Abstract
Youth antisocial behavior (AB) is an important public health concern impacting perpetrators, victims, and society. Functional neuroimaging is becoming a more common and useful modality for understanding neural correlates of youth AB. Although there has been a recent increase in neuroimaging studies of youth AB and corresponding theoretical articles on the neurobiology of AB, there has been little work critically examining the strengths and weaknesses of individual studies and using this knowledge to inform the design of future studies. Additionally, research on neuroimaging and youth AB has not been integrated within the broader framework of developmental psychopathology. Thus, this paper provides an in-depth review of the youth AB functional neuroimaging literature with the following goals: 1. to evaluate how this literature has informed our understanding of youth AB, 2. to evaluate current neuroimaging studies of youth AB from a developmental psychopathology perspective with a focus on integrating research from neuroscience and developmental psychopathology, as well as placing this research in the context of other related areas (e.g., psychopathy, molecular genetics), and 3. to examine strengths and weaknesses of neuroimaging and behavioral studies of youth AB to suggest how future studies can develop a more informed and integrated understanding of youth AB.
Keywords: Youth Antisocial Behavior, Conduct Disorder, Neuroimaging, fMRI, Developmental Psychopathology
A long history of research on children and adolescents has emphasized multiple pathways in the development and maintenance of antisocial behavior (AB) (e.g., Frick & White, 2008; Loeber & Dishion, 1983; Loeber & Stouthamer-Loeber, 1998; Moffitt et al., 2008; Moffitt, Caspi, Dickson, Silva, & Stanton, 1996). This heterogeneous group of behaviors, including physical and sexual aggression, destruction of property, theft, and violation of serious societal rules, has been of particular interest to researchers and the general public because of the large cost to society through their negative impact on perpetrators and victims, the chronic nature and trajectory of AB, and the difficulty in preventing and treating AB (Colman et al., 2009; Odgers et al., 2007; Scott, Knapp, Henderson, & Maughan, 2001). Theories on the etiology of AB from a wide array of disciplines have emphasized the contributions of biological (e.g., neural, hormonal, genetic) and/or environmental (e.g., parenting, poverty, peers) mechanisms, with recent nuanced views emphasizing the complex interplay between these domains of influence (e.g., D’Onofrio, Rathouz, & Lahey, 2011; Guo, 2011; Kendler, 2011b; Reiss, 2005; Rutter, 1997; Sameroff, 2010).
In the past two decades, advances in neuroscience and related biological sciences (e.g., molecular genetics) have furthered our ability to measure specific biological processes involved in psychopathology (e.g., Bogdan, Hyde, & Hariri, 2012; Cole, 2009; Rutter & Dodge, 2011; Stoltenberg & Burmeister, 2000). Improvements in, and greater accessibility of, neuroimaging techniques such as functional magnetic resonance imaging (fMRI) have made studies incorporating these techniques more practical in larger samples, which have increased our understanding of the brain’s role in psychopathology (e.g., Dolan, 2008; Hariri, 2009). Recently, research has been initiated that applies functional neuroimaging to the study of AB in both adults and children that can directly address biological theories of AB. For example, studies have linked dysfunction in several brain areas to adult psychopathy (e.g., Yang & Raine, 2008) using a variety of different fMRI paradigms to probe the neural correlates of specific behaviors implicated in psychopathy. Recent studies involving adolescents (Jones, Laurens, Herba, Gareth, & Viding, 2009; Marsh et al., 2008) have linked callous-unemotional (CU) traits (a downward extension of the interpersonal and affective components of the adult psychopathy construct) and AB to specific brain mechanisms, and have integrated these findings within the context of both developmental psychology and neuroscience. The existing studies of neural functioning in youth with AB share several important strengths that can inform our understanding of the neural correlates of AB, but also limitations that could be improved upon in future work. In this vein, the current paper seeks to integrate theory and research from basic neuroscience and developmental psychopathology and suggest future directions for studying the neurobiological mechanisms involved in the development of youth AB. Relevant work from forensic psychology, biological psychiatry, and genetics is incorporated with the goal of integrating converging findings across disciplines so that each area can inform the other.
While several authors have written recent reviews on similar topics (e.g., the neurobiology of psychopathy, the neurobiology of aggression in children: Blair, Peschardt, Budhani, & Pine, 2006a, 2006b; Glenn & Raine, 2008; Kiehl, 2006; Sterzer & Stadler, 2009; Yang & Raine, 2008), most of these reviews have been written more narrowly with the primary goal of describing an author’s theory of neural mechanisms involved in AB, with less emphasis on a critical examination of the reviewed studies’ methods and results. In contrast, the goals of the current review are as follows: (1) to provide a broad and in-depth literature review of the functional neuroimaging literature as it relates to youth AB with the goal of evaluating how this literature has informed our understanding of youth AB at the neural and behavioral level; (2) to evaluate the current neuroimaging studies of youth AB from a developmental perspective with an eye towards integrating research from neuroscience and concepts from developmental psychopathology, as little work has examined how behavioral and neuroimaging studies inform each other and how the integration of these studies may highlight areas for future research; (3) to examine strengths and weaknesses of neuroimaging and behavioral studies of youth AB to suggest how future studies can develop a more informed and integrated understanding of youth AB; and (4) to examine how other relevant literatures (i.e., structural MRI of youth AB, neuroimaging in psychopathy, neurotransmitter and genetics approaches, findings from normative adolescents) can inform current and future functional neuroimaging studies of youth AB.
This paper will begin by exploring definitions of AB and both developmental and measurement issues in the study of youth AB, describing important considerations in undertaking and evaluating neuroimaging studies, reviewing central tenets of developmental psychopathology that bear on understanding youth psychopathology, and providing an overview of brain areas implicated in youth AB. Studies that have used functional neuroimaging approaches to study youth AB will then be reviewed with an emphasis on their strengths and limitations, and a brief section on how structural MRI findings in this population fit in with functional findings. Next, relevant theoretical and empirical literature from other fields (e.g., adult psychopathy, molecular genetics) will be reviewed as they relate to youth AB with an emphasis on brain areas and plausible biological mechanisms involved in youth AB. Finally, the paper concludes with suggestions for intervention and empirical approaches to further integrate biological and environmental interactions in the study of AB.
Throughout, several themes will be emphasized. First, youth AB is a complex set of behaviors and subtypes or specific behaviors need to be targeted, especially those that have already been extensively characterized behaviorally (e.g., CU traits, age of onset). However, results should not be generalized beyond these specific groups as their etiology may be distinct. Second, specific details of studies are critical to interpreting results: fMRI task and stimuli, behavioral measures used, age, and characteristics of the sample being studied will influence observed findings. Third, neuroimaging studies of youth AB alone can highlight correlations between brain function and behavior but can best advance our understanding when integrated with work at the molecular and cellular level and/or at the broad behavioral and developmental level. Studies that propose differential functioning models with testable hypotheses will advance the field. For example, models that separate similar behavior and can specify different underlying neural correlates have the potential to lead to a more nuanced understanding of the etiology of youth AB across and within potential subtypes. Moreover, advances in neuroimaging of youth AB may help test questions that have been left unanswered by behavioral work and similarly, integrating between neuroimaging and behavioral work can help to elucidate the strengths and weaknesses of each approach.
Youth Antisocial Behavior
Definition and theories of youth AB
AB can be described by a host of terms in children, adolescents, and adults including legal definitions (delinquency), broad behavioral definitions (externalizing behavior problems), and specific types of behaviors (aggression). In the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994) and recent fifth edition (American Psychiatric Association, 2013), youth AB is categorized into oppositional-defiant disorder (ODD) and conduct disorder (CD), with ODD focused more on less severe forms of age-inappropriate angry and oppositional behaviors, and CD focused more on severe aggression and behaviors that involve inflicting pain on others (e.g., initiating fights, sexual assault), denying the rights of others (e.g., stealing), and status offences (Hinshaw & Lee, 2003). When these behaviors are persistent in adults, they are categorized as Antisocial Personality Disorder (APD), with APD requiring a prior diagnosis of CD. These disorders are quite common: a recent study has estimated the lifetime prevalence of CD in the United States to be 9.5% of the population (12% among males, and 7% among females), with a median age of onset of 11.6 years (Nock, Kazdin, Hiripi, & Kessler, 2006).
Within both child and adult antisocial populations, a wide heterogeneity of symptoms is prevalent often causing researchers to either subdivide these behaviors or study individual behaviors. For example, aggression and AB have been studied widely and can be divided into proactive versus reactive subtypes (Vitaro, Brendgen, & Barker, 2006), rule breaking versus aggressive behaviors (Burt, 2012), and overt versus covert behaviors (Loeber & Stouthamer-Loeber, 1998). In adults, a major distinction has been made between criminality (and the related diagnosis of APD) and a more severe form of personality disorder called psychopathy. Psychopathy involves a parasitic and antisocial lifestyle as well as affective and interpersonal deficits, such as lack of empathy, guilt, and remorse, along with superficial charm, conning, and manipulativeness (Kiehl, 2006).
There also appears to be heterogeneity even within a single diagnostic class (e.g., CD) based on presenting symptoms (e.g., in DSM-IV, only 3 of 13 items are needed for a diagnosis of CD, creating multiple clusters of symptoms) (e.g., Loeber & Stouthamer-Loeber, 1998), age of onset of symptoms (Moffitt, 1993a), and the presence of other related traits such as callousness (Frick & Ellis, 1999). This heterogeneity may lead to conflicting findings and increased measurement error in studying AB if there are subgroups with different underlying etiologies (e.g., those that start earlier or those that also have callous-unemotional traits). There are several meaningful ways to divide groups of antisocial youth and we briefly consider these strategies and their benefits and limitations before considering the fMRI literature that is based on these subtyping schemes.
Age of Onset
Some researchers have proposed to subdivide youth by age of AB onset: “early starters” (before age 10) and “late starters” (Aguilar, Sroufe, Egeland, & Carlson, 2000; Moffitt, 1993a). In correlational studies, early starting problems have been linked to neurocognitive deficits (Moffitt, 1993b), familial risk such as coercive parenting (Patterson, Reid, & Dishion, 1992), difficult temperament (e.g., high levels of negative emotionality, fearlessness), high levels of ADHD symptoms (Moffitt, Caspi, Harrington, & Milne, 2002), and a chronic and escalating trajectory of behavior (Shaw & Gross, 2008). Later starting AB has been correlated with deviant peer association (Dishion, Patterson, Stoolmiller, & Skinner, 1991), fewer proximal family risks, and a less elevated and less chronic trajectory of AB (Moffitt et al., 2002).
