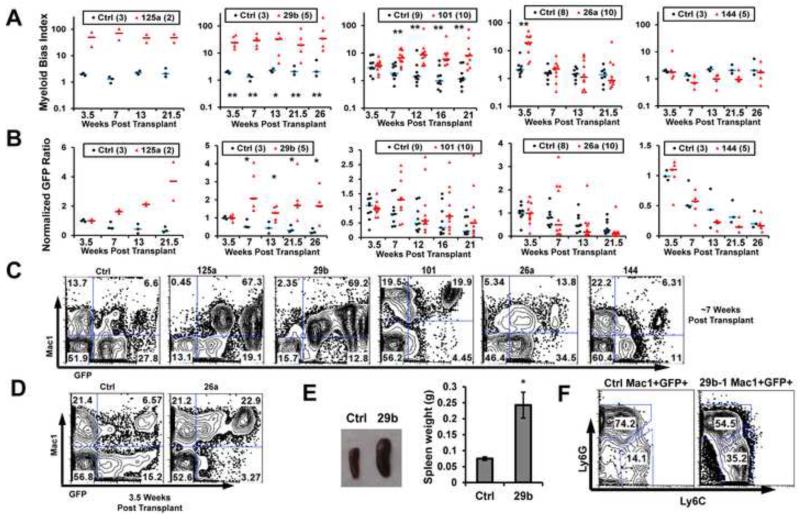
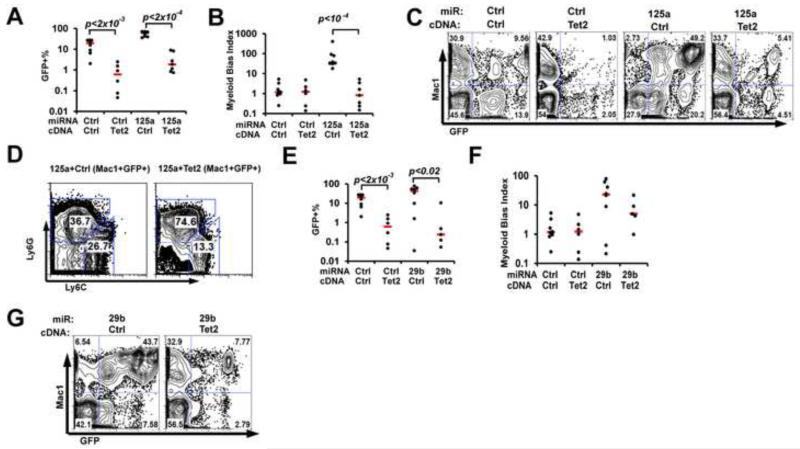
To determine whether TET2-targeting miRNAs can cause malignant hematopoiesis, we next examined the effect of 10 miRNAs in vivo. Forced expression of control or candidate miRNAs was delivered through viral transduction of wildtype bone marrow cells, with GFP marking transduced cells, followed by bone marrow transplantation into lethally irradiated host mice. We paid special attention to two hematopoietic traits associated with TET2 loss, namely biased differentiation into the myeloid lineage, as well as hematopoietic expansion (Moran-Crusio et al., 2011; Quivoron et al., 2011a; Li et al., 2011; Ko et al., 2010; Pronier et al., 2011). We used a myeloid bias index to reflect the biased differentiation, which was calculated by ratios of myeloid (Mac1+) versus non-myeloid cells in peripheral blood transduced (GFP+) versus non-transduced (GFP−) populations (see Experimental Procedures). To quantify hematopoietic expansion, we followed the peripheral blood GFP+% from 3 weeks post-transplantation and on. Among TET2-targeting miRNAs, the known oncogenic miR-125a served as a positive control, which led to both increased myeloid bias index, indicating skewed differentiation into myeloid lineage, and an increase in GFP+% over time, indicating hematopoietic expansion (Fig 4A-4C). On the other hand, miR-144, which does not strongly affect TET2 3′UTR, behaved similarly as the control vector (Fig 4A-4C). Consistent with miR-29b functioning as a bona fide oncogene, we observed both increased myeloid bias index and hematopoietic expansion (Fig 4A-4C). In addition, miR-29b mice had splenomegaly, with increased percentage of GFP+Ly6C+Ly6G− monocytes in bone marrow, revealing a CMML-like disease (Fig 4E, 4F). Other candidate miRNAs examined, except for miR-33, displayed these hematopoietic phenotypes to variable severities, and sometimes in unique manners (summarized in Fig S5D). For example, miR-101 expression led to a significant myeloid bias. Interestingly, although the GFP+ cell expansion was not statistically significant in the miR-101 cohorts, 2 out of 10 miR-101 recipients showed persistent hematopoietic expansion, suggesting incomplete penetrance. In contrast, these phenotypes were never observed in cohorts of 12 control recipients (Fig 4A-4C). Another example is miR-26a, which led to a reproducible transient myeloid bias at 3.5 weeks post transplantation (Fig 4A-4D), with 1 out of 10 mice showing hematopoietic expansion. Multiple other tested miRNAs (Fig S5A-S5D) also led to variable degrees of myeloid bias and hematopoietic expansion, often with incomplete penetrance. Of note, the levels of overexpression in vivo were similar to those observed in vitro (Fig S4C-S4F), and we confirmed that 5hmC levels in vivo could be suppressed by TET2-targeting miR-29b and miR-125a (Fig S5E). The differential phenotypes induced by these TET2-targeting miRNAs may reflect the different degrees of repression on TET2, the level of overexpression, and/or the effects from additional targets. Taken together, we identified previously unrecognized functions for miRNAs in derailing normal hematopoiesis. They converge on inhibiting TET2 and induce phenotypes associated with hematopoietic malignancy.
