Abstract
Miniature, self-contained biodevices powered by biofuel cells may enable a new generation of implantable, wireless, minimally invasive neural interfaces for neurophysiological in vivo studies and for clinical applications. Here we report on the fabrication of a direct electron transfer based glucose/oxygen enzymatic fuel cell (EFC) from genuinely three-dimensional (3D) nanostructured microscale gold electrodes, modified with suitable biocatalysts. We show that the process underlying the simple fabrication method of 3D nanostructured electrodes is based on an electrochemically driven transformation of physically deposited gold nanoparticles. We experimentally demonstrate that mediator-, cofactor-, and membrane-less EFCs do operate in cerebrospinal fluid and in the brain of a rat, producing amounts of electrical power sufficient to drive a self-contained biodevice, viz. 7 μW cm−2 in vitro and 2 μW cm−2 in vivo at an operating voltage of 0.4 V. Last but not least, we also demonstrate an inductive coupling between 3D nanobioelectrodes and living neurons.
Research on neural interfaces has the potential to revolutionise our understanding of fundamental neural mechanisms and is likely to engender new opportunities for clinical diagnosis and therapy1,2,3. Microscale autonomous, i.e. self-powered and wirelessly communicating, biodevices constitute a new generation of implantable neural interfaces. Such biodevices enable minimally intrusive, in vivo neurophysiological studies and therapies on a sub-cellular level. The overall dimensions and self-sufficiency of the biodevices significantly improve the implantable properties, thus enabling an unprecedented level of biodevice biocompatibility, reliability, and longevity due to complete physical and electrical decoupling from external devices. Indeed, such self-contained biodevices may reveal the full potential of nanostructure based neuronal interfaces for in vivo applications4,5.
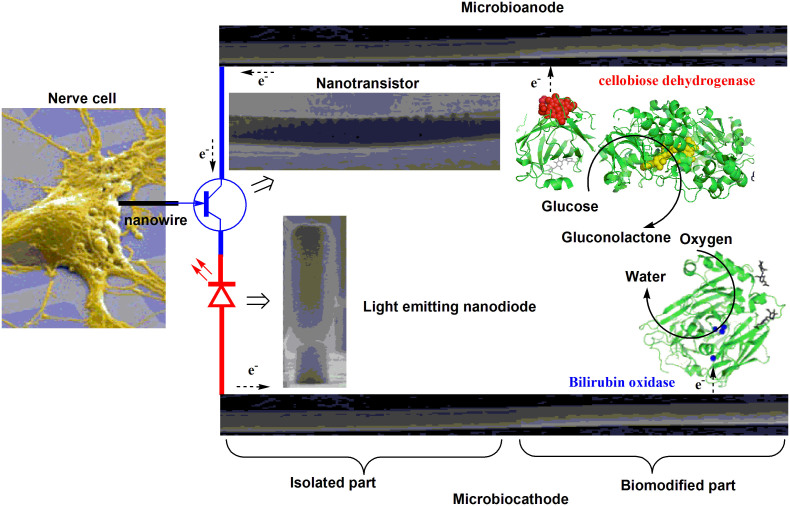
Wireless self-powered neural biodevices could for instance carry a probe for neuronal signal detection connected to a nano-amplifier and a nano-transmitter; all of which could be powered by a membrane- and mediator-less enzymatic fuel cell (EFC, Fig. 1). The functionality of these discrete nanoelectronic elements has already been demonstrated6,7,8. Moreover, in vivo studies of nanowire based neuronal electrodes have recently been performed9. Our work details the last element needed to design autonomous microscale biodevices, viz. an electric power source, specifically, a non-toxic and miniature EFC, capable of in vivo operation.
Figure 1. Minute autonomous biodevice for minimally invasive neuron interfacing.
Conceptual scheme of a wireless, self-powered biodevice for detection of neuronal signals which enables in vivo neurophysiological studies on a sub-cellular level with minimal intrusion and leads to a new generation of brain device interfaces. The images of a light emitting nanodiode, a nanotransistor, and a single neuron were adopted from Ref. 6, 8, 56. The structure of BOx was visualised based on the know crystal structure (PDB 2XLL) and CDH was designed using the cytochrome and the FAD domains of Phanerochaete chrysosporium cellobiose dehydrogenase (PDB 1D7D and 1NAA), respectively.
EFCs belong to a broad family of biofuel cells, which directly convert chemical energy into electric energy using biological catalysts, e.g. redox enzymes or even whole living cells10,11. Glucose/oxygen EFCs should provide a reliable source of electric energy for brain-device interfaces utilising the readily available biofuel, e.g. glucose, and biooxidant, e.g. molecular oxygen (O2), tapping into the same energy source as the biological entities of the brain. Recent calculations have demonstrated that the natural recirculation of the cerebrospinal fluid (CSF) in humans permits glucose energy harvesting at a rate of at least 1 mW, with no adverse physiological effects12. This suggests that EFCs can be used as a power source for minimally invasive wireless and self-powered biodevices in the brain. A few reports only exist on voltammetric studies of fully implantable EFCs operating in vivo in vertebrates13,14 including rats15,16. All of these EFCs were macroscale, substantially limiting their practical application to act as an electric power source for miniature self-contained biodevices. Below we detail a microscale glucose/O2 EFC operating in vivo in a rat brain, following our earlier reports17,18,19.
One way to design EFCs for possible implantable applications is to immobilise glucose oxidising and oxygen reducing enzymes on nanostructured electrode surfaces to create an efficient bioanode and biocathode, respectively20,21. By electrically connecting the enzymes directly to the electrode, i.e. relying on direct electron transfer (DET), the usage of separator membranes and potentially toxic mediators can be avoided22. Accordingly, several membrane-, cofactor-, and mediator-less carbohydrate/oxygen EFCs have been reported in the literature23,24,25,26, and the design principle simplifies the setup and is pertinent to miniaturisation27.
Recently, a simple fabrication method for three-dimensional (3D) nanostructured electrodes, based on gold nanoparticles (AuNPs) was reported28. These electrodes have already been used to create micro- and macroscale biocathodes, bioanodes, and even complete EFCs17,22,26,27,29,30. However, there was no understanding of the process underlying the 3D nanostructure formation, and consequently the potential of this process was not fully appreciated. Here we show that the 3D nanostructure formation is due to an electrochemical transformation of the AuNPs deposited on Au electrodes, changing the surface morphology. The process is very simple and potentially applicable for formation of complex nanostructures from different materials and for a wide range of applications.
We use the process to construct microscale 3D nanostructured Au electrodes, increasing the geometric surface area a hundred times compared to unmodified electrodes (i.e. without AuNPs). The electrode morphology and electrical properties were characterised with electrochemical impedance (EI) spectroscopy and cyclic voltammetry (CV) and the data thus gathered correlated with the results from atomic force microscopy (AFM) and scanning electron microscopy (SEM). In this way we show that the increased geometric area is due to 3D Au nanostructure formation and the electrodes were used to design high-performance, microscale glucose/oxygen EFCs. For the modification of the nanostructured electrodes thoroughly characterised anodic and cathodic bioelements were used.
A scheme of the EFC implemented in this study is shown in Fig. 1, where a glucose oxidising enzyme, cellobiose dehydrogenase (CDH), and an oxygen-reducing enzyme, bilirubin oxidase (BOx), are immobilised and directly wired to 3D microscale electrodes, resulting in a microbioanode and a microbiocathode, respectively. Glucose is oxidised to gluconolactone at the bioanode, transferring electrons to the electrode. At the biocathode electrons are transferred from the electrode to the enzyme, which reduces oxygen to water. By combining the microscale biocathode and bioanode, a miniature electric power generating EFC is realised. We experimentally prove that mediator- and membrane-less, microscale EFCs do generate electrical energy sufficient for a self-contained biodevice operating in CSF and in the rat brain. In addition, we also demonstrate that 3D microscale nanostructured electrodes establish well-pronounced inductive coupling with neurons in the brain, which is not only scientifically significant, but also permits active interactions, increasing the scope of implantable neural interfaces beyond that of passive monitoring.
Results
Fabrication and investigation of 3D nanostructured gold microelectrodes
To understand the formation process of 3D nanostructures and to firmly establish the structural features obtained, we performed detailed characterisation of the initially used AuNPs and 3D AuNPs based electrodes, as described below.