These behavioral studies inform hypotheses to be tested by neuroimaging studies. For example, it has been theorized (Moffitt, 1993a) that because of the relative dearth of risk factors for adolescent onset AB, late starters may have fewer biological correlates of AB relative to early starters. Additionally, based on the high level of environmental risk seen in early starters and emerging literature linking early maltreatment to behavioral, physiological, and neural changes in children indicating a heightened reactivity to threat (Cicchetti & Rogosch, 2001; Dannlowski et al., 2012; Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005; Pollak & Tolley-Schell, 2003; Pollak, Vardi, Putzer Bechner, & Curtin, 2005), we would expect neuroimaging studies of early starting youth to find differences in neural reactivity to threat. Whereas this subtyping scheme has direct implications for neural studies and is used widely through its inclusion in the DSM-IV (American Psychiatric Association, 1994), it should be noted that authors have recently questioned the usefulness of this approach on statistical grounds (Walters & Ruscio, 2012), in comparison to other subtyping approaches (e.g., physical aggression versus non-aggressive rule breaking) (Burt, Donnellan, Iacono, & McGue, 2011), and in relation to several biological studies demonstrating few differences between early and late starters (Fairchild, van Goozen, Stollery, & Goodyer, 2008; Fairchild, Van Goozen, Calder, Stollery, & Goodyer, 2009; Fairchild, van Goozen, Stollery, et al., 2009; Fairchild, van Goozen, Stollery, Brown, et al., 2008).
Callous-Unemotional (CU) Traits
A second emphasis in the study of AB, particularly neuroimaging studies of youth AB, has focused on the presence or absence of CU traits, with CU traits posited to be important in the etiology and course of AB for a subgroup of youth (Frick, Cornell, Bodin, et al., 2003; Frick & White, 2008). The presence of CU traits is now part of the diagnosis of AB disorders in the recently published DSM-5 (Moffitt et al., 2008; Pardini, Frick, & Moffitt, 2010), classified as “limited prosocial emotions” (American Psychiatric Association, 2013). CU traits are linked to the shallow affect and lack of empathy seen in adult psychopathy and can be seen theoretically as a downward extension of affective and interpersonal components of the psychopathy construct in youth. CU traits have been shown to predict a more severe course of AB that is more stable and linked to other affective and learning deficiencies in youth (Frick & White, 2008), especially those that would imply specific neural deficiencies (e.g., difficulty recognizing fearful facial expressions, perseveration in learning paradigms) (Blair, Colledge, Murray, & Mitchell, 2001; Marsh & Blair, 2008). Moreover, AB has been shown to be more highly heritable in the presence of CU traits (Fontaine, Rijsdijk, McCrory, & Viding, 2010; Viding, Blair, Moffitt, & Plomin, 2005; Viding, Jones, Paul, Moffitt, & Plomin, 2008), further emphasizing the need to explore neural correlates of this subgroup. Finally, as CU traits in youth and psychopathy in adults have been associated with high levels of proactive ABs (Cornell et al., 1996; Frick, Cornell, Barry, Bodin, & Dane, 2003), examining proactive (e.g., instrumental, planned) versus reactive (e.g., in response to a perceived or actual threat) AB may represent another overlapping way of subdividing patterns of AB with implications for neural functioning (Bezdjian, Tuvblad, Raine, & Baker, 2011; Stadler, Poustka, & Sterzer, 2010).
Although a majority of the neuroimaging studies to be reviewed focus on groups of AB+CU+ youth, there are several points to consider in evaluating research on CU traits, particularly neuroimaging of CU traits as it compares to adult psychopathy. First, CU traits have been shown to be stable during middle childhood and from adolescence to adulthood to some extent (Blonigen, Hicks, Krueger, Patrick, & Iacono, 2006; Frick, Kimonis, Dandreaux, & Farell, 2003). However, few studies have followed children with CU traits into adulthood and those that have, have found CU to be predictive of Antisocial Personality Disorder (Loeber, Burke, & Lahey, 2002) but not necessarily adult psychopathy. There is also some evidence that a substantial number of children initially high on CU traits decrease in these “traits” (or behaviors) through this period of development (Frick, Kimonis, et al., 2003). For example, in a study examining trajectories of CU traits and AB in an earlier age period (from age 7–12), 13% of children were found to be decreasing from high to low levels of CU, whereas only 5% had stable high CU (with 7% increasing CU), meaning that more youth are desisting on measures of CU “traits” than those that are stably high or even increasing in CU traits across this earlier age period (Fontaine, McCrory, Boivin, Moffitt, & Viding, 2011). These studies beg the questions: 1) In studying AB adolescents with high CU are we really studying adolescents who will go on to be adult psychopaths? 2) How much should the study of CU during childhood and adolescence be guided by the literature on adult psychopathy? 3) How persistent are CU traits across childhood and adolescence (and into adulthood) and how can we distinguish those who persist from those who desist from these behaviors or “traits”, especially from a neurobiological point of view? 4) How homogenous and stable is the group of youth with AB and CU traits? Are CU traits really “traits” (Waller, Gardner, & Hyde, 2013)? Fundamentally, much of the emerging neuroimaging literature on youth with AB and CU traits assumes that CU traits in adolescence are an early form of psychopathy; however, very little empirical data has tested this relationship longitudinally.
Second, beyond stability and developmental concerns, it also is not clear whether CU traits during childhood and adolescence should be conceptualized as a continuous dimension or a dichotomous trait. In the adult literature, psychopathy is often viewed as important only above a specific extreme threshold (e.g., PCL-R; Kotler & McMahon, 2005) and is typically measured in a dichotomous manner (Blonigen et al., 2006; Koenigs, Baskin-Sommers, Zeier, & Newman, 2011). Behavioral studies typically employ continuous measures of CU traits and less typically use cut-off scores in reporting associations, but recent neuroimaging studies have used only extreme groups (Jones et al., 2009; Marsh et al., 2008) despite having no standard cut point for establishing risk status on CU traits in children. Whereas the imaging studies reviewed here could imply that the extreme group may be biologically distinct, the lack of clarity about the conceptualization and measurement of the underlying construct makes interpreting these neuroimaging studies more difficult (for a discussion of similiar issues in adult psychopathy neuroimaging see Koenigs et al., 2011). This issue also has implications for informing intervention research, as recommendations would vary depending on whether neural differences in CU youth are present only when partitioned dichotomously versus examined continuously.
Important behavioral constructs not addressed in the neuroimaging literature
Just as it is important to note the limitations of constructs like CU traits that are widely used in neuroimaging studies, it is equally important to note what is not addressed in the neuroimaging literature but has been important in behavioral studies of youth AB. The first major omission is that the majority of neuroimaging studies of youth AB have focused on CU traits, very few on age of onset, and none on constructs such as proactive versus reactive aggressive behaviors (Vitaro, Gendreau, Tremblay, & Oligny, 1998), aggression versus rule breaking (Burt, 2012) or covert versus overt behaviors (Loeber & Stouthamer-Loeber, 1998), all of which have been shown to be valid approaches for subtyping this heterogeneous group. Moreover, although many assume that there is high overlap in youth who are early starters, high on CU traits, and more seriously and proactively aggressive, there is little prospective empirical evidence supporting the assumed overlap of all of these schemes for classifying AB youth. At best, those high on CU traits have been shown to be both proactive and reactively aggressive, whereas those low on CU traits have been shown to be mainly reactively aggressive (Bezdjian et al., 2011; Cornell et al., 1996; Frick, Cornell, Bodin, et al., 2003).
A second major omission is that no neuroimaging studies have examined neural reactivity in relation to Social Information Processing (SIP), a well-researched construct in relation to youth aggressive behavior. Dodge and others have proposed a theory of SIP that posits a series of steps in both interpreting and acting in social situations (e.g., interpretation of social cues, response evaluation) that are presumed to be proximal mechanisms that underlie children’s social behavior generally and aggression specifically (Dodge, 1993). A wealth of research supports evidence that aggressive boys display social information processing deficits (Dodge & Schwartz, 1997), particularly hostile attribution biases and response generation (Dodge & Schwartz, 1997; Orobio de Castro, Veerman, Koops, Bosch, & Monshouwer, 2002), including interpreting neutral faces as hostile (Dadds et al., 2006). Although SIP mechanisms have been found to be critical to understanding response to threat in aggressive youth, no neuroimaging studies to date have examined patterns of neural reactivity to threat in relation to SIP biases. This line of research could be quite fruitful in understanding the role of threat and reward in youth AB from a behavioral and neural level. For example, in one study reactively aggressive children demonstrated a greater history of maltreatment, slightly earlier onset of problems, and more difficulties in encoding (hostile attribution bias) and problem solving relative to their proactively aggressive peers (Dodge, Lochman, Harnish, Bates, & Pettit, 1997). In contrast, proactively aggressive children displayed differences in anticipating positive outcomes of aggressing (rather than hostile attribution bias), suggesting the possibility of different cognitive and neural mechanisms involved in these two overlapping but distinct forms of aggression (in relation to CU traits, see also Stickle, Kirkpatrick, & Brush, 2009). As such, reactive aggression could be associated with exaggerated response to threat or even neutral situations interpreted as threatening (hostile attribution bias), whereas proactive AB could be associated with poor evaluation of the outcomes of aggression (reward processing). This literature suggests that reactively aggressive youth would show exaggerated neural response to threat (or even neutral/ambiguous stimuli) in relevant neural regions, whereas proactively aggressive youth would be more likely to demonstrate greater response in neural regions associated with reward.
In summary, within the broad group of ABs, researchers have employed multiple ways of subdividing youth, theoretically leading to more homogenous groups that are likely to have distinct etiologies, which is particularly important when examining biological components of etiology. Two of these grouping methods, early versus late starting AB and the presence or absence of CU traits, are especially relevant for neuroimaging studies of youth with AB because most neuroimaging studies of youth AB have focused on these subgroups and these subtypes are the focus of diagnostic classification systems (i.e., the DSM). However, although these approaches have advantages, there are certainly limitations worth noting (e.g., the lack of replicated longitudinal research linking CU traits and adult psychopathy) when evaluating neuroimaging studies employing these constructs.
Overview of Developmental and Neuroimaging Methods
As the focus of this review is to bring together perspectives from multiple disciplines to provide a multi-faceted lens through which to evaluate current studies and suggest future directions for the study of youth AB, we first examine important points to consider within developmental and neuroimaging studies. We start with an overview of considerations from developmental psychopathology, provide a brief overview of important considerations in interpreting fMRI studies, and then describe the brain areas of focus in this review.