Figure 4. TET2-targeting miRNAs induce abnormal hematopoiesis in vivo.
(A, B) Wildtype bone marrow cells were transduced with control (ctrl) or indicated miRNAs, and transplanted into recipient mice, with GFP labeling transduced cells. Peripheral blood was analyzed in recipients at the indicated time points, with each dot representing one recipient. (A) Myeloid bias index (frequency ratios of (GFP+Mac1+/GFP+Mac1−)/(GFP−Mac1+/GFP−Mac1−)) was calculated to reflect biased presence of myeloid cells in transduced population. (B) Normalized GFP ratios were also calculated (by taking the ratio of GFP+/GFP− cells and normalized to the average at 3.5 weeks) to reflect hematopoietic expansion. Numbers of mice per group are indicated in parentheses. The short horizontal bars represent median levels. (C, D) Representative flow cytometry plots of recipients at ~7 weeks post transplantation (C) or 3.5 weeks post transplantation (D), showing myeloid marker Mac1 and GFP. (E) Splenomegaly in miR-29b recipients. A representative image is shown on the left, with pooled spleen weight data shown on the right. N=4 for ctrl and N=5 for 29b. (F) Bone marrow cells from ctrl or miR-29b recipients were analyzed for granulocyte (Ly6G+Ly6C−) and monocytes (Ly6G−Ly6C+). Representative flow cytometry plots are shown, after gating on transduced myeloid cell populations (GFP+Mac1+ population). Note the increased monocytic frequency in miR-29b transduced cells. Error bars represent standard deviations. *: p<0.05; ** p<0.01. See also Figure S5.
Expression of TET2 Rescues Malignant Phenotypes of TET2-targeting miRNAs
Given the in vivo phenotypes of TET2-targeting miRNAs, we asked whether TET2 expression can rescue miRNA-induced malignant hematopoiesis. We particularly focused on miR-125a, which potently induces a CMML-like disease in mice ((Guo et al., 2012) and reviewed in (Shaham et al., 2012)), and miR-29b, which we characterized above as a new oncogene. Compared to other TET2-targeintg miRNAs, another reason to focus on miR-125a and miR-29b was that both miRNAs induced strong malignant phenotypes in vivo, and it would be more challenging to revert such strong phenotypes. Since in vivo hematopoietic expansion is often correlated with increased hematopoietic colonies in serial methylcellulose cultures in vitro, we first examined the effect of TET2 expression on colony formation in the presence of miR-125a or miR-29b. Specifically, wildtype bone marrow cells were transduced with control, miR-125a or miR-29b (all marked with GFP), in combination with a control or 3′UTR-less TET2 cDNA. Consistent with miR-125a and miR-29b being oncogenes, significant increases in secondary colony formation were observed when either miRNA was expressed (Fig S5F). In contrast, TET2 co-expression potently suppressed miR-125a- and miR-29b-induced secondary colony formation to control levels (Fig S5F). To test the function of TET2 in suppressing the malignant phenotype in vivo, we next transplanted bone marrow cells transduced with both miRNAs and TET2 into recipient mice. Expression of TET2 significantly suppressed the miR-125a and miR-29b-induced increases in hematopoietic output (reflected by GFP+%), and completely normalized the myeloid-differentiation bias in miR-125a recipients (Fig 5A-5C, 5E-5G). In addition, miR-125a-TET2 co-expressing recipients showed the correction of monocytic differentiation bias among myeloid cells (Fig 5D). For miR-29b, myeloid bias index also trended lower with TET2 co-expression, although the data did not reach statistical significance (Fig 5F). This incomplete rescue of miR-29b-induced myeloid bias may be due to additional miR-29b targets or suboptimal stoichiometry of TET2 during rescue. Thus, our data demonstrate the importance of TET2-targeting in the oncogenic activities of miR-125a and miR-29b, and suggest that increasing TET2 expression could be a potential strategy to combat certain groups of hematopoietic malignancies.