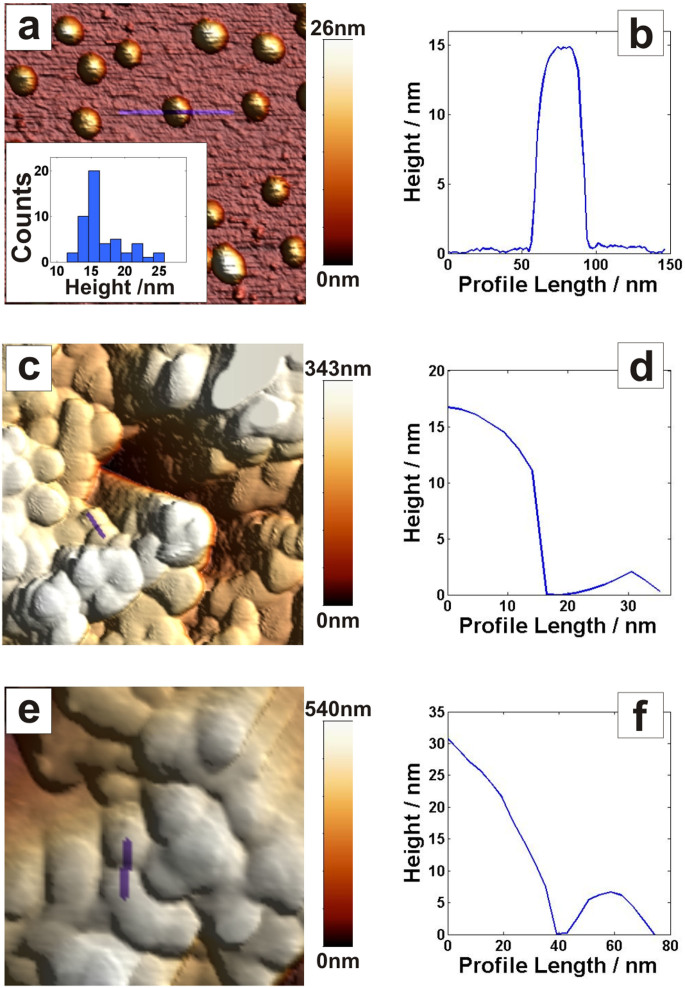
The size of the original AuNPs was determined by AFM; imaging of discrete NPs adsorbed on poly-lysine(PL)-coated mica surfaces (Fig. 2a and 2b) indicated an average NP height of 16.7 ± 3.2 nm (Fig. 2a, insert).
Figure 2. Atomic force microscopy studies of AuNPs and 3D nanostructured electrodes.
Images and corresponding height profiles of (a, b) AuNPs on a poly-lysine-coated mica surface, (c, d) microelectrode coated with AuNPs, (e, f) the microelectrode from panels c and d, after electrochemical cleaning. Scan areas for all images were 400 nm × 400 nm. Note that the height measured with AFM represents the diameter of the NPs; the width does not represent the diameter due to tip dilation effects57.
Microelectrodes coated with AuNPs were also imaged by AFM, before (Figs. 2c and 2d) and after (Figs. 2e and 2f) electrochemical treatment in 0.5 M H2SO4 using CV, a common procedure for cleaning Au surfaces, and also widely used to estimate the real surface area of Au electrodes31. It can be seen that the AuNPs modified surface undergoes a major transformation during the H2SO4 treatment, signified by a difference in the surface morphology. However, it was difficult to quantify the size of the transformed AuNPs, due to lack of a zero-height reference, but it was nonetheless clear that sizes increased with the electrochemical treatment, probably as a consequence of an aggregation process.
To obtain quantitative data of the features formed during the assembly of the 3D microscale electrodes, SEM studies were also performed. The images in Fig. 3a, b, c were recorded after electrochemical cleaning of AuNP modified Au microwires in 0.5 M H2SO4, whereas Fig. 3d corresponds to the original AuNPs. On the modified side the average grain size was 36 nm ± 12 nm, whereas the average feature size was as high as 77 nm ± 22 nm. For comparison, in Fig. 3d the original nanoparticles can be seen, and in agreement with AFM data (cf. Figs. 2a and 3d) the average AuNP size used for modification was measured to be only 17 nm ± 5 nm.
Figure 3. Scanning electron microscopy images of AuNPs modified microelectrodes.
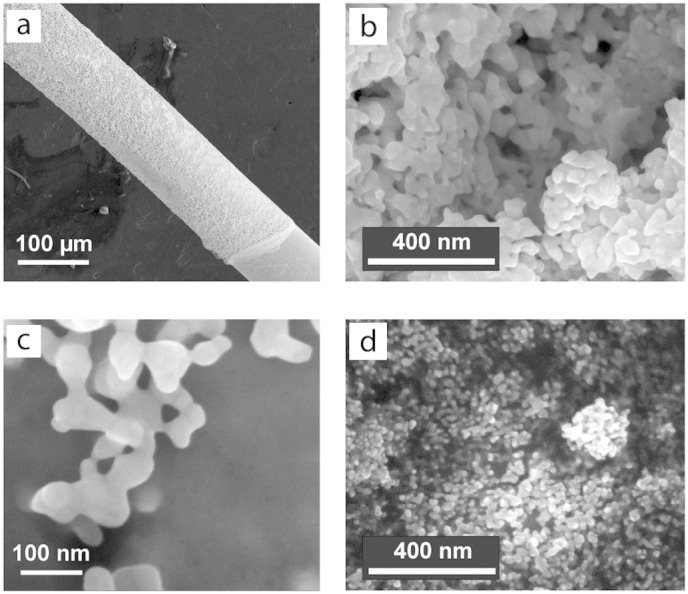
(a) Low magnification image of the AuNPs modified Au microwire: the microwire geometry and the AuNP coating border can be seen in the lower right corner. (b) High magnification SEM image in the middle of the electrode in panel a. (c) High magnification SEM image of the border of the electrode in panel a. The images in panels a, b, and c were taken after the electrode modification with AuNPs and treatment in 0.5 M H2SO4. (d) SEM image of a reference sample covered with AuNPs identical to those used for the construction of nanobiostructures, the original nanoparticle shape can be clearly seen.
In Fig. 3a the border between the AuNPs modified and bare Au microwire surface can be seen. It was observed (cf. Fig. 3b and 3c) that the particles merge into similar aggregates in the bulk, as well as on the border of the 3D nanostructured Au material, where the AuNPs coverage was limited to just a few monolayers. We conclude that the electrochemical treatment in H2SO4 transforms the particles into a porous 3D nanostructured Au surface with an average grain size of approximately 40 nm and an average feature size of approximately 80 nm, and since the border as well as the bulk show similar features, the transformation is independent of the AuNP layer thickness when a few monolayers of AuNPs up to thicker layers are employed.
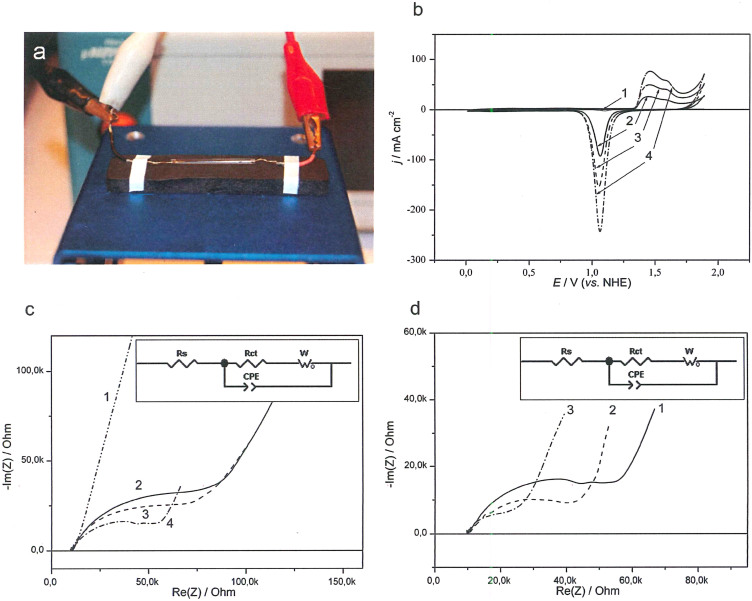
In conclusion, genuinely 3D, electrically conducting microscale electrodes were fabricated by immobilising AuNPs on Au microwires, followed by electrochemical treatment of the electrodes. The AuNPs modified microelectrodes were further characterised electrochemically (Fig. 4) in a custom-built electrochemical setup for microelectrode characterisation in small volumes of physiological fluids (Fig. 4a). Increasing the amount of AuNPs on the electrode decreases the charge transfer resistance, due to the increased surface area with increased amount of AuNPs (Fig. 4b). Impedance plots measured for Au microwires modified with different amounts of AuNPs are shown in Fig. 4c. The impedance can be described using a circuit with resistance of charge transfer and open circuit Warburg impedance (inset Fig. 4c and d). The system (Au/AuNPs) displays a leakage current (charge transfer resistance) connected to oxygen reduction on the unmodified AuNPs surface. This is highly dependent on the potential of the electrode and the amount of AuNPs (Fig. 4c and d).