Developmental Psychopathology Considerations
During the past quarter century a developmental psychopathology perspective (Sroufe & Rutter, 1984) has greatly changed the way clinical researchers approach the study of child psychopathology through an emphasis on constructs from developmental science and systems theory (Sameroff, 1995). Specifically, this approach suggests a greater appreciation of the multiple influences on adaptive and maladaptive child behavior, incorporating how biological and environmental forces impact children in a dynamic and transactional manner throughout development (Cicchetti, 1993; Cummings, Davies, & Campbell, 2000; Rutter, 1997; Sameroff, 2000; Sroufe & Rutter, 1984). As one goal of this review is to integrate these ideas and approaches into theory and interpretation of the present studies, several points of emphasis in developmental psychopathology that are important to consider are summarized briefly below.
Age and Developmental Stage
Aggression in a two-year old and a 15-year old are quite different phenomena. Aggression during the toddler years is normative and is not likely to be as destructive as it is in older children (Loeber & Hay, 1997; Tremblay et al., 1999). The developmental tasks and roles of youth are also different at different stages. For example, the relative influence of parenting and peers on child AB is likely to change as a function of youth’s developmental stage, with peers exerting an increasingly large influence as children approach adolescence (Dishion & Patterson, 2006; Shaw, Bell, & Gilliom, 2000). Therefore, a finding that a certain brain area is linked to AB at age 17 may tell us relatively little about the development of AB for a preschool child, as the following factors may vary as a function of developmental status: the presentation of AB (e.g., temper tantrums versus robbery), the influence of different environmental factors (e.g., parents versus peers), and the connections between and within brain areas (Casey, Tottenham, Liston, & Durston, 2005), especially as there are pronounced changes in myelination and development of the prefrontal cortex (PFC) that occur during adolescence (Durston et al., 2006; Giedd, 2008). Moreover, when considering risk factors for AB that may affect brain functioning (e.g., harsh parenting, neighborhood violence), it is important to consider that these risks may have different effects on the brain and the expression of these effects in terms of behavior may differ by age and developmental stage (Tottenham & Sheridan, 2009). This issue is broadly important to all behavioral studies, but we focus on it in relation to functional neuroimaging in particular because much of the extant literature has not been longitudinal in nature and includes a broad age range of participants spanning multiple development periods (e.g., 10 to 18 years).
Heterotypic Continuity
The underlying “phenotype” of problem behavior may present as different behaviors at different ages. For example, developmental models of AB emphasize early opposition and aggression that may then lead to later delinquent behaviors, which may then escalate to more serious violent offenses (Loeber, 1982; Loeber & Stouthamer-Loeber, 1998). Although these behaviors are different, they may be the changing presentation of the same underlying phenotype (e.g., the same brain circuitry, the same extreme dimension of temperament or personality).
Heterogeneity within Diagnostic Class
As emphasized previously, AB encompasses a wide array of behaviors. This heterogeneity may represent a broad but connected phenotype or it may represent related but distinct patterns of behavior with similar or dissimilar precursors. For example, some researchers have attempted to separate proactive and reactive aggression or “hot” versus “cold” behaviors (Dadds, Allen, et al., 2012; Kim, Nordling, Yoon, Boldt, & Kochanska, 2013; Vitaro et al., 1998). However, as these behaviors are highly statistically correlated it is often difficult to determine if many youth actually are distinctly reactive or proactive (Vitaro et al., 2006). New imaging techniques may therefore be an important means of identifying subgroups of individuals based on differences in biology within an otherwise heterogeneous group. Moreover, these approaches illustrate how using a person-centered approach (e.g., using developmental trajectories, separating by extremes scores on CU traits) to analyzing data can help delineate subgroups within those who appear similar at one point on one measure (e.g., two adolescents high on aggression at age 15 but one started at age 5 and the other at age 14) (Nagin & Tremblay, 2001; Shaw, Hyde, & Brennan, 2012).
Comorbidity
Childhood disorders are highly comorbid (Costello, Foley, & Angold, 2006). In particular, AB diagnosed as conduct disorder (CD) is highly comorbid with ADHD, depression, learning disabilities, and substance use disorders (Hinshaw & Lee, 2003). This comorbidity may reflect the overlap of symptoms in multiple disorders, underlying shared etiology or broader phenotype, shared genetic loading, and/or shared environmental risk (Banaschewski et al., 2005; Kendler, Aggen, & Patrick, 2013; Krueger & Markon, 2006). However, the presence of comorbidities makes design and interpretation of any clinical study difficult. For example, in most samples of boys with CD, the prevalence of ADHD will be quite high. If neural differences are found between a group of boys high on CD versus controls, the differences could reflect a difference specific and etiological to CD, a difference specific and etiological to a third variable (i.e., the high rate of ADHD in the CD sample) and/or a difference linked and etiological to broad externalizing behaviors (shared biological etiological factors to both ADHD and CD). This issue is particularly important in the case of ADHD, as studies have demonstrated that ADHD (even with no overlapping CD) is correlated with several neurobiological differences not seen in controls (Arnsten & Rubia, 2012; Durston, 2003; Rubia, 2011). Again, delineating subgroups of boys (those comorbid versus those with pure CD) is likely to have very important and practical implications. Additionally studies that emphasize how general versus specific their findings are (e.g., linked to CU traits only in CD and not in ADHD) will be helpful in defining the shared versus unique aspects to disorders within the externalizing spectrum (Lahey, Van Hulle, Singh, Waldman, & Rathouz, 2011).
Dimensional and Categorical Phenotypes
A developmental psychopathology perspective emphasizes the importance of understanding behavior as both categories (e.g., in diagnostic groups) and dimensions (Blonigen et al., 2006; Markon & Krueger, 2005). This point is especially important as increasing evidence mounts supporting the hierarchical and dimensional nature of psychopathology (Krueger & Markon, 2011; Ofrat & Krueger, 2012).
Complex Paths: Equifinality, Multifinality, Risk and Resilience
Children can arrive at the same developmental outcome (e.g., being arrested as an adolescent) from many different pathways (equifinality), and children with the same initial risk factors may show vastly different trajectories and outcomes (multifinality; Cicchetti & Rogosch, 1996). These concepts, along with the understanding that outcomes are probabilistic, are critically important in understanding the role of biology in behavior. Interactions with a complex social environment can dramatically change the impact of biologic risk, leading to multifinality (e.g., Hankin et al., 2011). For example, a child high on daring traits and testosterone has been shown to be at greater risk for delinquency in low income and dangerous neighborhoods (Dabbs & Morris, 1990; Trentacosta, Hyde, Shaw, & Cheong, 2009), but under different conditions these same “risk” factors may lead him to become a competent firefighter (Fannin & Dabbs, 2003). Moreover, it is important to understand that the interaction of these risks occur across the multiple levels of influence on the child (e.g., from city to neighborhood to familial risk: Bronfenbrenner & Ceci, 1994).
In contrast, multiple and different constellations of risk can influence the formation of the same behaviors, an example of equifinality. For example, a child exposed to early abusive parenting and a child with early warm parenting but later deviant peer affiliation may both exhibit the same symptoms of conduct disorder in adolescence. Similarly, a child that shows high neural reactivity to threat and a child with low levels of neural reactivity to threat may both exhibit AB but with different underlying etiologies (i.e., one characterized by high levels of reactive aggression and one with high levels of proactive aggression).
The brain itself can also be seen as probabilistic, and understanding biological differences between groups only helps us understand vulnerabilities towards certain behaviors. For example, studies of the serotonin system and the amygdala have shown that serotonin signaling and increased amygdala activity to threat are linked to trait anxiety and risk for depression (Fakra et al., 2009; Hariri et al., 2005; Monk, Klein, et al., 2008). However, most people with both increased amygdala activity to threat and with “risk” alleles in genes affecting the serotonin system are not clinically depressed or anxious (Dannlowski et al., 2007; Hyde, Manuck, & Hariri, 2011). These risk factors reflect one small part of a complex probabilistic chain, or perhaps, one small part of a very complex etiological chain for which we currently have an incomplete description, much less an understanding. Moreover, these risk factors may only be relevant or pathological in certain environments as demonstrated by a wealth of studies indicating the conditional and interactive nature of biological and environmental risk (Rutter et al., 1997; Sameroff, 2000), studies of gene by environment (G x E) interactions (Belsky et al., 2009; Caspi et al., 2002; Caspi et al., 2003; Jaffee et al., 2005; Manuck, 2009), and studies demonstrating that environments may moderate brain-behavior relationships (Hyde, Manuck, et al., 2011). One person may have several genes that put him/her at risk for AB, but in a protective environment these genes may not bias the system enough to play a role in pathology. Similarly, some genes or neural phenotypes may make individuals more or less susceptible to harsh or positive environments (Belsky & Pluess, 2009; Ellis & Boyce, 2011; Masten, 2001; Masten & Coatsworth, 1998). An appreciation of equifinality and multifinaliy, as well as risk and resilience, emphasizes that any one neurobiological correlate in isolation may not be meaningful for all children, may only matter in certain contexts, and may be a correlate of many different behaviors. These considerations also have important ethical implications, as findings about predictors of youth AB should not be seen as static and unchangeable because of their “biological” nature. Importantly, much of the behavioral and neuroimaging studies of youth AB are correlational in nature, and thus cannot be viewed as causal in nature either (Jaffee, 2011; Kendler, 2011a; O’Connor & Rutter, 1996; Rutter, 2000).
In sum, by applying a developmental psychopathology approach, we can better appreciate the nuances of studying youth within a complex system in which biology and the environment are constantly interacting (Hyde, Bogdan, & Hariri, 2011; Meaney, 2010). This appreciation of development, different pathways to pathology and health, and the complexity of diagnosis can all inform our evaluation and understanding of the neuroimaging research aimed at understanding youth AB.
Neuroimaging Considerations
fMRI
There are several ways to measure neurobiological activity and this review primarily focuses on fMRI studies because of various strengths that make it suitable to addressing questions of functional neurobiology: fMRI balances temporal and spatial resolution and is specific to tasks over brief periods of time. Thus, fMRI approaches create the possibility of making inferences that are specific to relatively small brain areas across relatively short time spans while also probing the interaction between brain areas. Although fMRI is focused on in this review, the best approach is to seek converging evidence across multiple methods (e.g., structural MRI - sMRI, lesion studies, animal studies). Other approaches (i.e., sMRI studies of youth AB, studies of adult psychopaths, genetic and neurotransmitter findings, findings from normative adolescents) are considered throughout this review where applicable but certainly not in an exhaustive manner.
An fMRI scanner is able to sample the entire brain every few seconds, giving a time series of BOLD (Blood Oxygen Level-Dependent) responses – a signal which reflects changes in regional neuronal activity (Lee et al., 2010; Logothetis & Pfeuffer, 2004). Understanding basic properties of fMRI is important because it underscores the role of contrast, task, and stimuli in the interpretation of fMRI findings.