Figure 5. TET2 expression rescues malignant phenotypes by oncogenic miRNAs.
Bone marrow cells were transduced with ctrl or miR-125a or miR-29b in combination with a cDNA ctrl vector or TET2. Same numbers of sorted transduced cells were transplanted into each recipient in each experiment. (A, B) For miR-125a/TET2 rescue, GFP+% and myeloid bias index at 3.5 weeks post-transplantation are shown, with each dot representing one recipient. (C) Representative flow cytometry plots for (A, B). (D) Bone marrow cells from miR-125a+ctrl or miR-125a+TET2 recipients were analyzed for granulocyte (Ly6G+Ly6C−) and monocytes (Ly6G−Ly6C+). Representative flow cytometry plots are shown, after gating on transduced myeloid cell populations (GFP+Mac1+ population). Note that the monocytic bias in the miR-125a+ctrl recipient was largely corrected by TET2 expression. (E, F) For miR-29b/TET2 rescue, GFP+% and myeloid bias index at 3.5 weeks post-transplantation are shown, with each dot representing one recipient. (G) Representative flow cytometry plots for (E, F). The short horizontal bars represent median levels. P values are indicated. See also Figure S5.
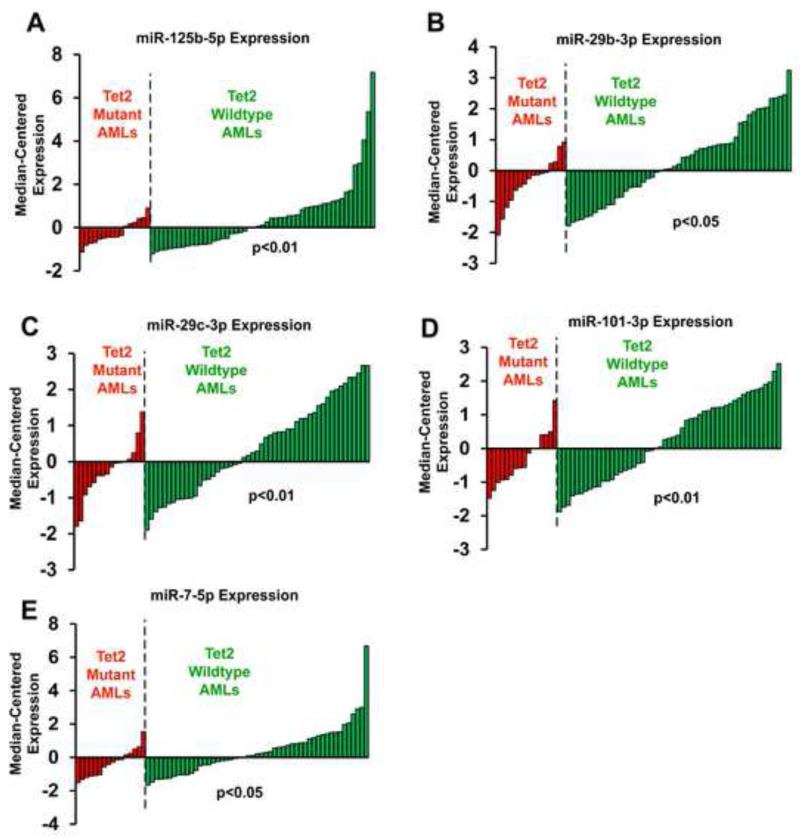
TET2-targeting miRNAs are Preferentially Overexpressed in TET2-wildtype AMLs
We assessed the miRNA-TET2 mechanism in the pathogenesis of human leukemia. Since decreased TET2 function could be a result of either genetic TET2 mutations or elevated expression of its targeting miRNAs, we reasoned that these two mechanisms likely occur independently, rather than redundantly. If true, we would expect TET2-targeting miRNAs to be more frequently overexpressed in TET2-wildtype leukemia, as compared to those harboring TET2 mutations. To test this possibility, we profiled miRNA expression for a cohort of 67 cases of cytogenetically normal AMLs, among which 16 patients carry protein-sequence-altering TET2 mutations (Table S3). To assess miRNA overexpression outliers, we used a method similar to COPA (Tomlins et al., 2005), and quantified the frequency of TET2-wildtype and TET2-mutant samples with outlier overexpression levels, using false-discovery rate <0.15 to define significant association (see Experimental Procedures). Among the 17 TET2-targeting miRNAs that we measured and passed detection threshold, overexpression of miR-125b, miR-29b, miR-29c, miR-101 and miR-7 were more frequently observed in TET2-wildtype cases than TET2-mutant cases, at two different outlier cutoffs (Fig 6, Tables S4). Interestingly, the overexpression spectra of these miRNAs were not fully overlapped in TET2-wildtype AMLs (Fig S6E), suggesting that TET2-targeting miRNAs are differentially utilized in different AMLs in a largely non-redundant manner. Other TET2-targeting miRNAs were significant at a single outlier cutoff (miR-30e), or had a single strong expressing sample in the TET2-wildtype but not TET2-mutant cohort (e.g. miR-520a-5p and miR-202), or not significant in this cohort (Fig S6A-S6D, Table S4).