Figure 4. Electrochemical characterisation of AuNPs modified microelectrodes.
(a) Image showing the electrochemical setup for characterisation of microelectrodes. (b) Cyclic voltammograms, (c) Nyquist plots for Au microwires with different amount of AuNPs: 0 μl (1), 3 μl (2), 6 μl (3), 12 μl (4). (d) Nyquist plots at different potentials: −50 mV (1), −100 mV (2), −150 mV (3). Inset (c,d): Equivalent circuit Rs – solution resistance, W – Warburg impedance, Rct – charge transfer resistance, CPEpl – constant phase element. Conditions: 0.5 M H2SO4, 100 mV s−1 (b); 50 mM PBS pH 7.4, −50 mV (c) 50 mM PBS pH 7.4, 12 μL of AuNPs (d).
Bioelements purification and characterisation
To design efficient and stable microbioanodes and microbiocathodes Corynascus thermophilus cellobiose dehydrogenase (CtCDH) and Myrothecium verrucaria bilirubin oxidase (MvBOx) were deployed as anodic and cathodic biocatalysts, respectively. These enzymes have already been exploited in our previous work to fabricate DET based membrane-less glucose/oxygen EFCs operating in plain glucose containing buffers, as well as complex human physiological fluids, such as blood, plasma, serum, saliva, and tears22,23,26,27. However, detailed biochemical and kinetic characterisation of the redox enzymes was not done, leaving questions regarding the reliability and reproducibility of the results unanswered. Thus, to address these questions, both bioelements were isolated, purified on a preparative scale, and investigated in detail as described in SI (section 1 of Supplementary Information (SI), Figs. S1–S6, Tables S1–S2).
Concentrated high purity enzyme preparations in weak buffers (10–20 mM) were used for the construction of EFCs. For proper enzyme immobilisation on the electrode surface, the ionic strength, pH, and buffer composition were carefully controlled. The pH dependent catalytic activity of both biocatalysts used for EFC construction was obtained both in homogeneous solution and in the adsorbed state for biocatalytic and bioelectrocatalytic reactions. Obviously, the enzymes need to retain high activity within the EFC operational pH range (Supplementary Figs. S2 and S5).
The pH of the brain interstitial fluid (ISF) has been determined to be about 7.3, with pH values down to 7.1 at the centre of brain slices32. In the most extreme cases with severe ischemia or tissue hypoxia, the pH can drop to 6.033. Accordingly, the activity of MvBOx and CtCDH was monitored in a wide pH range, when enzymes were immobilised on AuNPs modified electrodes, and compared to homogeneous reactions using different enzyme substrates, resulting in two different mechanisms of intramolecular electron transfer in the case of CDH34 or different driving forces during homogeneous reactions catalysed by BOx35.
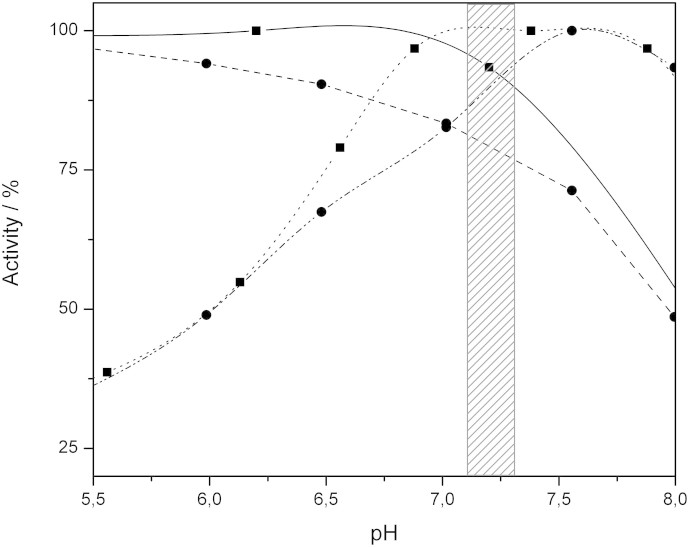
Briefly (all details can be found in SI), the results, as summarised in Fig. 5, indicate a good correlation between homogeneous and heterogeneous processes; the usual physiological pH range of ISF is highlighted in Fig. 5. In this region both enzymes retain at least 75% of their maximum activity. However, as the pH decreases the activity of CtCDH is significantly lowered, but about 50% of the activity remains even at pH 6.0.
Figure 5. Activity of Corynascus thermophilus cellobiose dehydrogenase and Myrothecium verrucaria bilirubin oxidase at different pH values.
Activity MvBOx in solution with K4[Fe(CN)6] as the substrate (solid line, boxes) and immobilised on a RDE (dashed line, circles), as well as CtCDH in solution with cytochrome c as the substrate (dotted line, boxes) and immobilised on a RDE (dash-dotted line, circles), with the pH region 7.1–7.3 highlighted. The rotation rate of the RDE was 2000 rpm.
Fabrication and electrochemical study of biomodified 3D microscale Au electrodes
Biomodification procedures of the 3D Au microelectrodes were conducted following a previously developed procedure for macroscale AuNPs modified electrodes26, adapted to microscale electrodes27.
Detailed electrochemical investigations of microbioanodes and microbiocathodes were performed in carbohydrate containing buffers and rat CSF. Chemical compounds are quite freely exchanged between CSF and ISF, making the composition of the ISF highly influenced by the CSF36. The amount of glucose in the CSF correlates with the concentration of glucose in blood, containing roughly two thirds of the blood amount, with a normal range of 2.5–4.5 mM37.
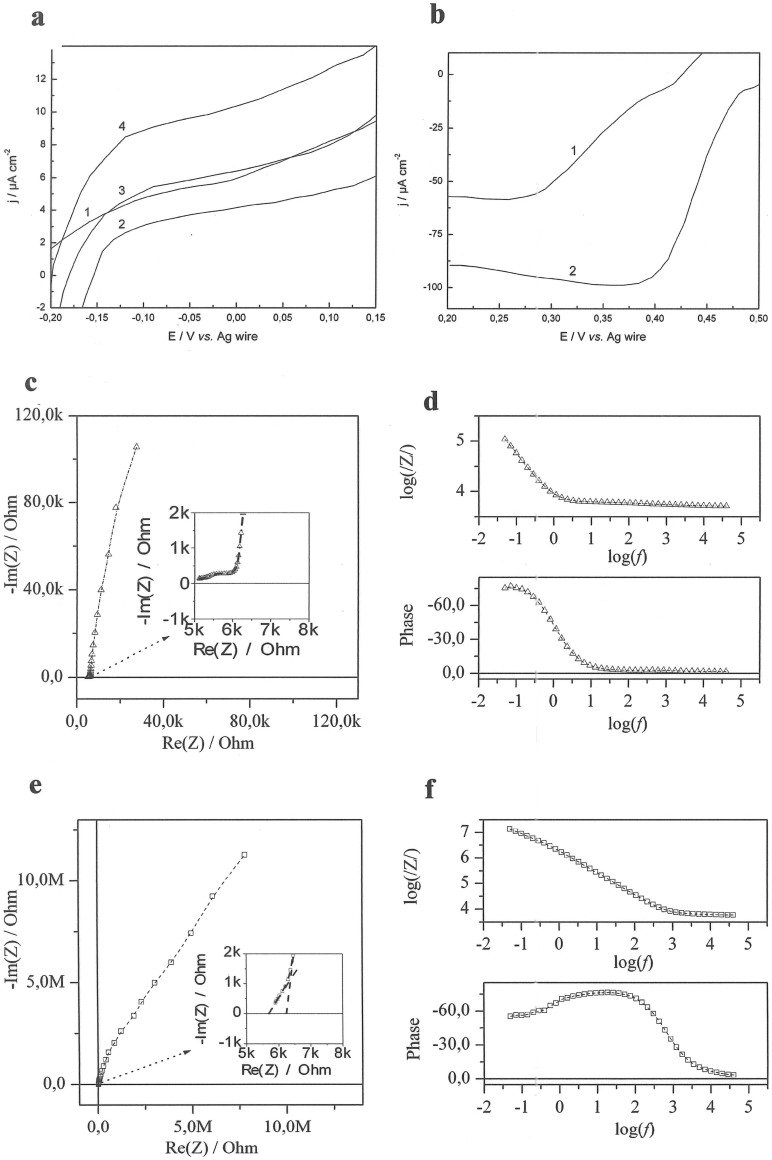
Linear sweep voltammograms (LSVs) of microbioanodes and microbiocathodes operating in different fluids are shown in Fig. 6a, b with a variation in the onset potential of ±5 mV and maximum current outputs of ±12%. For bioanodes, increasing the glucose concentration naturally increases the current generated by the anode (cf. curves 2–4 in Fig. 6a).
