Abstract
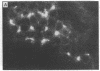
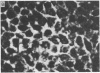
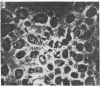
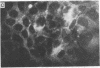
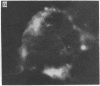
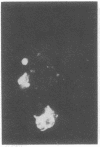
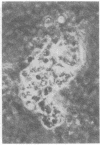
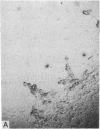
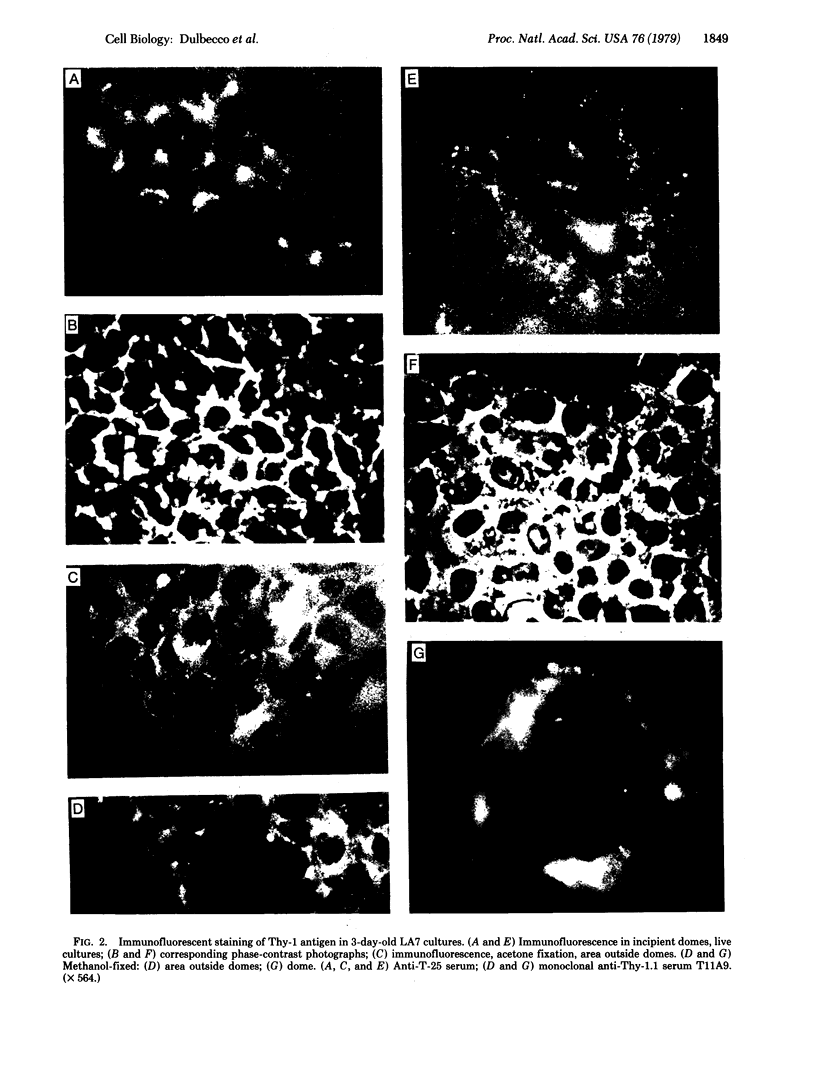
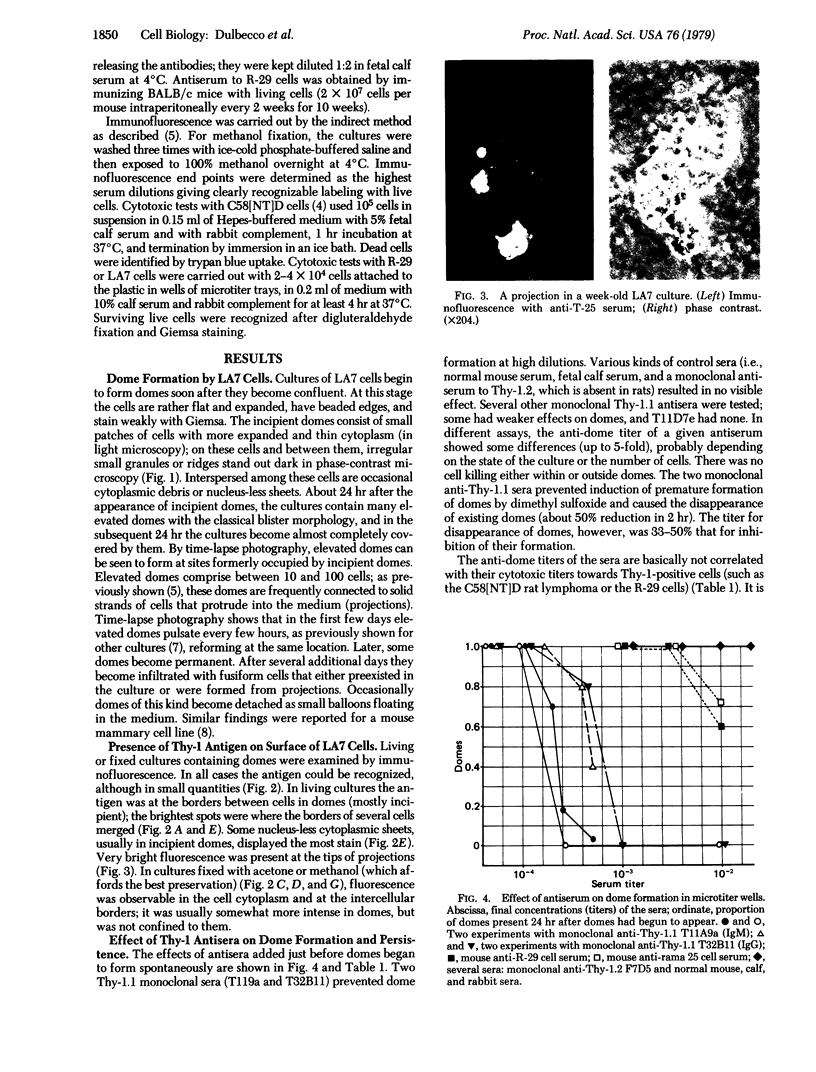
A line of rat mammary cells, LA7, undergoes differentiation into domes or ridges or projections under partial medium control. Immunofluorescent studies show that at the time of dome formation the LA7 cultures express the Thy-1 antigen at the borders between cells. Exposure of the cultures to certain monoclonal anti-Thy-1.1 sera prevents formation of new domes and causes disappearance of preexisting ones; antisera to whole cells produce no visible effect. Cultures exposed to anti-Thy-1 sera at effective concentrations of antibody against domes show morphological changes and ridge formation. It seems that the specific interactions of these antisera with the Thy-1 antigen redirects the differentiation program of the cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett D. C., Peachey L. A., Durbin H., Rudland P. S. A possible mammary stem cell line. Cell. 1978 Sep;15(1):283–298. doi: 10.1016/0092-8674(78)90104-6. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Bologna M., Unger M. Differentiation of a rat mammary cell line in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1256–1260. doi: 10.1073/pnas.76.3.