Figure 6. Preferential overexpression of TET2-targeting miRNAs in TET2-wildtype acute myeloid leukemia.
The expression of TET2-targeting miRNAs was measured in a cohort of 67 cytogenetically normal AMLs, among which 16 samples were TET2-mutant. Expression data were normalized by subtracting cohort median and dividing by median average deviation to reflect outlier expression patterns. The data for miR-125b-5p (A), miR-29b-3p (B), miR-29c-3p (C), miR-101-3p (D) and miR-7-5p (E) were plotted, with higher bars indicating higher expression. P values reflect the probability of observing more frequent higher expression for the indicated miRNA in TET2-wildtype AMLs than TET2-mutant AMLs. The specific cutoff applied for these p-values is examining the top 33% samples with the highest expression of the indicated miRNA. See also Figure S6 and Tables S3, S4 and S5.
An alternative explanation for the preferential overexpression of TET2-targeting miRNAs in TET2-wildtype leukemia is that TET2 functionally upregulates these miRNAs. To examine this possibility, we overexpressed TET2 cDNA in BaF3 and K562 cells and knocked down TET2 in BaF3 cells (Fig S3B, 2D, S3D), and then profiled miRNA expression. Results showed that although TET2 level modulation altered the expression of some miRNAs, the five TET2-targeting miRNAs that correlated with TET2 mutational status were not positively regulated by TET2 (Table S2). Another possibility is that many other miRNAs can score significantly in this statistical test, and our observation of the association with TET2-targeting miRNAs is solely random. To exclude this possibility, we examined >580 miRNAs profiled in this cohort. Excluding the TET2-targeting miRNAs, there were 196 miRNAs passing detection threshold, among which 9 miRNAs were similarly associated with TET2 wildtype status (Table S5). These 9 miRNAs include miR-99a that is located in the same genomic cluster as miR-125b-2, and thus co-expressed with the TET2-targeting miR-125b (Fig S6F). In addition, three of the miRNAs (miR-18a, miR-18b and miR-19a) are known to be co-expressed and were correlated with each other in our dataset (Table S5). Even without eliminating such influences, our observation of 5 out of 17 (29%) TET2-targeting miRNAs scoring in this test is significant (as compared to 9 out of 196, or 4.6%, p<0.003, Fisher’s exact test). Taken together, these data support the notion that overexpression of a subset of TET2-targeting miRNAs identified in this study can be an important mechanism in human leukemogenesis.
Discussion
Using a high-throughput reporter screen, our study systematically identified miRNA-mediated regulation of TET2 through its 3′UTR, and revealed the roles of TET2-targeting miRNAs in malignant hematopoiesis. We found that in a cohort of cytogenetically-normal human AMLs, multiple TET2-targeting miRNAs, including miR-29b, miR-101, miR-125b, miR-29c and miR-7, were preferentially overexpressed in TET2-wildtype specimens than those with TET2-mutations. These data support a role of miRNA-TET2 pathway in the pathogenesis of human AML and other malignancies, adding a new layer to the existing paradigms of loss-of-function mutations in TET2 and gain-of-function mutations in the IDH genes. Our data also argue that in addition to routine genetic mutational analyses on TET2 and related IDH1/2 genes, which are currently being developed and implemented in clinics, the expression status of our identified TET2-targeting miRNAs could be considered as an additional diagnostic parameter to inform the deregulation of the TET2 pathway. In this regard, measuring TET2-targeting miRNAs has advantages over directly measuring TET2 protein or mRNA levels, due to the limited range of differential TET2 expression and difficulty in its protein measurements. For example, we noticed that the range of differential TET2 RNA expression in AML samples is ~ 3-fold (data not shown) and thus is susceptible to interference by measurement noise. TET2-protein measurements suffer from the same restrains, and western-based measurements require a large number of cells. In contrast, TET2-targeting miRNAs displayed much larger dynamic range of expression. For example, miR-125b expression has a range over 10,000-fold in the same data sets (Fig S4C). Our findings also raise several important questions to be further examined in future diagnostic and prognostic studies. For example, do TET2-targeting miRNAs contribute differently in the pathogenesis of single-allelic TET2 mutants versus bi-allelic mutants (which our study cohort was not statistically powered to address, given that a much larger cohort will be needed)? In addition, it is important to point out that other TET2-targeting miRNAs demonstrated in this study may also have a role in human hematopoietic malignancies, even though they were not significantly associated in this cytogenetically-normal AML cohort, since they may be involved in other AML types or other hematopoietic malignancies (Shih et al., 2012).