Figure 6. Electrochemical investigations of microelectrodes.
(a) LSVs of microbioanode operating in CSF (1) and buffer containing 1 mM (2), 2.5 mM (3) and 5 mM glucose (4). (b) LSVs of microbiocathode operating in CSF (1) and air saturated buffer containing 2.5 mM glucose (2); Conditions: 50 mM PBS, pH 7.4; scan rate 0.3 mV s−1. (c) Nyquist plots and (d) Bode diagrams for AuNP modified Au microelectrodes in CSF. (e) Nyquist plots and (f) Bode diagrams for unmodified Au microelectrodes in CSF. Conditions for c,d,e,f: U = 0.45 V; amplitude = 10 mV.
Compared to CSF, quite similar current levels are obtained in buffer solutions containing 2.5 mM glucose, (cf. curve 1 and 3, Fig. 6a). Contrary to bioanodes, the biocathode performance was essentially unaffected by the amount of glucose, but a large difference in both onset potential and current generated was observed between buffer and CSF (cf. curves 1 and 2, Fig. 6b). Most likely, the observed reduced performance of the EFCs in CSF is due to electrocatalytic oxidation (i.e. side reactions) of some other compounds present in this complex fluid (e.g. ascorbate, which is present at significant concentrations, ~160 μM compared to ~40 μM in blood38), catalysed by the underlying supporting material (vide infra); e.g. AuNPs are known to efficiently catalyse electrooxidation of different substrates including biofules of EFCs39,40,41.
In addition to the voltamperometric studies, impedance measurements were performed to further investigate the influence of the supporting material on (bio)electrocatalytic reactions. For this purpose both unmodified and AuNPs-modified Au microwires were investigated in CSF (Fig. 6c–6f). The spectrum for a system consisting of two unmodified Au microelectrodes, displays a low charge transfer resistance and quite low total capacitance. For modified electrodes, one element in the high frequency region can be described by two parallel R-CPE circuits, corresponding to Faradaic processes, e.g. oxidation of interfering compounds present in CSF. Many different biocompounds that can be electrooxidised on the Au nanostructured surface are present in both ISF and CSF, e.g. antioxidants such as ascorbate, glutathione, uric acid, and neurotransmitters such as dopamine, serotonin and epinephrine42,43. These electrooxidative processes are much faster compared to the O2 reduction reaction occurring on the same electrode (cf. Fig. 4 and Fig. 6c–6f). The capacitance of 3D electrodes is 100 times higher compared to unmodified ones. Note that after the modification with both self-assembled monolayers and enzymes, the capacitance of 3D electrodes is insignificantly reduced (vide supra; Fig. 8c). Nevertheless, impedance studies also confirm the possibility of efficient non-enzymatic electrooxidation of biomolecules present in CSF by 3D Au microelectrodes.
Figure 7. Enzymatic fuel cell implanted into the rat brain.
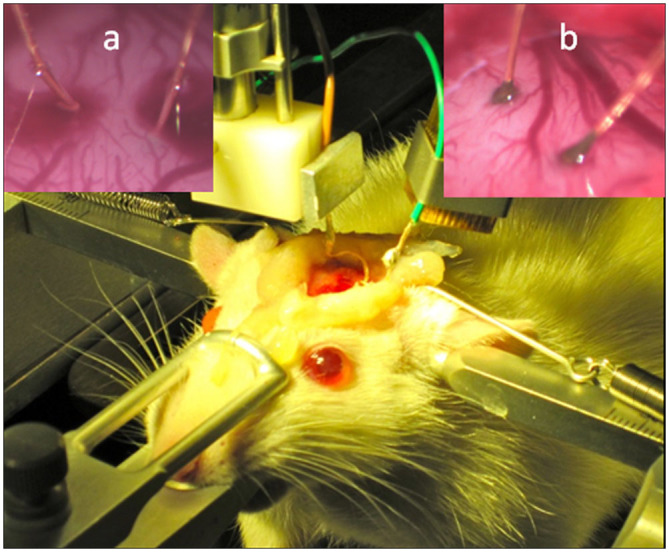
Photographs of the experimental arrangement for implantation of microbioelectrodes into the rat cortex, as well as close up pictures of the implantation with (a), and without (b), localised surface bleeding.
Figure 8. Enzymatic fuel cell characterisation.
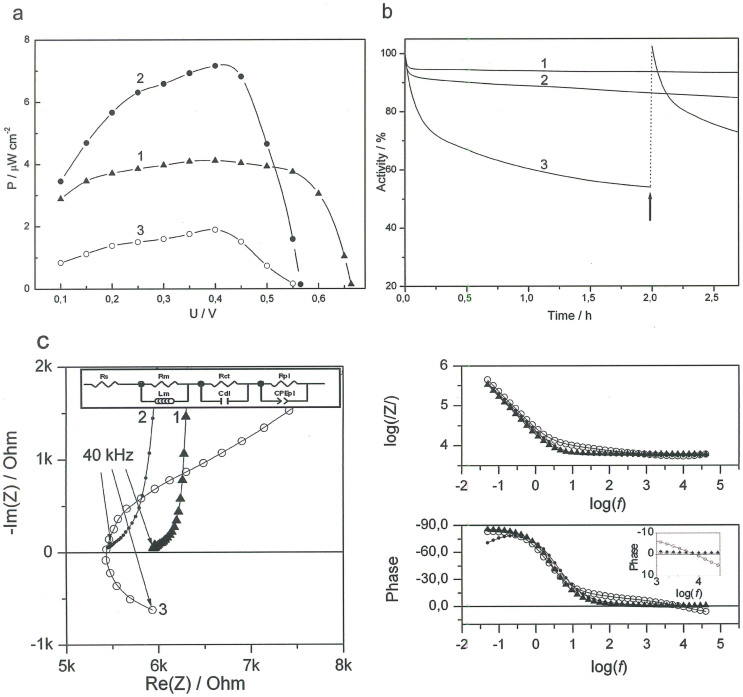
(a) Typical power density plots of EFCs operating in buffer with 2.5 mM glucose (1), CSF (2), and rat brain (3). (b) Operational stability of EFCs operating in buffer with 2.5 mM glucose (1), CSF (2), and rat brain (3). At the time indicated by an arrow, a slight manipulation of the implanted electrodes was performed, moving them 0.1–0.2 mm. (c) Nyquist plots and Bode diagrams measured for EFCs in buffer (1, triangles), CSF (2, full circles), and rat brain (3, open circles). Insets: Equivalent circuit and magnified phase dependence. Rs – solution resistance, Rm – membrane resistance, Rct – charge transfer resistance, Rpl – resistance of distribution element impedance, Lm – membrane inductivity, Cdl – double layer capacity, CPEpl – constant phase element. Conditions: 50 mM PBS, pH 7.4; power density plots were obtained using both calibrated resistors of different resistance values and LSVs recorded with a scan rate of 0.3 mV s−1. The operational stability was measured at a constant load of 35.86 MΩ. Impedance measurements were done at U = 0.45 V and an amplitude of 10 mV.