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Unger M., Dulbecco R. Thy-1: a differentiation marker of potential mammary myoepithelial cells in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6093–6097. doi: 10.1073/pnas.75.12.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J. F., Lennon V. A. Transitory expression of Thy-1 antigen in skeletal muscle development. Nature. 1977 Jul 14;268(5616):163–165. doi: 10.1038/268163a0. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raedler A., Arndt R., Raedler E., Jablonski D., Thiele H. G. Evidence for the presence of Thy-1 on cultured thymic epithelial cells of mice and rats. Eur J Immunol. 1978 Oct;8(10):728–730. doi: 10.1002/eji.1830081011. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Thiery J. P., Brackenbury R., Sela B. A., Edelman G. M. Mechanisms of adhesion among cells from neural tissues of the chick embryo. Proc Natl Acad Sci U S A. 1976 Feb;73(2):577–581. doi: 10.1073/pnas.73.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser A. S., Prop F. J. "Domes," periodically expanding and collapsing secretory structures in cell cultures of mouse mammary tumors. J Natl Cancer Inst. 1974 Jan;52(1):293–295. doi: 10.1093/jnci/52.1.293. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Differentiation antigens of the lymphocyte cell surface. Contemp Top Mol Immunol. 1977;6:83–116. doi: 10.1007/978-1-4684-2841-4_3. [DOI] [PubMed] [Google Scholar]
- Yagi M. J. Cultivation and characterization of BALB-cfC3H mammary tumor cell lines. J Natl Cancer Inst. 1973 Dec;51(6):1849–1860. doi: 10.1093/jnci/51.6.1849. [DOI] [PubMed] [Google Scholar]