Contrast, task, and stimuli
Most fMRI studies are focused on examining a contrast of one condition within a task to another. The brain is constantly active and using oxygen, thus the BOLD signal at any one point in time may be difficult to interpret unless compared to activity at another point in time. If two similar stimuli are contrasted (fearful versus neutral faces) then the difference in BOLD signal can be interpreted as the change in brain activity in response to the differences between the stimuli. Note that task characteristics (e.g., labeling the gender of faces vs. labeling their affect) and specific features of stimuli (e.g., width of the pupils) all affect neural responses (Demos, Kelley, Ryan, Davis, & Whalen, 2008; Lieberman et al., 2007). Thus, even tasks and stimuli (e.g., pictures of scary or gross scenes, angry faces) that may tap into theoretically similar constructs (e.g., distressing and threatening stimuli) may engage the brain differently.
Limitations
Finally, it is also important to note some general limitations of fMRI in drawing conclusions from neuroimaging studies of youth AB. First, fMRI studies on humans are typically correlational. Without being able to actually manipulate the brain, conclusions cannot be causal. This observation reflects a broad issue within most of developmental psychopathology, as well as sMRI and non-randomized clinical data, as most of these studies are also limited by their correlational nature. Second, while much research has been aimed at understanding the BOLD signal, it is still not clear whether these changes in blood flow represent input or output of the particular brain area (Lee et al., 2010; Logothetis & Pfeuffer, 2004).
In sum, evaluating the strengths, weaknesses, and validity of the task, stimuli, and contrast (as well as the imaging modality) is critically important in understanding neuroimaging studies of youth AB. Moreover, just as the “devil is in the details” in terms of evaluating neuroimaging approaches, the details (e.g., subtype of AB measured, developmental stage of participants) in understanding the development of youth AB are equally important as we apply findings from behavioral and neuroscience approaches to youth AB.
Overview of Brain Areas Implicated in Youth AB
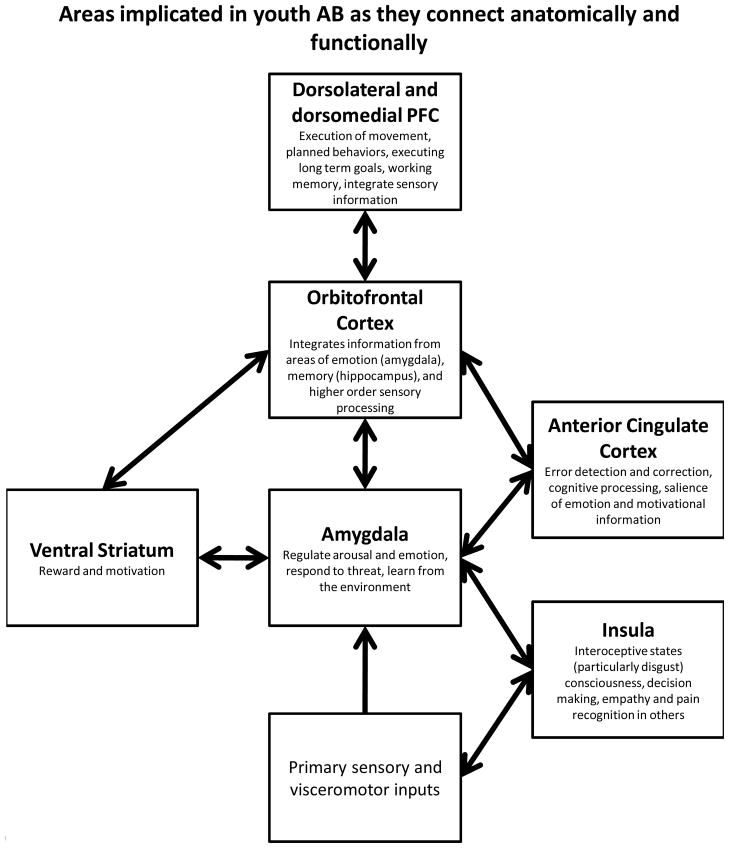
Before reviewing individual studies, we first describe several of the brain areas that have been regions of interest (ROIs) within neuroimaging studies (for a brief review see Sterzer & Stadler, 2009) from both an anatomical and systems perspective to help understand how they may be implicated in youth AB (see Figure 1). Second, we briefly describe several overarching theories and hypotheses put forward in the field of AB that underline which areas of the brain are likely to be implicated in youth AB and why.
Figure 1.
Areas implicated in youth AB as they connect anatomically and functionally
Areas with the most support for their role in youth AB. (See Sterzer & Stadler, 2011; Forbes & Grafman, 2010).
Understanding brain areas implicated in youth AB
The amygdala has been a primary structure of interest in AB and psychopathology more broadly. The amygdala is a subcortical structure and a major hub connecting both subcortical and cortical areas (LeDoux, 2000). It is thus important in many disparate processes such as regulating arousal and emotion, responding to threat, and learning from the environment (e.g., fear conditioning) (Cardinal, Parkinson, Hall, & Everitt, 2002; Whalen & Phelps, 2009). The prefrontal cortex (PFC) has also been broadly implicated in youth AB, as well as theories of inhibition and aggression more generally (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Best, Williams, & Coccaro, 2002; Blair, 2004). The PFC is composed of structurally and functionally distinct areas (Fuster, 2008): The orbitofrontal cortex (OFC) and ventromedial PFC (vmPFC) are junctures that integrate converging information from areas of emotion (amygdala), memory (hippocampus), and higher-order sensory processing and relay this information on to the dorsolateral and dorsomedial PFC (dlPFC, dmPFC) (Fuster, 2001; Wood & Grafman, 2003). Moreover, these areas (OFC, vmPFC) have a role in sensory integration, representing affective values of reinforcers, and decision making (Cardinal et al., 2002; Finger et al., 2011; Kringelbach, 2005). Dorsal and lateral areas (dlPFC, dmPFC) are involved in the execution of movement and planned behaviors (and executing long-term goals), as well as the integration of sensory information and working memory (Wood & Grafman, 2003). In terms of connections and the stream of information, the amygdala is poised to relay initial information from primary sensory and visceromotor inputs to medial and orbitofrontal regions (e.g., OFC), which in turn send information on to dmPFC and dlPFC. The OFC is thus integral for monitoring internal states and motivations and relaying that information on to more dorsal and lateral regions for behavioral action (Forbes & Grafman, 2010). In turn, prefrontal regions, including medial PFC and perigenual anterior cingulate cortex (ACC; see below), are critically involved in providing negative feedback to the amygdala through excitatory projections which regulate the amygdala’s impact on arousal.
While such distributed circuitry is critical for complex behavioral responses, it is also important to note that local circuitry within each of these regions plays an important role in shaping behavior, particularly within the amygdala (e.g., intra-amygdala circuitry including the basolateral complex, central nucleus and intercalated cell masses is critical for fear conditioning) (Cardinal et al., 2002; Davis, Johnstone, Mazzulla, Oler, & Whalen, 2010; LeDoux & Sciller, 2009; Whalen et al., 2001). A recent theory of psychopathy has emphasized the importance of differential responses within two major subregions of the amygdala (Moul, Killcross, & Dadds, 2012), a point not addressed by any current studies of youth AB, though shown to be important in studies of AB in adults (Carré, Fisher, Manuck, & Hariri, 2012; Gopal et al., 2013).
Another important structure in processing information about emotional states and shifting contingencies in the environment is the ACC (Devinsky, Morrell, & Vogt, 1995). The ACC has a role in error detection and correction (monitoring when outcomes differ from what was expected) (Botvinick, Cohen, & Carter, 2004), with dorsal regions implicated in cognitive processing (top-down and bottom up processing) and ventral regions implicated in assessing the salience of emotion and motivational information (Bush, Luu, & Posner, 2000). It is important to note that the ACC has dense connections with the amygdala and prefrontal areas noted above (particularly the OFC) (Mega, Cummings, Salloway, & Malloy, 1997), and many of these areas (e.g., portions of the ACC and PFC, the amygdala) are extremely rich in serotonergic projections (Varnäs, Halldin, & Hall, 2004). Finally, in regard to youth AB, it is important to consider the role of other brain areas such as the insula and ventral striatum. The insula has been implicated in integrating interoceptive states into conscious feelings, decision making, and empathy and pain recognition in others (Craig, 2009; Decety & Jackson, 2006; Naqvi & Bechara, 2009), and thus may be important in understanding empathy deficits seen in youth AB. The ventral striatum has been linked to reward and motivation (Berridge & Robinson, 2003; Kable & Glimcher, 2007) and is anatomically linked to structures such as the amygdala, hippocampus, and medial prefrontal cortex (Cardinal et al., 2002; Pierce & Kumaresan, 2006).
Not surprisingly, given the interconnections between these areas, many authors have grouped these brain regions together by function. For example, the amygdala, OFC, and insula have been proposed as critical for recognizing emotions in others (Adolphs, 2002). More broadly, the amygdala, ACC and OFC have been implicated in implicit social cognitive processes (Forbes & Grafman, 2010), and all of the areas reviewed in this section are likely critical to navigating a social world through their role in emotion, social cognition, and moral judgment (Forbes & Grafman, 2010), as well as reward processing. These roles are important for understanding youth AB, as these behaviors typically involve harming others and violating social norms, and pursuing behaviors with high risk and reward.
Overarching theories of neural function in AB and psychopathy
Psychopathy: Paralimbic dysfunction
In regards to adult psychopathy and violence more broadly, some authors (Davidson, Putnam, & Larson, 2000; Kiehl, 2006) have pointed out that the array of brain areas implicated in adult psychopathy and violence are not simply random areas but part of a distributed “paralimbic” network crucially involved in emotion processing and learning. These areas include the OFC, insula, anterior and posterior cingulate, amygdala, parahippocampal gyrus, temporal pole and anterior superior temporal gyrus. As Kiehl (2006) proposed, these areas have been grouped by neuroanatomists into a paralimbic cortex based on their cytoarchitectural similarities, and are all broadly implicated in emotion which is seen as both lacking and primary to psychopathy. However, as others have pointed out, it is not clear if all of these structures are dysfunctional or if, based on their interconnectivity, one or two dysfunctional areas may be causing disruption across the entire circuit (Glenn & Raine, 2008). Regardless, this line of thinking would suggest we would see dysfunction in these brain areas in AB+CU+ youth to the extent to which CU traits are an indicator of early psychopathy and these areas of dysfunction are specific to psychopathy and not broad AB.