Experimental evaluation of the performance of miniature glucose/oxygen in vitro and in vivo
In vitro measurements with CSF were performed using the microcell developed (Fig. 4a; vide supra), whereas the experimental setup used for in vivo studies can be seen in Fig. 7.
EFCs were investigated by connecting the bioanode (CtCDH/AuNP/Au) as a working electrode and the biocathode (MvBOx/AuNP/Au) as a combined reference and counter electrode. Steady-state measurements were performed by connecting calibrated resistors and measuring the voltage after the equilibration of the system, which was monitored by observing the stabilisation of the measured voltage. When the EFC was operated in vitro, open circuit voltages (OCVs) of 0.65 ± 0.03 V and 0.57 ± 0.01 V were obtained in glucose containing buffers and CSF, respectively, averaging from five different EFCs. The observed reduction in voltage of 0.08 V, when the EFCs operated in CSF, was expected due to the drop in the onset potential of microbiocathodes (Fig. 6b; vide supra). When the EFC was implanted in the brain of a rat, OCV values of 0.54 ± 0.03 V were registered, i.e. very close to the OCV obtained in CSF, confirming that ISF and CSF have similar compositions36,44,45. During some implantation procedures a localised surface bleeding occurred (cf. Fig. 7a and 7b). However, no difference in the performance of EFCs was observed (vide infra), whether a localised bleeding occurred during implantation or not.
It is important to emphasise that no changes in the voltage was registered, when several EFCs were connected in series in the brain. Thus, the operating voltage of a single EFC is a crucial parameter when designing practically applicable power sources for implantable electronic biodevices.
Typical power profiles of EFCs operating both in vitro and in vivo are compared in Fig. 8a. A maximum power density of around 4 μW cm−2, ranging between ~3.2–4.0 μW cm−2 at 0.4 V was generated by the EFC in a 2.5 mM glucose containing buffer and over 7 μW cm−2 at a similar voltage in CSF, ranging between ~6.3–7.2 μW cm−2. In the brain, the maximum power density dropped to 2 μW cm−2, ranging between ~1.3–2.1 μW cm−2 at a voltage of 0.4 V. Since no significant differences were observed, when actual steady-state measurements were used to evaluate the performance of EFCs, compared to the widely used LSVs measurements at low scan rates, the results presented in Fig. 8a were obtained under equilibrium conditions. Thus, one can easily re-plot the data based on a well-known and very simple equation, viz. power = voltage × current.
The high power density and the different appearance of the power density profiles of EFCs operating in CSF compared to buffer solutions (cf. curves 1 and 2 in Fig. 8a) were obtained due to the combination of bioelectrocatalytic and electrocatalytic processes occurring on microbioanodes in the complex physiological fluid. Since significant power was generated when no enzyme was present on the anode, the electrooxidation of CSF on nanostructured microEFCs is most likely triggered by the supporting material (see details in section 3 of SI, Fig. S8)39,41. Thus, in addition to bioelectrooxidation of glucose, electrooxidation of antioxidants and neurotransmitters resulted in significant increase of power densities generated by EFCs at low voltages, i.e. in the 0.1 V–0.5 V range (curve 2 in Fig. 8a).
Operational stability tests of EFCs in vivo in the brain, as well as in vitro using buffer solutions and CSF, were performed by applying a constant load of 35.86 MΩ, and monitoring the voltage drop. The resistance value was chosen to perform stability tests in the appropriate way, i.e. at the maximal performance of EFCs, when biodevices deliver maximal power: ca. 11 nA at 0.4 V, or 2 μW cm−2. Typical stability curves of EFCs operating in buffer, CSF, and brain tissue are presented in Fig. 8b. A reduction in voltage of roughly 50% after 2 h of continuous EFC operation in the brain was observed (Fig. 8b, curve 3). At the time marked with an arrow in Fig. 8b, the electrodes were carefully displaced 0.1–0.2 mm vertically while inserted in the brain. This resulted in the voltage returning to the original value, which indicated that the observed drop in performance is not related to bioelements deactivation. Most likely the diffusion properties of the brain tissue limit the power of the EFC. When no load was applied to the EFC the OCV remained unchanged for at least 3 hours. In buffer solutions and CSF, EFCs displayed excellent stability with over 90% and 85% of the initial voltage remaining after 2 h of continuous operation, respectively (curves 1 and 2 in Fig. 8b). It is worthwhile to mention a significant increase in power density (from 2 up to 4 μW cm−2), when the cortical depth for implanted EFCs was decreased from 2 mm to 1 mm.
In additional experiments OCVs of implanted EFCs after an injection of a lethal dose of barbiturate was continuously monitored. As the heart of the animal stopped beating, a rapid drop in OCV values was observed: in 10 min the OCV value settled at ~0.3 V, which is almost 50% lower than the original OCV value. This can be attributed to the ceased flow of blood, and thereby depletion of O2 in the tissue surrounding the EFC. These measurements also confirm the bioelectrocatalytic origin of the electric power obtained from the implanted EFCs.
The typical electrochemical impedance (EI) values measured for EFCs in the brain, in CSF and buffer are presented in Fig. 8c. An ohmic resistance of about 5.5 kΩ in the brain tissue and in CSF was observed. EI values were also measured for EFCs in different buffer solutions (section 4 of SI, Fig. S9). The capacitance and the shape of impedance spectrum were found to be similar for EFCs in the brain and in CSF. However, the solution resistance along the electrodes was different, which can be attributed to the difference in the measurement conditions in the brain tissue and in the microcell. The main parameter here is the thickness of the electrolyte layer along the microelectrodes, the electrolyte layer along the microelectrodes inserted in he brain is narrow and consequently the solution resistance is higher.
Modelling of the EI data measured for implanted EFCs was performed with the equivalent circuit presented in the Fig. 8c inset. The data fit for the low frequency range (50 mHz–13 Hz) indicated that in this range the electric properties are mainly determined by the charge transfer resistance and the double layer capacitance (Rct and Cdl in Fig. 8c, the inset)46. The data fit for the intermediate frequency range (13 Hz–7 kHz) indicated that in this range the electrical properties are mainly determined by the resistance of the distribution element impedance and the constant phase element (Rpl and CPEpl in Fig. 8c, the inset), which was attributed to the distribution of electrical parameters along the electrodes and the depth of the porous layer. This is in a good agreement with theoretical prediction for highly porous electrodes47. The data fit for the high frequency range (7 kHz–40 kHz) indicated that in this range the electrical properties are mainly determined by the elements representing neurons (Rm and Lm in Fig. 8c, the inset), according to the theory developed by Hodgkin and Huxley (see detailed description in section 4 of SI, Fig. S10)48,49. Importantly, the inductive loop on the impedance spectrum appeared only when the EFCs were implanted in the brain (cf. curves 1–3 in Fig. 8c). We attributed this to the inductive properties of neuronal membranes, which contain voltage-gated ion channels that allow the neuron to generate and propagate an electrical signal by ion conduction49.
Discussion
In the present study we show that the process underlying the simple method for fabrication of 3D nanostructured Au electrodes is, in all likelihood, based on an electrochemically driven transformation of physically deposited AuNPs into a genuinely 3D nanostructured material, in contrast to the previously suggested AuNPs aggregation during solvent evaporation28. However, the underlying mechanism of this process is still unclear and calls for further investigations.
Using this process we fabricated 3D nanostructured electrically conducting microelectrodes with a geometric area increased 100 times. We confirmed that the geometrical area increase is indeed due to the formation of a genuinely 3D nanostructured Au surface. The electrochemical transformation of separate AuNPs into a solid nanostructured material with the formation of strong metallic bonds implies that 3D nanostructures fabricated with this simple method are mechanically robust, more sustainable in harsh conditions, and more biologically friendly compared to nanostructured materials made of physically agglomerated separate nanostructures. Formation of genuine and solid 3D Au nanostructure with an increase of the roughness factor28 up to 300 times is remarkable and makes this type of material very desirable for neural prosthetics applications50. Moreover, the same simple approach can be employed for formation of complex nanostructures for different applications, and not necessarily only Au based materials. The finding reported here is also important for the electrochemical community, as the process exploited is commonly used for cleaning of Au electrode surfaces and also estimation of the effective (electrochemical or real) surface area. Until now this method has also been applied to nanostructured electrodes without the understanding that the process itself leads to the transformation of nanostructured Au surfaces.