While this work was being revised, it was published that miR-22 targets TET2 through 3′UTR and regulates hematopoietic stem cells (Song et al., 2013a, 2013b). While our work systematically complements and extends these findings on TET2 regulation, it is also interesting to note that we did not detect a repressive effect of miR-22 on TET2 3′UTR (Table S1), even though we confirmed miR-22 was overexpressed >12-fold (Fig S4G). In our AML cohort, miR-22 did not show significant association with TET2 mutational status (Table S5). The difference in 3′UTR data may be due to the use of full length TET2 3′UTR in our study versus a much shorter 500-bp 3′UTR fragment (Song et al., 2013b). Since it is recognized that the location of miRNA binding site within 3′UTR and target RNA structure can determine the effectiveness of miRNA binding sites (Bartel, 2009; Long et al., 2007), it raises the possibility that additional mechanisms regulate the presentation of the miR-22 binding site.
Our data also uncovered multiple miRNAs with unrecognized oncogenic potential, and revealed TET2-targeting as a novel relevant mechanism of previously known oncogenic miRNAs. For example, miR-29b was previously recognized as a tumor suppressor in myeloid leukemia (Garzon et al., 2009a, 2009b), but our study demonstrated an opposite oncogenic role of this miRNA. The differences observed may be related to the level of miR-29b expression or the duration of expression. Interestingly, the miR-29 family and miR-26 family miRNAs regulate TET1 and TET3 in addition to TET2, suggesting a miRNA-mediated master regulatory program in shaping cellular 5hmC landscape. The in vitro and in vivo data presented here also showed that miRNAs, such as miR-101, miR-29c, miR-26a/b and miR-520d, can function as previously unappreciated oncogenes by derailing normal hematopoietic differentiation processes, and provided a new molecular mechanism for the known oncogenic miRNAs including miR-125 family and miR-29a. It is also interesting to note that although all these miRNAs were capable of targeting TET2, their in vivo overexpression phenotypes were variable. One possibility for such differences is the involvement of other targets of the specific miRNAs. For example, miR-125 family miRNAs regulate multiple pathways (Shaham et al., 2012), such as enhancing growth factor signaling and inhibiting apoptosis (Guo et al., 2012, 2010), which may cooperate with TET2 repression by this miRNA. Similarly, miR-101 can also regulate the PRC2 component EZH2 (Varambally et al., 2008), suggesting a broad effect of this miRNA in regulating multiple enzymes that control the epigenome. Another example is miR-26 family, which led to transient myeloid differentiation bias, but only caused hematopoietic expansion in a small number of mice. Notably, miR-26 has been shown to target cyclin D2 and E2, and inhibit cell cycle in other cancer types (Kota et al., 2009), a mechanism that may modify the TET2-targeting effect of this miRNA. Alternatively, the different efficiencies of TET2-targeting by different miRNAs may itself contribute to the varying phenotypes. In this regard, it is important to note that minor changes in TET2 expression can not only lead to functional consequences in malignant hematopoiesis, but also be associated with longer latency or incomplete penetrance. For example, heterozygous TET2 knockout in mouse, which led to ~50% loss of TET2 gene expression (Li et al., 2011), results in significant but slower and less frequent malignant transformation than double-allele knockout (Li et al., 2011; Quivoron et al., 2011b; Moran-Crusio et al., 2011). As a third possibility, it is also conceivable that such in vivo phenotype differences were due to different levels of overexpression of TET2-targeting miRNAs (Fig S4). It will be interesting to dissect these possibilities in the future.
Lastly, our data raise the prospect of enhancing TET2 expression to combat certain subgroups of hematopoietic malignancies, and implicate modulating TET2-targeting miRNAs as a strategy for both solid and hematopoietic cancers. Recently, decreased TET gene expression and cellular 5hmC levels have been found as a hallmark of multiple solid cancer types (Yang et al., 2013; Lian et al., 2012; Hsu et al., 2012), whereas elevating TET1 or TET2 gene expression has been proposed as a strategy against melanoma and breast cancer (Lian et al., 2012; Hsu et al., 2012). When we expressed TET2 together with oncogenic miR-29b and miR-125a, we observed strong suppression of miRNA-mediated malignant phenotypes. In the case of miR-125a, TET2 expression not only suppressed hematopoietic expansion, but remarkably corrected multiple differentiation biases induced by miR-125a. These data suggest that targeting mechanisms that inhibit TET2 gene expression may be a useful strategy to overcome certain hematopoietic malignancies. Our findings of an extensive network of TET2-targeting miRNAs and several pan-TET-inhibitory miRNAs raise the possibility and opportunity for future therapeutic intervention in this pathway.