Psychopathy: Emotion, AB, and Lying
Raine and colleagues have focused on specific brain areas associated with particular deficits seen in psychopathy by dividing core deficits into three major categories - emotion, AB, and lying - each with corresponding brain deficits (Glenn & Raine, 2008; Raine, 2002; Raine & Yang, 2006; Yang & Raine, 2008). Emotional deficits are seen to arise from dysfunction in the OFC, amygdala, hippocampus, ACC, and insula. AB is connected to problems with impulsivity, attention selection, and response inhibition, which may be the result of dysfunction in the ACC, OFC, dlPFC, and superior temporal gyrus. Pathological lying is seen as being connected to differences in the ACC, OFC, and vlPFC (Raine & Yang, 2006; Spence et al., 2004; Yang et al., 2007).
Reactive versus Instrumental Aggression in Children
Blair and colleagues have proposed similar models to those above, however Blair has specifically emphasized the OFC-amygdala connection in adult and child psychopathy and how a violence inhibition mechanism may go awry in psychopaths (Blair, 2003, 2004). This model implies that genetic variability disrupts neural systems (e.g., OFC, amygdala) that impair the ability to form stimulus-reinforcement associations early in life. Genetic influences alter these stimulus-reinforcement associations as a function of contingency change, disrupting the typical effects of socialization efforts (e.g., parenting) and leading to later forms of extreme aggression and psychopathy (Blair, Peschardt, et al., 2006a). This model, based on a wealth of behavioral work, predicts that youth with AB and CU traits should have specific deficits in identification and reactivity to fearful faces due to amygdala dysfunction (Blair, 1999; Blair et al., 2001), as well as deficits in stimulus-reinforcement learning and reinforcement expectancies (Blair et al., 2004; Blair, 2004) due to OFC (and amygdala) dysfunction (Blair, 2007a).
Beyond identifying specific structures involved in AB+CU+, Blair and colleagues also offer a model in which childhood aggression is divided into proactive and reactive aggression with putatively different origins (Crowe & Blair, 2008). Proactive aggression is viewed as the result of innate brain differences that result in an inability to learn resulting in both blunted emotion and deficient cost calculation. In contrast, reactive aggression is thought to be mediated by threat circuitry (including 5-HT and cortisol functioning) that may be disrupted through experience (e.g., child abuse) and/or biology (e.g., genetic differences in the amygdala or 5-HT signaling, poor PFC regulation of threat circuitry) (Blair, Peschardt, et al., 2006b; Crowe & Blair, 2008). This theory is worth noting in reviewing studies of youth AB because it proposes differential biological correlates for different subtypes of AB with testable hypotheses that can be born out through more studies of children using functional imaging.
Direct Evidence – Functional Neuroimaging in Youth
The Amygdala
The amygdala has emerged as a focus of research on youth with AB for several reasons. First, as noted above, the amygdala has been implicated in emotional learning, fear response, memory consolidation, and general arousal (LeDoux & Sciller, 2009). Deficits in each of these processes have been correlated with AB (Glenn & Raine, 2008). Second, neuroimaging studies of antisocial adults have implicated the amygdala in this disorder (Birbaumer et al., 2005; Kiehl et al., 2001). Third, children and adults with AB and related disorders such as psychopathy display various psychophysiological differences that are similar to patients with amygdala lesions (Blair, Peschardt, et al., 2006a) and several psychophysiological studies of adult and youth AB have implicated differences in amygdala functioning (van Goozen, Fairchild, Snoek, & Harold, 2007).
Thus, there has been a recent explosion of studies that have explored the link between amygdala functioning and youth AB using fMRI paradigms that generally contrast negative stimuli to neutral stimuli (see Table 1)1. These studies have helped inform our understanding of youth AB and also demonstrate the utter complexity and nuance of the literature. Therefore we spend much of the review considering this set of studies as a model for issues affecting the broad evaluation of neuroimaging studies of youth AB.
Table 1.
Functional neuroimaging studies of youth antisocial behavior
| Study | Sample (groups contrasted; recruitment; age; gender; comorbidity and exclusions) | Key Methods (fMRI task and stimuli; measures of AB) | Contrast | Key Findings; coordinates (x, y, z)1[LWH1] |
|---|---|---|---|---|
| Emotion focused designs | ||||
| White, Marsh, et al., 2013 | 15 DBD+PT vs. 17 controls; community; age 10 – 17; mostly males; excluded substance use, anxiety and mood disorders; 8 DBD youth also had ADHD when excluded findings weakened but were in same direction |
“Bars task” – fear and neutral faces flanked by bars. Participants rate if bars are parallel. Amount of deviation from parallel decreases attention load; K-SADS + PT assessed via total APSD scores. |
Low load > high load | Control > DBD+PT : L amygdala/lentiform nucleus (−20, −14, −3) (DBD group shows reverse: > response to fear under high versus low load) |
| Fear > neutral | Control > DBD+PT: L middle temporal gyrus (BA 21) (−53, −3, −13 & −58, −8, −11) CU traits correlated with amygdala response to fearful expressions under low attentional load Main effect of group (control > DBD+PT: R middle frontal gyrus, bilateral superior frontal gyrus, L inferior frontal cortex, R posterior cingulate cortex, L inferior temporal cortex, L declive, L lingual gyrus[LWH2] |
|||
| Viding et al., 2012 | 15 CP+CU+ vs. 15 CP+CU− vs. 16 controls; community; age 10 – 16; males; controlling for ADHD, anxiety, and depression did not alter findings | Backward masked pictures of facial affect. Calm or fear were presented preattentively followed by calm faces; CASI-4R + CU traits assessed via the ICU (median split). |
Masked fear > masked calm | CP+CU− > control > CP+CU+ : R amygdala (−20, −3, −18); CP+CU− > control, CP+CU+ significant post-hoc; control and CP+CU+ not significant post-hoc; CU correlated negatively with 1 voxel in R amygdala (−24, −23, −15). |
| White, Williams, et al., 2012 | 17 DBD+ PT vs. 19 controls; community; age 10 – 17 ; mostly males; no substance use, anxiety or mood disorders; 9 DBD youth also had ADHD; when excluded some findings remained significant |
Eye gaze task with probes towards or away from the eyes using neutral, angry, and fearful faces; K-SADS+PT assessed via total APSD scores. |
Fear > Neutral during incongruent trials > congruent | Controls > DBD+PT : bilateral superior parietal lobule (BA 7) (23, −58, 44; −20, −60, 44), bilateral inferior parietal lobule (BA 40) (29, 41, 44; −44, −35, 40), R posterior cingulate cortex (BA 31) (17, −32, 40), L cuneus (BA 18) (−5, −80, 21). |
| Neutral > anger | DBD+PT > Controls: L superior frontal cortex (BA 9) (−29, 50, 28); R middle frontal cortex (BA6) (32, −5, 52). | |||
| Incongruent > congruent trials | Controls > DBD+PT: R middle temporal cortex (38, −11, −5); R thalamus (14, −16, 4). | |||
| Kalnin et al., 2011 | 22 CD or ODD vs. 22 controls (50% media violence exposed); community and clinic fliers; age 13 – 17; males and females; comorbidity not reported | Emotional stroop task; K-SADS |
“emotional” (violent) words > non-emotional words | AB youth who were also high on media exposure to violence have less activation in the R rostral ACC (13, 37, −8), R amygdala (22, 0, −21), and R posterior superior frontal gyrus (18, −1, 48). |
| Sebastian et al., 2012 | 31 CP+ vs. 16 controls; community; age 10 – 16; males; controlling for ADHD, GAD, MDD, and alcohol use disorder symptoms weakened but did not change the direction of the findings; overlapping subjects with Viding et al., 2012 | Cartoons asking participants to use cognitive or affective ToM or physical causality; CASI-4R + CU traits assessed via the ICU. |
Affective ToM > Cognitive ToM | Controls > CP: R amygdala (driven by greater response to affective – cognitive ToM in control group; no differences in CP group) (24, −12, −8) & R anterior insula (32, 16, 8) Supressor effects in R amygdala showing divergent relations with CU traits (− partial correlation) and CPs (+ partial correlation) |
| Passamonti et al., 2010 | 27 EO CD vs. 25 AO CD vs. 23 healthy controls; schools, clinics, youth offending services vs. schools and colleges; age 16 – 21; males; ADHD and MDD past and present in some but not all participants; controlling for ADHD did not alter findings; Both CD groups higher on CU traits than controls |
Gender categorization using pictures of facial affect with additional fixation cross trials a baseline measure; K-SADS + CU traits assessed via the YPI; No correlations found between CU traits and any fMRI activations. |
Anger > neutral | Both CD groups < control: L & R amygdala (−24, −4, −18; 26, −4, −22), vmPFC (10, 30, −17), L insula (−26, 19, −6), L & R OFC (46, 42, −9; 42, 44, −12). |
| Sad > neutral | Both CD groups < control: L & R amygdala (−20, −6, −13; 22, −6, −11), vmPFC (0, 26, −20). EO-CD < AO-CD: L & R amygdala (−20, −7, −16; 18, −6, −11), R anterior superior temporal sulcus (53, −11, −16). | |||
| Anger > fixation | Results in L & R amygdala & L insula driven by increased response to neutral faces in CD group, other areas driven by differences in neutral and anger faces | |||
| Sad > fixation | Results driven by differences in both neutral and anger faces | |||
| Marsh et al., 2008 | 12 CU + CD or ODD vs. 12 ADHD vs.12 control; community; age 10 – 17; males and females; excluded mood or anxiety disorders. | Gender categorization using pictures of facial affect; K-SADS + CU traits via > 20 score on the APSD and PCL− YV. |
Fearful > neutral | C/U < ADHD, C: R Amygdala (20, −7, −26); Less functional connectivity in CU group between amygdala and vmPFC (12, 35, −25). |
| Angry > neutral | Only the ADHD group differed from controls and the CU group | |||
| Jones et al., 2008 | 17 CU+ CP vs. 13 age and IQ matched; part or larger community study of twins; age 10 – 12; males; findings consistent when controlling for hyperactivity symptoms. | Gender categorization using pictures of facial affect; Combination of SDQ and APSD scores. |