We design 3D microbiocathodes and microbioanodes by biomodification of 3D nanostructured Au electrodes with suitable bioelements, viz. MvBOx and CtCDH, respectively, and study the performance of nanobioelectrodes in CSF and in the rat brain. Prior to biomodification, the bioelements were thoroughly characterised in order to avoid any ambiguities regarding the results obtained. Combining a 3D microbiocathode with a 3D microbioanode realises a mediator-, cofactor-, and membrane-less, microscale EFC. Owing to the simplicity of the design, substantial miniaturisation of the EFCs was possible, making the EFC implantation less traumatic and thus allowing adequate in vivo studies.
Only two reports exist on voltammetric studies of fully implantable EFCs operating in vivo in rats13,14,15,16. The basic parameters of the EFC detailed herein (OCVs, operating potentials, and power densities) are at least comparable, or even superior, to recently reported implanted biodevices (Table 1). However, the reported EFCs were macroscale and could not be used as low distress level EFCs operating in the brain.
Table 1. Comparison of basic characteristics of EFCs implanted in rats. EFCs recently detailed in Refs. 15, 16 were built from carbon nanotubes (CNTs) and buckypaper (BP) using glucose oxygen (GOx) or glucose dehydrogenase (GDH) as anodic and laccase (Lc) as cathodic bioelements, respectively.
Considering limitations, one could expect that the performance of the EFC designed by the combination of microbiocathodes and microbioanodes, would be clearly limited by anodic bioelectrocatalytic reactions (cf. generated currents in Fig. 6a, b), at least when the EFC is operating in vitro using simple glucose containing solutions. Moving to physiological situations, e.g. from simple buffers to CSF, cathodes would definitely be responsible for a lowering of the voltage of the EFCs (cf. curves 1 and 2 in Fig. 6b) because of their depolarisation in CSF. Moreover, one could also expect significant decrease in current densities obtained from microbiocathodes operating in vivo, because of the significantly lower concentration of available O2 in both brain tissue and CSF in the living organism, ca. 50 μM51, compared to the air saturated solutions used in our in vitro studies, ca. 250 μM52. In the latter case, a decrease of the O2 concentration from 250 to 50 μM resulted in a pronounced decrease of the bioelectrocatalytic currents (section 2 of SI, Fig. S7).
The obtained results unequivocally show an electric connection between nanostructured Au surfaces and neurons, evidenced by the appearance of inductive loops in the impedance spectra of bioelectrodes, a consequence of the inductive properties of neuronal membranes. This is a very interesting and practically applicable finding in the field of implantable nanostructure based neural interfaces, which is discussed in further detail in section 4 of SI. To the best of our knowledge, this is the very first impedance investigation of EFC-brain interfaces.
In summary, we present unequivocal experimental proof that microscale, mediator- and membrane-less EFCs do generate significant amounts of electrical energy for a self-powered biodevice, when operating in cerebrospinal fluid (in vitro) and when implanted in the rat brain (in vivo). To conclude, the present study shows without a doubt that miniature autonomous biodevices, i.e. wireless and self-powered biodevices, for minimally invasive neuron interfacing, which use nanostructured EFCs as electric power sources, are practically realisable. This paves the road for a new generation of self-contained minimally invasive neural interfaces and brain-device applications.
Methods
Chemicals
All chemicals were of analytical grade and used without further purification. All buffers were prepared with deionized water. 100 μm diameter Au wires and 130 μm silver wires were purchased from Goodfellow Cambridge Ltd. (Huntingdon, England).
AuNPs were prepared following a previously reported citrate-reduction procedure53. To concentrate the AuNPs, the AuNPs dispersion was centrifuged at 10000 × g for 30 min on a 5402 Eppendorf centrifuge (Eppendorf, Hamburg, Germany) in 1 mL Eppendorf tubes. Then, 98% of the supernatant was removed, the pelleted AuNPs were re-suspended by ultrasonication using an XB2 Ultrasonic Cleaner from VWR International Ltd. (East Grinstead, West Sussex, UK), and stored as a 50 times concentrated AuNPs dispersion at 4°C.
CtCDH was obtained from a culture of Corynascus thermophilus CBS 405.69 (Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands) and MvBOx was obtained as a kind gift from Amano Enzyme Inc. (Nagoya, Japan).
Electrode fabrication
3D microbioanodes and microbiocathodes were fabricated from Au microwires with a diameter of 100 μm. Firstly, wires were insulated using cellulose acetate (10 mg mL−1) dissolved in pure acetone. A section of roughly 3 mm was left un-insulated and used as a working surface. Secondly, electrodes were gently polished with 0.05 μm aluminium oxide powder (Struers A/S, Ballerup, Denmark), and cleaned electrochemically by cycling in the potential interval −0.2 V–1.7 V (vs. Ag/AgCl) for 20 cycles in 0.5 M H2SO4, with a scan rate of 100 mV s−1. Thirdly, several drops of concentrated AuNPs (ca. 1 μL) were applied to the surface of the cleaned electrodes, modifying roughly 1.5 mm of the working surface (projected [geometric] area of about 0.5 mm2). Thereafter, the electrodes were electrochemically cleaned again. The AuNPs-modified cathode was incubated in a 2 mg mL−1 MvBOx solution for 2 h, washed with phosphate buffer saline (PBS) and was then ready for use. The AuNPs-modified anodes were incubated for 1 h in a mixed thiol solution (1:1 mixture of 1 mM 4-aminothiophenol and 1 mM 4-mercaptobenzoic acid), washed with ethanol, and incubated in CtCDH with glutaraldehyde as a crosslinker for 2 h (6 μL 23 mg mL−1 CDH + 3 μL 2% glutaraldehyde). Finally, the anodes were carefully washed with PBS and were then ready for use.
A silver micro-reference electrode (Ag wire) was formed by cycling a silver microwire in the potential interval −0.5 V–0.5 V (vs. Ag/AgCl); 5 cycles in PBS. The thus obtained pseudo reference electrode had a potential of about 270 mV (vs. NHE) in PBS.
For heterogeneous activity measurements, macroscale Au rotating disk electrodes (RDEs) (Bioanalytical Systems, West Lafayette, IN, USA) were prepared following a procedure similar to the assembly of microbioelectrodes.
Animal handling and implantation
A total of 4 adult female Sprague Dawley rats weighing 260–320 g were used. The animals received food and water ad libitum, and were kept in a 12-h day-night cycle at a constant environmental temperature of 21°C (rel. humidity 65%). Approval for the experiments was obtained in advance from the Lund/Malmö local ethical committee on animal experiments, regulated by the code of regulations of the Swedish Board of Agriculture. These regulations, including directives from the European Union, follow the law on animal welfare legislated by the Swedish parliament. The County Administrative Board governs the implementation of the rules. Further, the experiments were in accordance with the policies and guidelines reported previously54.
The rats were anesthetised via intraperitoneal injections with a mixture (20:1, 6.3 ml kg−1) of Fentanyl Citrate (Fentanyl, B Braun, Melsungen Germany) and Medetomidine (Domitor, Orion Pharma, Espoo Finland) and attached to a stereotactic frame for the duration of the experiment. To measure electrode performance, the cortex surface (4–5 mm2) was exposed with a craniotomy followed by removal of the dura. Care was taken to keep the brain surface moist with 0.9% saline solution, to prevent drying of the brain.
The electrode probes were attached to micromanipulators, allowing slow and controllable insertion during implantation, and electrically connected to a potentiostat. The implantations were made under visual inspection using a microscope to a maximum depth of approximately 2 mm. In a separate set of experiments, an incision was made between the skull and the first cervical vertebra. CSF was carefully drained through a small hole in the dura matter by aid of a glass pipette. The CSF was centrifuged at 10000 × g at 4°C for 20 min and the supernatant was used for electrochemical characterisation of electrodes. After the experiments, each rat was sacrificed by a lethal dose of Sodium Pentobarbital (Apoteket, Sweden).