Experimental Procedures
Luciferase Reporter Assay and Analysis
Reporter assays were carried out in 384 well plates. Specifically, 460 miRNA constructs were individually assayed in combination with 3′UTR luciferase reporters. 293T cells were transfected with 6 ng of 3′UTR reporter and 54 ng of a miRNA construct in each well. After two days, luciferase assays were performed using Dual-Glo Luciferase kit (Promega). We built three types of control assays into each 384-well plate, including one or more CtrlUTR-CtrlMiR assays (a control reporter assayed with a control vector for miRNA expression (pMIRWAY-puro vector)), two UTR-CtrlMiR assays (TET2 reporter assayed with a control vector), and multiple CtrlUTR-miR assays (a control reporter assayed with each of the miRNAs on the plate).
Data analysis was performed using custom Matlab codes. We first took the ratio of renilla luciferase versus firefly luciferase readings (RvF ratios). The RvF ratio of any given well, including controls, was then normalized using the following formula. Normalized Luciferase Activity=(RvFWell / mean(RvFUTR-CtrlMiR))/(mean(RvFCtrlUTR-miR)/mean(RvFCtrlUTR-CtrlMiR)). After normalization, the means of all three control assays become 1. We used mean data to identify miRNA-3′UTR relations that resulted in >25% downregulation. To identify TET2-targeting miRNAs, we excluded constructs for clusters of miRNAs, and categorize the same mature miRNA appearing at different genome loci as only one TET2-targeting miRNA.
See Extended Experimental Procedures for more details.
Murine Bone Marrow Transplantation and Related Experiments
All mouse experiments were approved by Yale IACUC committee and followed federal, state and institutional guidelines.
Bone marrow transplantation with single miRNAs cloned into the pMIRWAY-GFP-based vectors was performed as described previously (Guo et al., 2012). These miRNAs include miR-29b-1, miR-125a, miR-26a-1, miR-29c, miR-101-1, miR-767, miR-520d, miR-33, miR-153-2, miR-144 and a vector control.
For Tet2 rescue experiments, 5-FU-primed bone marrow cells were co-transduced with mouse Tet2 cDNA (with puromycin marker), or a corresponding vector control together with a specific miRNA expression construct in pMIRWAY-GFP backbone. Transduced cells were cultured, selected with puromycin,and sorted for GFP+ cells for transplantation. 50,000 cells (per mouse) were injected for miR-125a-related rescue and control groups, and 10,000 cells (per mouse) were injected for the miR-29b rescue and control groups.
Assessment of hematopoietic phenotypes were performed as previously described (Guo et al., 2012; Adams et al., 2012; Guo et al., 2010). Myeloid bias index was used to quantify biased differentiation into myeloid lineages, which was calculated by (%GFP+Mac1+)/(%GFP+Mac1−)/((%GFP−Mac1+)/(%GFP−Mac1−)).To examine monocytic differentiation bias, Mac1+ cells from GFP+ fraction (transduced) were gated before examining Ly6C and Ly6G distribution. To examine hematopoietic expansion, peripheral %GFP+/%GFP− ratios were taken and normalized to the ratio at 3.5 weeks post transplantation.
Assessment of in vivo effect of miRNA overexpression levels or 5hmC levels, Mac1+GFP+ cells were FACS-sorted from recipient mice, and subjected to qRT-PCR or 5hmC analysis.
All flow cytometry antibodies were from BD Biosciences or eBioscience.
Clinical Samples and TET2 Sequence Analysis
All human AML samples were obtained with informed consent and approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (FAHSU). 67 Chinese AML patients with normal cytogentics were enrolled between March 2005 and September 2009 at FAHSU, with a median age of 43 years (range: 18-76), and a female:male ratio of 28:39. Diagnosis and classification of these patients were defined according to the French-American-British (FAB) and World Health Organization (WHO 2008) classifications. Bone marrow samples were collected at presentation. Mononuclear cells were separated by Ficoll Hypaque, frozen and banked, and subjected to genomic DNA and total RNA preparations. TET2 mutations were analyzed by PCR amplification of the entire coding region spanning exon 3 to exon 11 followed by direct bidirectional DNA sequencing as previously described (Delhommeau et al., 2009). For analysis purpose, samples with non-synonymous coding sequence alterations were classified as TET2-mutant, whereas those without amino-acid-altering coding region changes were defined as TET2-wildtype (Table S3).