Fearful > neutral | Control > CU+/CP+: R amygdala (30, 2, −25) |
| Herpertz et al., 2008 | 22 early onset CD vs. controls (with follow up comparison 13 “pure” ADHD to controls); clinic vs. community; age 12 – 17; males; all of CD group was diagnosed with ADHD. | Passive viewing IAPS pictures (negative, positive, & neutral); K-SADS. |
Negative > neutral | CD > control - L Amygdala (−30, −7, −25); CD group found IAPS pictures less arousing Present when controlling for IQ, anxiety/depression. Measures of internalizing symptoms correlate with amygdala reactivity in the CD group. |
|
Sterzer et al., 2005 Also see: Stadler et al., 2007 |
13 CD vs. 14 age matched controls; inpatients admitted for abnormal aggressive behavior vs. community; age 9 – 15; males; 62% comorbid for ADHD and high levels of anxiety/depression noted; some CD group on mediction. | Passive viewing IAPS pictures (negative & neutral); DSPDCA. |
Negative > neutral | Control > CD – R Dorsal ACC (9, 36, 33); ACC activation correlated with aggressive behavior on CBCL; CD group found IAPS pictures less arousing; Control > CD when controlling for anxiety/depression symptoms – L Amygdala (−21, −12, −18); |
| Studies focused on attention and inhibition | ||||
| Rubia, Halari, et al., 2009 | 13 pure early onset CD (+ODD) vs. 20 pure ADHD vs. 20 control (all medication naïve); clinic, advertisements; parent support groups; age 9 – 16; males; all comorbid for ODD; Same sample as Rubia et al., 2008. | Simon task (CD group made most overall errors, ADHD group was most variable); Maudsley diagnostic interview + SDQ. |
Incongruent vs. oddball trails (interference inhibition) | C > ADHD, CD – R superior/middle temporal lobe (BA 21, 42, 22) (54, −7, −13), R Precuneus (BA 7/19/31) (14, −66, 36). |
| Oddball vs. congruent trials (attention) | C, CD > ADHD – L inferior/middle PFC (BA 45/47/46/10) (−32, 52, −2); C > ADHD, CD – R DLPFC (BA 8/9/6) (28, 33, 48). | |||
| Rubia, Smith, et al., 2009 | 14 pure early onset CD (+ODD) vs. 18 pure ADHD vs. 16 control (all medication naïve); clinic, advertisements; parent support groups; age 9 – 16; males; all comorbid for ODD; Same sample as Rubia et al., 2008. | Rewarded continuous performance task; Maudsley diagnostic interview + SDQ. |
Non-rewarded target trials versus non-target trials (Sustained attention) | C, CD > ADHD – L & R vlPFC (BA47/45/44/10 & BA 47, 45, 11) (−47, 44, −13; 43, 41, −13); C, ADHD > CD - R insula (& premotor cortex; BA6) (36, −15, −13; 26, −15, −13), R hippocampus (32, 19, −7), L postgenual/dorsal anterior cingulate (BA[LWH3] 32) (−4, 19, 42). |
| Rewarded versus nonrewarded target trials (effect of reward) | C, ADHD> CD - R lateral and medial OFC (BA 47) (32, 37, −2); C, CD > ADHD - L precuneueus and posterior cingulate gyrus (BA 29) (−4, −52, 15). | |||
| Rubia, et al, 2008 | 13 pure early onset CD (+ODD) vs. 20 pure ADHD vs. 20 control (all medication naïve); clinic, advertisements; parent support groups; age 9 – 16; males; all comorbid for ODD; | Visual tracking stop task; Maudsley diagnostic interview + SDQ. |
Successful trial versus failed stop | C, CD > ADHD – L middle/inferior frontal gyrus (BA 46/10) (−25, 59, 15). |
| Failed trial versus go | C > CD, ADHD – R posterior cingulate/precuneus (BA 29/31/7) (11, −48, 26); C, ADHD > CD – L inferior parietal (BA 40) (−40, −33, 37), R posterior/superior temporal/inferior parietal lobe (BA 3/4/42/40) (58, −19, 20). | |||
| Studies focused on learning, reward and other | ||||
|
Sharp et al., 2011 See also White et al., 2013 |
10 externalizing vs. 10 controls; community (recruited from the boy scouts); age 11 – 16; males; no information on comorbidity. | “Trust game” involving interaction with a virtual peer in exchange of money; Combined parent, peer, and self-report of externalizing on the CBCL. |
Share decisions > keep decisions | Externalizing > non-externalizing in bilateral insula (44, −8, 4; −29, 25, 9 & −39, 17, 4). |
| Outcome phase > fixation x neutral > kind/mean peer | Non-externalizing > externalizing in bilateral caudate (7, 4, 12); Non-externalizing showed greater response to neutral (vs. kind/mean) peer; externalizing group shows no moderation in bilateral insula (54, 13, −12; −51, 19, −11). | |||
| Finger et al., 2011 | 15 DBD vs. 15 controls; community fliers & referrals; mean age 13–14; males and females; 67% of CU/CD group comorbid for ADHD. | Passive avoidance task; K-SADS + CU traits assessed via APSD & PCL-YV. |
Early > late trials | CU/CD group have less activation in “network of attention” including R OFC (23, 36, −13) and L caudate (−2, 16, 13) among others. |
| Rewarded correct hits > punished commission errors | CU/CD < controls: R OFC (5, 64, −8), L middle frontal gyrus (−44, 12, 49) and parahippocampal gyrus (35, −41, −10); Amygdala (20, −10, −26), insula (−41, 14, 0), fusiform gyrus (32, −81, −20) and caudate (−8, 7, 16)(& others) responsiveness lower throughout entire task for those in the CU/CD group. | |||
| Crowley et al., 2010 | 20 abstinent ASD vs. 20 controls; boys in current substance treatment vs. controls from community; age 14 – 18; males; All subjects diagnosed with CD and substance abuse or dependence. | Colorado Balloon Game (a decision and reward task); DISC, CBCL, CIDI-SAM. |
Decision making > direction following | ASD < control for many areas including OFC, DLPFC, ACC, basal ganglia, insula, amygdala, & hippocampus (coordinates not provided as many regions were overlapping and part of same large cluster). |
| Wins > Loss | ASD < control in ACC, temporal regions, & cerebellum. | |||
| Loss > Win | ASD > control in OFC, DLPFC, brain stem & cerebellum. | |||
| Decety et al., 2009 | 8 childhood onset CD vs. 8 age, sex, & race matched control; part of larger community study; age 16 – 18; males and females; 88% of CD group comorbid for ADHD, | Animations of Painful situations: cause by accident, on purpose, help, non-painful; DISC + CADS. |
Pain by accident > non-painful Pain on purpose vs. accident |
CD group differential activation of “pain matrix” with greater activation in: L Amygdala (−18, −8, −8), Bilateral temporal pole (62, −2, −5; −50, −4, 0), ACC (2, 10, 23), R Caudate (12, 18, 3). CD > C- L anterior insula (−26, −23, −2), R SMA (12, −7, 56), L Precentral gyrus (−30, 18, 49); C > CD - L DLPFC (−42, 42, 18), R superior frontal gyrus (18, 64, 0). CD youth have less functional connectivity between amygdala and prefrontal areas while watching pain inflicted on others. Amygdala activity correlated with number of aggressive acts and ratings of daring. |
| Gatzke-Kopp et al., 2009 | 19 CD + ADHD boys vs. 11 controls; community fliers; age 12 – 16; males; almost all boys had ADHD and most had CD in “EXT” group. | Reward task (rewarded for reporting which side a light was on); DISC. |
Rewarded vs. non-rewarded blocks | CD/ADHD group continued to activate the caudate during non-reward blocks while controls shifted to bilateral dorsal ACC (14, 36, 24) activation; (CD/ADHD < Controls ACC during non-reward). |
| Finger et al., 2008 | 14 CU +CD ODD, 14 ADHD, 14 control; community fliers & referrals; age 10 – 17; males; excluded mood and anxiety disorders. | Probabilistic learning reversal task; K-SADS + CU traits assessed via the APSD. |
Punished reversal errors vs. all correct responses | C/U > C, ADHD – Bilateral medial frontal gyrus (BA 10) (−17, 47, 5; 23, 46, 9); C/U > C – caudate (20, −23, 26). |
All Coordinates are from the system of Talairach and Tournoux (Talairach & Tournoux, 1988). All coordinates that were reported in other coordinate systems (e.g., MNI) were converted to Talairach using the Wake Forest PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas). Note that anatomical labels provided in this table are the ones used by the original authors regardless of the labels assigned by the Talairach daemon.
Abbreviations: ACC – Anterior Cingulate Cortex, AO-CD – Adolescent-Onset Conduct Disorder, APSD – Antisocial Process Screening Device, ASD – Antisocial Substance Disorder, BA – Brodmann’s Area; CASI-4R - Child and Adolescent Symptom Inventory – 4 R, CBCL – Child Behavior Checklist, CD – Conduct Disorder, CP – Conduct Problems, CIDI-SAM – Compsite International Diagnostic Interview – Substance Abuse Module, CU – Callous/Unemotional, DBD – Disruptive Behavior Disorder (CD and/pr ODD), DISC – Diagnostic Interview Schedule for Children, DPSPDCA – Diagnostic System for Psychiatric Disorders in Childhood and Adolescence, EO-CD – Early Onset Conduct Disorder, ICU – Inventory of Callous-Unemotional Traits; KSADS-PL – Schedule for Affective Disorders and Schizophrenia for School aged children; MNI – Coordinates based on the system of the Montreal Neurological Institute, PCL-YV – Psychopathy Checklist: Youth Version, PFC – Prefrontal Cortex, PT – Psychopathic Traits; SDQ – Strengths and Differences Questionnaire, SMA – Supplementary Motor Area; ToM – Theory of Mind; YPI – youth Psychopathic Traits Inventory.
Early Studies
For the most part researchers have proceeded with the general hypothesis that children with AB (particularly those with CU traits) will show less amygdala reactivity than controls to negative as compared with neutral stimuli, consistent with a deficiency in general and threat-related arousal. Two early studies painted a mixed picture using pictures from the International Affective Picture System (IAPS). First, Sterzer and colleagues (2005) initially found no differences in amygdala functioning when comparing a group of CD adolescent boys with controls (age 9–15), but did find lesser right dorsal ACC activity to a task contrasting negative to neutral pictures (Lang, Greenwald, Bradley, & Hamm, 2007). However, when the authors controlled for the high degree of anxiety/depression symptoms in the sample, they found that the CD group displayed less left amygdala reactivity to the negative/neutral contrast than the control group (Sterzer et al., 2005). In a follow-up study, differences in dorsal ACC activity were also found to be attributed to differences in novelty seeking (Stadler et al., 2007), consistent with literature implicating dorsal ACC deficits and poor error processing in impulsivity and novelty seeking (Fineberg et al., 2009). In a second related study, boys (age 12–17) with CD displayed greater left amygdala reactivity than controls to a paradigm contrasting negative and neutral IAPS images (Herpertz et al., 2008).