Activity measurements
The activity of MvBOx was determined using 5 mM K4[Fe(CN)6] solutions at different pH; the activity was assessed using an Anthelie Advanced Spectrophotometer (Topac Inc., Cohasset, MA, USA). When immobilised on RDEs, the MvBOx activity was assessed using a BASi RDE-2 system (Bioanalytical Systems, West Lafayette, IN, USA) with a rotation rate of 2000 rpm. Similarly, the activity of CtCDH was determined in 20 μM cytochrome c solutions at different pH, also when immobilised on RDEs.
Electrochemical investigations
Electrochemical measurements
Electrochemical measurements were carried out using a μAutolab Type III/FRA2 potentiostat/galvanostat from Metrohm Autolab B.V. (Utrecht, The Netherlands). Electrochemical impedance was measured with a voltage amplitude perturbation of 5 mV using a 50 kHz–10 mHz frequency range. Fitting of the impedance data was done using the ZView 2.0 programme from ZView 2.80, from Scribner Associates Inc. (Southern Pines, NC, USA), in the frequency interval 1 kHz to 10 mHz. Unless otherwise noted, all in vitro characterisations were performed in a microcell made from a glass capillary with a volume of roughly 15 μL and an internal diameter of 1.5 mm.
The electrodes were characterised individually in PBS, pH 7.4, with different glucose concentrations as well as in CSF using the Ag wire reference electrode as a combined counter and reference electrode. The microbioelectrodes were evaluated by LSV using a scan rate of 0.3 mV s−1. Basic EFC parameters were evaluated in PBS, CSF, and in vivo in the brain, and obtained using the anode as the working electrode and the cathode as a combined counter/reference electrode, without any separating membrane. The performance was evaluated by LSV, changing the voltage with a scan rate of 0.3 mV s−1 from the OCV to lower voltage regions, while recording the current in the circuit. The low scan rate eliminated charging currents of the double layer. As a complementary method to characterize the EFC performance, measurements were performed with different calibrated resistors, 1–100 MΩ, from Velleman Inc. (Forth Worth, TX, USA). The resistors were calibrated using a μAutolab Type III/FRA2, input impedance >100 GΩ. The system was allowed 3 min to reach equilibrium for each point. The stability of the EFC was determined by monitoring the change in voltage, when a constant load of 35.86 MΩ was applied to the EFC.
Verification of electrochemical measurements
About 100 bioelectrodes in total were fabricated during our studies. Averaged OCV and power density values are based on at least three measurements in vitro. Typical plots showing in vitro studies were calculated averaging the results from at least three different measurements. Four different animals were used in our in vivo studies. Thus, averaged OCV and power density values are based on four different measurements in vivo. Typical plots showing in vivo studies were calculated by averaging the results from two different measurements. Importantly, no significant differences were observed, when actual steady-state measurements were used to evaluate the performance of EFCs, compared to LSVs measurements at low scan rates.
AFM and SEM studies
AFM images of the nanoscopic surfaces were obtained using a Multimode VIII SPM with a Nanoscope V control unit (Bruker AXS, Santa Barbara, CA, USA). The AFM was operated in the ScanAsyst mode. All images were obtained in air and at room temperature. Triangular silicon nitride cantilevers with a nominal spring constant of 0.4 N m−1 (ScanAsyst Air probes, Bruker AXS) were employed in all measurements. Analysis and processing of AFM images was performed with the WSxM software package55. Image processing consisted of plane subtraction, equalization and 3D representation.
Poly-lysine (PL) coated mica sheets (Electron Microscopy Sciences, Hatfield, PA, USA) were used as a substrate for the visualization of single AuNPs. Coating was done by pipetting a 10 μL PL (aqueous solution 0.01% w/w) drop onto a freshly cleaved mica sheet in order to provide a positively charged surface on which the negatively charged AuNPs would adsorb. After incubation for 30 sec, the surface was gently rinsed with UHQ water and dried in a nitrogen stream, to ensure that only strongly adsorbed PL chains remained on the surface. Afterwards, the surface was incubated in a AuNPs suspension for 1 min, then rinsed with UHQ water, and dried in a N2 stream before being placed in the microscope.
SEM images were obtained using a high resolution SEM FEI Nova NanoLab 600 in field immersion mode at 15 kV accelerating voltage and 36 pA beam current. The reference samples for original AuNPs visualisation with SEM were made of silicon wafers covered with 2000 Å of Au film on top of 25 Å titanium adhesion layer, the deposition was done in a Balzers UMS 500 P system (Balzers, Neugrüt, Lichtenstein) by electron-beam evaporation. The AuNPs coating was done by pipetting of 5 μL of a AuNPs suspension and drying in air.
Author Contributions
S.S., D.B.S., V.O.P., Z.B., and Je.Sc. conceived and initiated the project. S.S. conceived and supervised the experiments. V.A. and M.F. prepared and electrochemically characterised EFCs. Ja.So. and D.B.S. performed AFM and SEM studies, respectively, and analysed the data. S.S., Je.Sc., M.G. D.B.S., V.A., and M.F. performed in vivo experiments. M.G. and Je.Sc. obtained ethical approval for experiments. R.L. isolated, purified, and characterised bioelements. S.S., Z.B., Je.Sc., D.B.S., and V.O.P. wrote the manuscript. All authors discussed the results and implications, and commented on the work.
Supplementary Material
Supplementary Information
Acknowledgments
The authors thank Amano Enzyme Inc. for a kind gift of Amano 3 preparation. The authors would like to acknowledge also D. Csontos for helpful suggestions and fruitful discussions. The work has been supported financially by the Swedish Research Council (2013-6006), Linnaeus grant (80658701), the Knut and Alice Wallenberg Foundation (KAW 2004.0119), the Nanometer Structure Consortium at Lund University (nmC@LU), and by the Russian Foundation for Basic Research (12-04-33102 and 13-04-12083).