Statistical Analysis
Student’s t-test was used to assess statistical significance, unless otherwise stated. False discovery rate was calculated following Benjamini Hochberg method.
Additional Procedures
Constructs, computational target analysis, cell culture, western blot and dot blot analysis, quantitative RT-PCR, colony formation assays, and miRNA profiling and data analyses are described in Extended Experimental Procedures.
Supplementary Material
Systematically identifies TET2-targeting miRNAs through a high-throughput 3′UTR screen
TET2-targeting miRNAs regulate cellular 5hmC and malignant hematopoiesis
TET2 expression corrects phenotypes induced by TET2-targeting microRNAs
TET2-targeting miRNAs are preferentially overexpressed in TET2-wildtype leukemia
Acknowledgment
We thank Hao Zhang and Judy Wang for excellent technical assistance with the miRNA expression library preparation. We thank Dianqing Wu, Diane Krause and Haifan Lin for sharing equipment. We thank Cybio for helping setting up the screen. This work was supported in part by NIH grants R01CA149109 (to J.L.), R01GM099811 (to Y.D. and J.L.) and K01DK082982 (to S.G.), NSF grant DBI-0650991 (to Y.D.), Stewart Trust Fellowship (to J.L.), Connecticut Stem Cell Fund award 09SCBYALE27 (to J.L.), and Yale Liver Center Pilot grant (to J.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams BD, Guo S, Bai H, Guo Y, Megyola CM, Cheng J, Heydari K, Xiao C, Reddy EP, Lu J. An In Vivo Functional Screen Uncovers miR-150-Mediated Regulation of Hematopoietic Injury Response. Cell reports. 2012;2:1048–1060. doi: 10.1016/j.celrep.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome biology. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino L, Abdel-Wahab O, Levine RL, Aifantis I. TET Family Proteins and Their Role in Stem Cell Differentiation and Transformation. Cell stem cell. 2011;9:193–204. doi: 10.1016/j.stem.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Massé A, Kosmider O, Le Couedic J-P, Robert F, Alberdi A, et al. Mutation in TET2 in myeloid cancers. The New England journal of medicine. 2009;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nature methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon R, Heaphy C. E. a, Havelange V, Fabbri M, Volinia S, Tsao T, Zanesi N, Kornblau SM, Marcucci G, Calin G. a, et al. MicroRNA 29b functions in acute myeloid leukemia. Blood. 2009a;114:5331–5341. doi: 10.1182/blood-2009-03-211938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon R, Liu S, Fabbri M, Liu Z, Heaphy C. E. a, Callegari E, Schwind S, Pang J, Yu J, Muthusamy N, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009b;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, Farh KK-H, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Molecular cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Bai H, Megyola CM, Halene S, Krause DS, Scadden DT, Lu J. Complex oncogene dependence in microRNA-125a-induced myeloproliferative neoplasms. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16636–16641. doi: 10.1073/pnas.1213196109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Lu J, Schlanger R, Zhang H, Wang JY, Fox MC, Purton LE, Fleming HH, Cobb B, Merkenschlager M, et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14229–14234. doi: 10.1073/pnas.0913574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jungkamp A-C, Munschauer M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y-C, Park CY, Bhagat G, Zhang J, Wang Y, Fan J-B, Liu M, Zou Y, Weissman IL, Gu H. microRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. The Journal of experimental medicine. 2010;207:475–489. doi: 10.1084/jem.20090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y-F, Li B-Z, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, et al. Tet-Mediated Formation of 5-Carboxylcytosine and Its Excision by TDG in Mammalian DNA. Science (New York, N.Y.) 2011 doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-H, Peng K-L, Kang M-L, Chen Y-R, Yang Y-C, Tsai C-H, Chu C-S, Jeng Y-M, Chen Y-T, Lin F-M, et al. TET1 Suppresses Cancer Invasion by Activating the Tissue Inhibitors of Metalloproteinases. Cell reports. 2012;2:568–579. doi: 10.1016/j.celrep.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Huang X, Schwind S, Yu B, Santhanam R, Wang H, Hoellerbauer P, Mims A, Klisovic RB, Walker A, Chan KK, et al. Targeted Delivery of microRNA-29b by Transferrin Conjugated Anionic Lipopolyplex Nanoparticles: A Novel Therapeutic Strategy in Acute Myeloid Leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 doi: 10.1158/1078-0432.CCR-12-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg J. a, He C, Zhang Y. Tet Proteins Can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science (New York, N.Y.) 2011 doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin EM, Rodríguez-Ubreva J, Christensen J, Cimmino L, Aifantis I, Helin K, Ballestar E, Graf T. Tet2 Facilitates the Derepression of Myeloid Target Genes during CEBPα-Induced Transdifferentiation of Pre-B Cells. Molecular cell. 2012:266–276. doi: 10.1016/j.molcel.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, An J, Lamperti ED, Koh KP, Ganetzky R, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O’Donnell K. a, Wentzel E. a, Montgomery CL, Hwang H-W, Chang T-C, Vivekanandan P, Torbenson M, Clark KR, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langemeijer SMC, Kuiper RP, Berends M, Knops R, Aslanyan MG, Massop M, Stevens-Linders E, van Hoogen P, van Kessel AG, Raymakers R. a P., et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nature genetics. 2009;41:838–842. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]