More recent studies
After these studies using IAPS pictures, several similar studies emerged focusing on response to facial affect. In two studies with very similar methods (Jones et al., 2009; Marsh et al., 2008), boys (ages 10–12 and 10–17 respectively) high on both AB and CU traits were found to have less right amygdala reactivity than controls in a task contrasting fearful to neutral/calm faces. Most recently, a study of older adolescents (age 16–21) within a larger sample (n = 75) of both early and late starting AB, found less bilateral amygdala reactivity (and decreased activity across many other related areas including the OFC, vmPFC, and insula among others) in tasks contrasting angry faces to neutral faces and sad faces to neutral faces (Passamonti et al., 2010) but found that CU traits were not correlated to amygdala response.
Beyond IAPS pictures and affective faces, studies have been using other paradigms to explore the neural correlates of emotional processing in youth AB. For example, in a study examining the role of media violence exposure (measured by self-report of TV and video game violence seen over the past year) (Kronenberger et al., 2005), a group of youth with AB (age 13 – 17) were compared to control youth while undergoing an emotional Stroop task (Kalnin et al., 2011). Youth high on AB who had also been exposed to high levels of media violence, demonstrated less reactivity within the right amygdala, rostral ACC and posterior superior frontal gyrus.
Using a very different type of stimulus, the role of empathy (Decety, 2010) was probed in a group of adolescents with early starting CD who were compared to healthy controls (Decety, Michalska, Akitsuki, & Lahey, 2009). Participants watched animations of other people experiencing pain caused by accident or on purpose and people not experiencing pain. Whereas both groups displayed increases in activity in brain areas associated with pain (Jackson, Rainville, & Decety, 2006), the AB group showed even greater activation when contrasting accidental pain to no pain animations in limbic and frontal regions (amygdala, temporal pole, striatum). When contrasting pain caused on purpose versus pain caused by accident, the CD group showed greater activation in some areas (e.g., insula) and lesser activation in frontal areas (dlPFC and right superior frontal gyrus). Additionally, connectivity analyses implicated decreased coupling between the left amygdala and PFC areas, and aggressive CD symptoms and dimensions of daring and sadism were positively correlated with activity in the amygdala.
Studies examining moderators of outcome
At this point, the empirical literature was quite murky. Across a variety of tasks and groups of youth, studies had either demonstrated decreased (Jones et al., 2009; Kalnin et al., 2011; Marsh et al., 2008; Passamonti et al., 2010; Sterzer et al., 2005) or increased (Decety et al., 2009; Herpertz et al., 2008) amygdala reactivity to a variety of tasks involving negative emotion (with a particular emphasis on affective faces). Thus, the most recent studies in this area have examined possible moderating factors to explain conflicting findings. These studies have proceeded on the hypotheses that the presence or absence of CU traits and aspects of attention to the task may all affect the direction of results.
Heterogeneity and subgroups
In attempting to understand possible divergent effects between CU+ (e.g., Jones et al., 2009; Marsh et al., 2008) and CU− AB youth (e.g., Decety et al., 2009; Herpertz et al., 2008), Viding and colleagues (2012) demonstrated that adolescent boys (age 10–16) high on conduct problems but low on CU traits (AB+CU−) had a greater response to pre-attentively presented fear faces (relative to calm faces) than healthy controls or youth high on conduct problems and callous traits (AB+CU+ who had the lowest amygdala reactivity) and callousness scores were negatively correlated with amygdala response. These results suggest that AB youth without CU traits may show an opposite pattern of amygdala reactivity than those with CU traits (see also Posner et al., 2011). Moreover, the study suggests that AB youth low on CU may be hypersensitive to emotional faces, even when presented below awareness (Viding, Fontaine, & McCrory, 2012).
In a study addressing that AB youth (particularly those with CU) demonstrate deficits in affective but not cognitive theory of mind tasks (Jones, Happé, Gilbert, Burnett, & Viding, 2010), boys (age 10 – 16) high on AB were found to have less reactivity in the amygdala and anterior insula to animations eliciting affective versus cognitive theory of mind (Sebastian et al., 2012). Within the AB group, the authors found statistical suppression effects, whereby AB was positively correlated, and callousness was negatively correlated, with amygdala reactivity but only when controlling for the overlap of these two variables (i.e., AB and CU traits) (see also Feilhauer, Cima, Korebrits, & Kunert, 2011). Though this study and the one described before it (Viding, Sebastian, et al., 2012) come from a similar sample and suggest divergent relationships between AB+CU−, AB+CU+ and amygdala reactivity, they support similar but distinct models of amygdala reactivity in youth AB: one in which CU is a moderator (AB+CU+ youth are low on amygdala reactivity; AB+CU− youth are high on amygdala reactivity) and one in which the divergent relationship between AB and CU traits with amygdala reactivity is statistically suppressed (only when dimensional overlapping variance is accounted for do these variables predict the outcome) (MacKinnon, Krull, & Lockwood, 2000; Paulhus, Robins, Trzesniewski, & Tracy, 2004). Regardless, both suggest that the prevalence of CU traits within a sample may have profound impacts on the effects observed and that those with CU traits versus those without may have distinct and divergent neurobiological profiles.
Task effects
Given the evidence already presented that hypoactivity in the amygdala may be specific to AB+CU+ youth, two recent studies from White, Blair and colleagues has explored whether aspects of the neuroimaging task may affect amygdala reactivity in AB+CU+ youth. In the first study, the authors aimed to test the effect of attentional load on amygdala reactivity to fearful versus neutral faces. Research and theory in the field has debated whether emotion deficits (and amygdala hyporeactivity) are primary deficits that lead to many of the symptoms of psychopathy (Blair, 2003, 2007a) or whether these emotion deficits may be secondary to aberrant attentional control (e.g., paying attention to irrelevant information that distracts from important emotion information) (Newman & Baskin-Sommers, 2011). Using a task that asks subjects to determine if lines flanking emotional faces were parallel or not, with increasingly ambiguous lines leading to greater attentional load, the authors found that, in a group of adolescents (age 10 – 17), under low but not high attentional load youth with AB and “psychopathic traits” (measured by total scores on the Antisocial Process Screening Device (APSD) a commonly used parent and child report measure containing factors measuring CU traits, narcissism, and impulsivity) showed lower amygdala reactivity than controls and this response in the AB group was correlated with CU traits (White, Marsh, et al., 2012). This study emphasizes that the attentional demands of the task may affect the results seen and suggests that results in many of the initial studies in this area may have demonstrated differences in AB+CU+ youth (lower amygdala reactivity) because attentional demands were quite low.
Consistent with an emphasis on attention and work underscoring abnormal attention to the eyes (attention that is crucial for emotion recognition) in youth AB, particularly those with CU (Dadds, Allen, et al., 2012; Dadds, El Masry, Wimalaweera, & Guastella, 2008; Dadds, Jambrak, Pasalich, Hawes, & Brennan, 2011; Dadds et al., 2006), a recent study examined the effect of cued eye gaze task (a probe appeared on a congruent or incongruent side as eye gaze) using fear, angry, and neutral faces in a sample of adolescents (age 10 – 17 years) with or without AB and “psychopathic traits” (White, Williams, et al., 2012). Using this task, the authors found reduced recruitment/activation of what they labeled an “endogenous attention orienting network” (superior parietal lobule, inferior parietal sulcus) in the AB youth, but they did not observe the hypothesized hypoactivity in amygdala reactivity to fear (or angry) versus neutral faces in AB youth. This outcome suggests that aspects of an emotional faces task can modulate whether deficits in amygdala reactivity are seen in AB+CU+ youth.
Issues in Interpretation
Although the results from these studies appear to address a relatively straightforward question of the role of amygdala reactivity in youth AB, they demonstrate the complexity of the question. Moreover, beyond their value for addressing this research question, the comparison between these studies illustrates many of the issues present in studying the neural correlates of youth AB. Given the complexity inherent in comparing these studies, we consider a few points before drawing conclusions.
Who is being studied?
A primary issue in the study of youth AB is the heterogeneity of this group. An advantage of several studies (Jones et al., 2009; Marsh et al., 2008; White, Marsh, et al., 2012; White, Williams, et al., 2012) was that youth were selected to be both high on AB and CU traits, theoretically leading to a more homogeneous group being studied. While this sub-typing is very helpful in interpretation, when used in small extreme-group comparisons it leads to two main disadvantages: the inability to partition the contribution of AB versus CU traits and the inability to test whether these differences exist only at the extreme versus dimensionally throughout the population. CU traits were assessed because of similar findings in adult psychopaths; however, in these studies there is no way to know whether amygdala hyporeactivity is related directly to the presence of CU or AB. For example one study now suggests that CU versus non-CU AB youth may have entirely divergent patterns of amygdala reactivity (Viding, Fontaine, et al., 2012) and that amygdala hyporeactivity is specific to CU traits rather than AB more broadly; whereas another study suggests that amygdala hyporeactivity is not specific to those high on CU traits but is associated more specifically with the severity of AB (Passamonti et al., 2010). Studies that are larger and have dimensional measures of AB and CU could disentangle the relative contribution of AB versus CU traits in driving amygdala reactivity.
As several studies did not measure CU traits (Decety et al., 2009; Herpertz et al., 2008; Kalnin et al., 2011), it is difficult to know how to compare their findings to those that did measure CU traits, especially given that 2 of the 4 studies demonstrated increased, while the other 2 studies demonstrated decreased, amygdala reactivity to a variety of tasks in youth with AB. Though both the Passamonti and Herpertz studies measured age of onset in subtyping youth AB, the two studies found opposite results (i.e., lesser versus greater amygdala reactivity in those with AB respectively). Moreover, all studies had small samples sizes (i.e., Ns ranges from 13 to 52 in the AB group), causing worry about the ability to replicate modest effects.
Interestingly, the study by Passamonti and colleagues (2010) addresses some of the limitations in this literature: the sample size was larger (approximately 25 in each group), the study examined early-onset versus adolescent-onset AB, and CU traits were measured continuously. In this study, CU traits were not correlated with any dimensions of brain activity, suggesting that CU may not be driving individual differences in neural function. However, it should be noted that both AB groups were higher on CU traits and thus could still be considered AB+CU+. A great advantage of this study was that the authors examined differences between early and later onset AB, finding few differences in neural reactivity. Both groups demonstrated similar under-reactivity to the tasks in many brain areas but the early-onset group did show even greater reduced response in the amygdala to the sad versus neutral contrast. This study demonstrates how two subgroups of AB youth (early versus late onset) with different sets of risk factors show similar but not identical patterns of neural reactivity (i.e., equifinality).