References
- Kipke D. R. et al. Advanced neurotechnologies for chronic neural interfaces: new horizons and clinical opportunities. J. Neurosci. 28, 11830–11838 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov N. A. et al. Nanomaterials for neural interfaces. Adv. Mater. 21, 3970–4004 (2009). [Google Scholar]
- Vaadia E. & Birbaumer N. Grand challenges of brain computer interfaces in the years to come. Front. Neurosci. 3, 151–154 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Karni T., Langer R. & Kohane D. S. The smartest materials: The future of nanoelectronics in medicine. ACS Nano 6, 6541–6545 (2012). [DOI] [PubMed] [Google Scholar]
- Schouenborg J. Biocompatible multichannel electrodes for long-term neurophysiological studies and clinical therapy--novel concepts and design. Prog. Brain Res. 194, 61–70 (2011). [DOI] [PubMed] [Google Scholar]
- Bryllert T., Wernersson L.-E., Froeberg L. E. & Samuelson L. Vertical high-mobility wrap-gated InAs nanowire transistor. IEEE Electron Device Lett. 27, 323–325 (2006). [Google Scholar]
- Lieber C. M. Semiconductor nanowires: a platform for nanoscience and nanotechnology. MRS Bull. 36, 1052–1063 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C. P. T. et al. Monolithic GaAs/InGaP nanowire light emitting diodes on silicon. Nanotechnology 19, 305201/305201–305201/305206 (2008). [DOI] [PubMed] [Google Scholar]
- Suyatin D. B. et al. Nanowire-based electrode for acute in vivo neural recordings in the brain. PLoS One 8, e56673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton S. C., Gallaway J. & Atanassov P. Enzymatic biofuel cells for implantable and microscale devices. Chem. Rev. 104, 4867–4886 (2004). [DOI] [PubMed] [Google Scholar]
- Freguia S., Virdis B., Harnisch F. & Keller J. Bioelectrochemical systems: Microbial versus enzymatic catalysis. Electrochim. Acta 82, 165–174 (2012). [Google Scholar]
- Rapoport B. I., Kedzierski J. T. & Sarpeshkar R. A glucose fuel cell for implantable brain-machine interfaces. PLoS One 7, e38436 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinquin P. et al. A glucose biofuel cell implanted in rats. PLoS One 5, e10476 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T. et al. Enzymatic biofuel cells designed for direct power generation from biofluids in living organisms. Energy Environ. Sci. 4, 5008–5012 (2011). [Google Scholar]
- Castorena-Gonzalez J. A. et al. Biofuel cell operating in vivo in rat. Electroanalysis 25, 1579–1584 (2013). [Google Scholar]
- Zebda A. et al. Single glucose biofuel cells implanted in rats power electronic devices. Sci. Rep. 3, 1516, 10.1038/srep01516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk M., Narvaez Villarrubia C. W., Babanova S., Atanassov P. & Shleev S. Biofuel cells for biomedical applications: colonizing the animal kingdom. ChemPhysChem 14, 2045–2058 (2013). [DOI] [PubMed] [Google Scholar]
- Shleev S. et al. High-perfromance, size-optimized, nanostructure-based biofuel cell operating in rat brain. 63rd Annual ISE Meeting, August 19–24, Czeck Republic, (2012). [Google Scholar]
- Falk M. et al. In vivo operating miniature, direct electron transfer based, membrane-less glucose/oxygen biofuel cell in rat brain. 222nd ECS Meeting, October 7–12, Hawaii, (2012). [Google Scholar]
- Minteer S. D. Nanobioelectrocatalysis and its applications in biosensors, biofuel cells and bioprocessing. Top. Catal. 55, 1157–1161 (2012). [Google Scholar]
- Tamaki T. Enzymatic biofuel cells based on three-dimensional conducting electrode matrices. Top. Catal. 55, 1162–1180 (2012). [Google Scholar]
- Falk M., Blum Z. & Shleev S. Direct electron transfer based enzymatic fuel cells. Electrochim. Acta 82, 191–202 (2012). [Google Scholar]
- Coman V. et al. A direct electron transfer-based glucose/oxygen biofuel cell operating in human serum. Fuel Cells 10, 9–16 (2010). [Google Scholar]
- Coman V. et al. A membrane-, mediator-, cofactor-less glucose/oxygen biofuel cell. Phys. Chem. Chem. Phys. 10, 6093–6096 (2008). [DOI] [PubMed] [Google Scholar]
- Pan C. et al. Generating electricity from biofluid with a nanowire-based biofuel cell for self-powered nanodevices. Adv. Mater. 22, 5388–5392 (2010). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. Mediatorless sugar/oxygen enzymatic fuel cells based on gold nanoparticle-modified electrodes. Biosens. Bioelectron. 31, 219–225 (2012). [DOI] [PubMed] [Google Scholar]
- Falk M. et al. Biofuel cell as a power source for electronic contact lenses. Biosens. Bioelectron. 37, 38–45 (2012). [DOI] [PubMed] [Google Scholar]
- Murata K. et al. A simple fabrication method for three-dimensional gold nanoparticle electrodes and their application to the study of the direct electrochemistry of cytochrome c. Electroanalysis 22, 185–190 (2010). [Google Scholar]
- Murata K., Kajiya K., Nakamura N. & Ohno H. Direct electrochemistry of bilirubin oxidase on three-dimensional gold nanoparticle electrodes and its application in a biofuel cell. Energy Envir. Sci. 2, 1280–1285 (2009). [Google Scholar]
- Dagys M. et al. Laccase-gold nanoparticle assisted bioelectrocatalytic reduction of oxygen. Electrochem. Commun. 12, 933–935 (2010). [Google Scholar]
- Trasatti S. & Petrii O. A. Real surface area measurements in electrochemistry. Pure Appl. Chem. 63, 711–734 (1991). [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol. Rev. 83, 1183–1221 (2003). [DOI] [PubMed] [Google Scholar]
- Rehncrona S. Brain acidosis. Ann. Emerg. Med. 14, 770–776 (1985). [DOI] [PubMed] [Google Scholar]
- Ludwig R., Harreither W., Tasca F. & Gorton L. Cellobiose dehydrogenase: A versatile catalyst for electrochemical applications. ChemPhysChem 11, 2674–2697 (2010). [DOI] [PubMed] [Google Scholar]
- Shleev S. et al. On the possibility of uphill Intramolecular electron transfer in multicopper oxidases: electrochemical and quantum chemical study of bilirubin oxidase. Electroanalysis 24, 1524–1540 (2012). [Google Scholar]
- Sherwood L. Human physiology: from cells to systems. 558–569 (Brooks/Cole, Pacific Grove, 2001). [Google Scholar]
- Mak W., Cheng T. S., Chan K. H., Cheung R. T. F. & Ho S. L. Cerebrospinal fluid to serum glucose ratio in non-hypoglycorrhachic neurological conditions. Hong Kong Med. J. 11, 457–462 (2005). [PubMed] [Google Scholar]
- Reiber H., Ruff M. & Uhr M. Ascorbate concentration in human cerebrospinal fluid (CSF) and serum. Intrathecal accumulation and CSF flow rate. Clin. Chim. Acta 217, 163–173 (1993). [DOI] [PubMed] [Google Scholar]
- Shaidarova L. G., Chelnokova I. A., Romanova E. I., Gedmina A. V. & Budnikov G. K. Joint voltammetric determination of dopamine and uric acid. Russ. J. Appl. Chem. 84, 218–224 (2011). [Google Scholar]
- Vidakovic-Koch T. et al. Impact of the gold support on the electrocatalytic oxidation of sugars at enzyme-modified electrodes. Electroanalysis 23, 927–930 (2011). [Google Scholar]
- Teng H., Liu Y. & You T.-Y. Facile synthesis of gold nanoparticle-loaded carbon nanofiber composites and their electrocatalytic activity towards dopamine, ascorbic acid and uric acid. Chem. Res. Chin. Univ. 27, 496–499 (2011). [Google Scholar]
- Bayir H. et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr. Res. 51, 571–578 (2002). [DOI] [PubMed] [Google Scholar]
- Hall J. E. & Guyton A. C. Textbook of medical physiology. 562–564 (Saunders/Elsevier, Philadelphia, 2011). [Google Scholar]
- Agus D. B. et al. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J. Clin. Invest. 100, 2842–2848 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F. et al. Change in tissue concentrations of lipid hydroperoxides, vitamin C and vitamin E in rats with streptozotocin-induced diabetes. Clin. Sci. 96, 185–190 (1999). [PubMed] [Google Scholar]
- Lasia A. Electrochemical impedance spectroscopy and its applications. Mod. Aspects Electrochem. 32, 143–248 (1999). [Google Scholar]
- Aurbach D., Levi M. D. & Levi E. A review on the solid-state ionics of electrochemical intercalation processes: How to interpret properly their electrochemical response. Solid State Ionics 179, 742–751 (2008). [Google Scholar]
- Hodgkin A. L. & Huxley A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J. Voltage-gated ion channels in dendrites. Dendrites. Stuart G., , Spruston N., , Hausser M. (eds.) 139–160 (Oxford University Press, New York, 1999). [Google Scholar]
- Zhang H., Shih J., Zhu J. & Kotov N. A. Layered nanocomposites from gold nanoparticles for neural prosthetic devices. Nano Lett. 12, 3391–3398. 10.1021/nl3015632 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzu G. et al. Real-time monitoring of brain tissue oxygen using a miniaturized biotelemetric device implanted in freely moving rats. Anal. Chem. 81, 2235–2241 (2009). [DOI] [PubMed] [Google Scholar]
- Truesdale G. A. & Downing A. L. Solubility of oxygen in water. Nature 173, 1236 (1954). [Google Scholar]
- Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature, Phys. Sci. 241, 20–22 (1973). [Google Scholar]
- Drummond G. B. Reporting ethical matters in The Journal of Physiology: standards and advice. J. Physiol. 587, 713–719 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horcas I. et al. WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78, 013705/013701–013705/013708 (2007). [DOI] [PubMed] [Google Scholar]
- Fromherz P. Electrical interfacing of nerve cells and semiconductor chips. ChemPhysChem 3, 276–284 (2002). [DOI] [PubMed] [Google Scholar]
- Keller D. Reconstruction of STM and AFM images distorted by finite-size tips. Surf. Sci. 253, 353–364 (1991). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information