- Li Z, Cai X, Cai C, Wang J, Zhang W, Petersen BE, Yang F-C, Xu M. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118:4509–4518. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, Xu W, Tan L, Hu Y, Zhan Q, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150:1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipchina I, Elkabetz Y, Hafner M, Sheridan R, Mihailovic A, Tuschl T, Sander C, Studer L, Betel D. Genome-wide identification of microRNA targets in human ES cells reveals a role for miR-302 in modulating BMP response. Genes & development. 2011;25:2173–2186. doi: 10.1101/gad.17221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D, Lee R, Williams P, Chan CY, Ambros V, Ding Y. Potent effect of target structure on microRNA function. Nature structural & molecular biology. 2007;14:287–294. doi: 10.1038/nsmb1226. [DOI] [PubMed] [Google Scholar]
- Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. The New England journal of medicine. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronier E, Almire C, Mokrani H, Vasanthakumar A, Simon A, da Costa Reis Monte Mor B, Massé A, Le Couédic J-P, Pendino F, Carbonne B, et al. Inhibition of TET2-mediated conversion of 5-methylcytosine to 5-hydroxymethylcytosine disturbs erythroid and granulomonocytic differentiation of human hematopoietic progenitors. Blood. 2011;118:2551–2555. doi: 10.1182/blood-2010-12-324707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivoron C, Couronné L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, Do Cruzeiro M, Delhommeau F, Arnulf B, Stern M-H, et al. TET2 Inactivation Results in Pleiotropic Hematopoietic Abnormalities in Mouse and Is a Recurrent Event during Human Lymphomagenesis. Cancer cell. 2011a:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Quivoron C, Couronné L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, Do Cruzeiro M, Delhommeau F, Arnulf B, Stern M-H, et al. TET2 Inactivation Results in Pleiotropic Hematopoietic Abnormalities in Mouse and Is a Recurrent Event during Human Lymphomagenesis. Cancer cell. 2011b:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Shaham L, Binder V, Gefen N, Borkhardt A, Izraeli S. MiR-125 in normal and malignant hematopoiesis. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2012;26:2011–2018. doi: 10.1038/leu.2012.90. [DOI] [PubMed] [Google Scholar]
- Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nature reviews. Cancer. 2012;12:599–612. doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
- Song SJ, Ito K, Ala U, Kats L, Webster K, Sun SM, Jongen-Lavrencic M, Manova-Todorova K, Teruya-Feldstein J, Avigan DE, et al. The Oncogenic MicroRNA miR-22 Targets the TET2 Tumor Suppressor to Promote Hematopoietic Stem Cell Self-Renewal and Transformation. Cell Stem Cell. 2013a;13:87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SJ, Poliseno L, Song MS, Ala U, Webster K, Ng C, Beringer G, Brikbak NJ, Yuan X, Cantley LC, et al. MicroRNA-Antagonism Regulates Breast Cancer Stemness and Metastasis via. Cell. 2013b;2:1–14. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor W. a, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science (New York, N.Y.) 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins S. a, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun X-W, Varambally S, Cao X, Tchinda J, Kuefer R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science (New York, N.Y.) 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- Varambally S, Cao Q, Mani R-S, Shankar S, Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science (New York, N.Y.) 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes & development. 2011;25:2436–2452. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Guo Z, Liu Y, Tang B, Wang Y, Yang L, Du J, Zhang Y. Oct4 and the small molecule inhibitor, SC1, regulates Tet2 expression in mouse embryonic stem cells. Molecular biology reports. 2012 doi: 10.1007/s11033-012-2305-5. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu Y, Bai F, Zhang J-Y, Ma S-H, Liu J, Xu Z-D, Zhu H-G, Ling Z-Q, Ye D, et al. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene. 2013;32:663–669. doi: 10.1038/onc.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Huang B, Xu X, Sessa WC. Ten-eleven translocation (Tet) and thymine DNA glycosylase (TDG), components of the demethylation pathway, are direct targets of miRNA-29a. Biochemical and biophysical research communications. 2013;437:368–373. doi: 10.1016/j.bbrc.2013.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.