A final critical point is sample selection. Ultimately a sample is used to generalize to a population, but it is unclear to what extent the populations represented by each study overlap. For example, the following four studies each differ in the type of participants and/or the methods used to identify participants: the Jones et al. (2009) study is a community sample of twins selected from a larger sample by scores on behavior scales; the Marsh et al. (2008) study is a community sample but it is unclear how the subjects were recruited to find youth high on CU traits; the Herpertz et al. (2008) study contrasts clinic versus community recruited youth; and the Sterzer et al. (2005) study contrasts inpatient youth versus community youth (see Table 1). Clearly these studies are not contrasting groups that are equivalent and thus any differences in findings may be due to any number of third variables associated with differences in the samples being compared (e.g., level of depressive symptoms, education, IQ, environmental adversity) or even simple differences in AB symptom severity. These differences in sample characteristics also affect how findings may be generalizable and compared to existing behavioral studies.
This point is underscored when examining the Passamonti et al. (2010) study. A clear goal is made within this study to evaluate the early versus late starting models of youth AB to determine if these groups differ in respect to neural reactivity. The antisocial adolescents (and young adults – up to age 21) were recruited from schools, pupil referral units, and youth offending services, with the healthy control group recruited from schools and colleges. In and of itself, this recruitment strategy calls into question what population this sample represents, how appropriate it is for an evaluation of a theory based on representative population studies (Moffitt, 1993a), and in how many ways the healthy controls may differ from the AB youth based on differences in recruitment methods. Moreover, an examination of the average IQ of the early-onset AB group (i.e., mean IQ = 101.6) makes clear that these youth are not of the same population studied in epidemiological samples where IQ has often been shown to be below average (Moffitt et al., 1996). Thus, it is important to consider sampling approaches when evaluating any study of youth AB, but particularly within these small neuroimaging studies. As fMRI becomes less expensive, representative, or at least high risk samples, may be included more often, allowing for better inference between sample and population and the ability to exclude or control for other important third variables.
Comorbidity
A similar issue to sample selection is dealing with co-occurring psychopathology (Banaschewski et al., 2005). For example, the Marsh study directly addressed this issue by including a CU+/AB+ group who were comorbid for ADHD in addition to an ADHD-only comparison group. This comparison was important, as ADHD has been associated with differences in neural functioning in fronto-striatal structures (Bush, Valera, & Seidman, 2005; Durston, 2003; Rubia, 2011) and there is a high level of comorbidity between ADHD and CD (Banaschewski et al., 2005). The Sterzer and Passamonti studies addressed comorbidity in a different way by statistically controlling for depression/anxiety and ADHD symptoms (and the Blair and White studies generally exclude all those with internalizing disorders). Interestingly, in the Sterzer study by controlling for possible confounding internalizing symptoms, new findings emerged (less amygdala reactivity only when controlling for internalizing symptoms) that may be the result of statistical suppression (also seen in the Sebastian et al., 2012 study). In the case of depression and/or anxiety, the overlap with AB may affect fMRI results given that some studies of child and adult anxiety and depression have found greater amygdala reactivity to similar emotional faces paradigms (Monk, Klein, et al., 2008; Monk, Telzer, et al., 2008), as have studies of ADHD+AB+ youth (Posner et al., 2011). While AB itself could be linked to lesser amygdala reactivity, an adolescent with comorbid depression or ADHD could have a different pattern of neural reactivity and there may also be subgroups of youth who demonstrate AB due to underlying or comorbid depression (which may have shared or separate neural correlates) (Cole & Carpentieri, 1990; Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Monk, Klein, et al., 2008). This problem illustrates the broader issue in the study of youth AB of defining who to study: If a study excludes those with comorbid conditions, they may exclude much of the sample of AB youth, thus lessening the study’s generalizability. However if comorbidity is not addressed, it remains unclear whether findings can really be linked specifically to AB. Approaches such as those used in the Marsh study can be particularly helpful: defining a more homogenous subgroup at risk for worse outcomes (CU+/AB+) while also identifying contrast groups that may address issues of comorbidity (i.e., the ADHD group). Eventually neuroimaging itself may help address this problem if different subtypes or different diagnostic groups are found to have different brain correlates.
Task stimuli
As illustrated by some of the most recent studies (White, Marsh, et al., 2012; White, Williams, et al., 2012), even minor changes in similar tasks can have dramatic effects on the outcome. Thus it is difficult to assess how these studies with various tasks fit together. Given that amygdala differences have been seen across a variety of tasks, are differences in amygdala reactivity in AB youth simply a function of arousal and are any tasks that elicit arousal comparably useful and analogous? Or, is there something unique about faces (particularly fear faces) (Marsh & Blair, 2008) or highly negative pictures that may bear on our understanding of AB? For example, emotional faces, which are ubiquitous in our lives and are conditioned to various emotions, may drive amygdala reactivity because of their ecological conditioning to threat and emotion, whereas IAPS pictures and an emotional stroop as relatively novel stimuli may drive amygdala through novelty and more general arousal to gross or disturbing images (see Hariri & Whalen, 2011 for a more lengthy explanation of how differnt types of stimuli may drive amygdala reactivity in different ways).
The Passamonti and White studies further demonstrate the complexity of interpreting even a relatively “simple” task. In past studies in which emotional faces have been employed as stimuli within a very simple task (often asking for the gender of the face) (Jones et al., 2009; Marsh et al., 2008), authors have assumed that the affective faces (rather than the neutral faces) were driving differences in neural response. However, in the Passamonti et al. (2010) study the authors found that for angry versus neutral faces, the difference in the AB group was driven by greater response to neutral faces (versus baseline) rather than lesser response to angry faces (when contrasted to neutral faces) (see also Bobes et al., 2012). Response to sad versus neutral faces was driven by differential activation (relative to baseline) to both types of faces. These results emphasize that even neutral faces may be differentially processed in youth high on AB possibly because neutral faces are relatively novel (we see them less often than emotional faces), have been shown to be perceived as non-neutral (representing emotion) in youth with psychopathology and AB (Dadds et al., 2006; Rich et al., 2006), and have been shown to produce different levels of amygdala reactivity at different ages across childhood and adolescence (Somerville, Fani, & McClure-Tone, 2011). Though the effect of neutral faces is at odds with theory in the field (Blair, 2007a; van Honk & Schutter, 2007), it is consistent with a recent study finding greater amygdala reactivity to neutral faces in a group of chronically violent adult men (Pardini & Phillips, 2010). However, it is also difficult to interpret the different group responses to neutral faces in the Passamonti study because they were compared to a relatively non-informative baseline (a fixation cross) that differed in a number of ways from the faces. Moreover, the effect of the neutral faces was only present when presented in the same block as angry but not sad faces. Thus, future studies need to examine the effect of all faces and not assume that neutral faces are a comparable “baseline” condition.
Beyond the effects of neutral faces, both studies from White and colleagues emphasize the effects of attention on neural response: in one study high attentional load made the AB+CU+ youth amygdala hypoactivation disappear (White, Marsh, et al., 2012) and in another study the AB+CU+ group did not demonstrate any differences in amygdala reactivity compared to healthy controls (White, Williams, et al., 2012) possibly due to the high attentional demands of the task. Thus, even minor differences in task may lead to divergent effects, especially differences that engage attention away from the face.
More broadly than task effects, it is important to consider Dadds and colleagues’ (2008) work in regards to attention to facial emotion processing in youth AB and its implication for neuroimaging (Dadds, Allen, et al., 2012). Extensive work has demonstrated that youth with AB and CU traits have deficits in processing and identifying facial emotions (particularly fear faces) (Blair et al., 2001; Marsh & Blair, 2008) generally hypothesized to emerge from amygdala dysfunction in relation to reactions to these faces (e.g., Blair, 2003; Dadds & Rhodes, 2008). However, several studies (Dadds, Allen, et al., 2012; Dadds et al., 2008; Dadds et al., 2011; Dadds et al., 2006) have shown that differences in eye gaze (e.g., youth high on AB and CU look much less at the eyes when viewing faces) underlie this emotion recognition deficit. As attention to the eyes is critical to emotion recognition and amygdala response to emotional faces (Adolphs et al., 2005; Morris, DeBonis, & Dolan, 2002) and ultimately to developing empathy for others (Dadds, Allen, et al., 2012), it could be that youth high on AB and CU show decreased amygdala reactivity to emotional face paradigms in fMRI studies because they look at the eyes less, rather than because their amygdala are “dysfunctional” in responding less to emotional faces. In this case, how task stimuli are processed visually (i.e., the eyes being the most stimulating portion of emotional faces) may underlie neuroimaging differences in this group (and behavioral differences in identifying fear in others), rather than a more broad deficit in the amygdala responding to emotional stimuli (though eye gaze differences themselves may emerge from amygdala deficits: Adolphs et al., 2005). In fact, in a recent fMRI study of community adults high and low on callous traits participants completed an emotion recognition task in which the eyes of the faces shown were occluded or isolated (decreasing or increasing the salience of the eyes). Individuals with higher callous traits showed less neural reactivity in key brain areas (e.g., the amygdala, medial PFC) when the eyes were occluded but not when they were highlighted (Han, Alders, Greening, Neufeld, & Mitchell, 2011). This study implies that when the eyes were given more salience and subjects attended to them more, the neural reactivity differences between CU and non-CU participants disappeared. This study also suggests that results from the recent study by White and colleagues (2012) in youth AB may be attributable, not only to attentional load, but also to increasing or decreasing youth’s attention to the eyes (e.g., amygdala reactivity differences only emerge in AB+CU+ youth when it is easier for all participants to focus on the eyes). Thus, how faces are viewed in many of the reviewed studies may not be equivalent across CU and non-CU groups and differences seen in neural reactivity may be the result of different patterns of viewing rather than fundamental differences in reactivity to emotional faces. Studies asking youth to look directly at the eyes or using eye tracking while they complete neuroimaging tasks could help to further address how eye gaze is affecting amygdala reactivity to emotion.
Age
As emphasized above, developmental stage and age are critically important in interpreting these studies. For example, with the exception of the Jones study, all studies have an age range of at least 5 years and together span from age 9 to 21. Those youth engaging in these activities at younger ages may represent a very different group than those who have initiated this behavior later (Moffitt et al., 2002; though see Passamonti et al., 2010). Moreover, amygdala reactivity varies by age (Hare et al., 2008; Somerville et al., 2011), and the brain, especially prefrontal areas that receive and provide substantial feedback to the amygdala, shows marked structural changes throughout adolescence and thus the brain of a 10 year old is likely to be quite different than that of an 18 year old (Giedd, 2008; Giedd et al., 1996; Shaw et al., 2008; Toga, Thompson, & Sowell, 2006). Future studies that link amygdala functioning to AB longitudinally may uncover a more complex developmental course and studies may benefit from examining age as a moderator.
Conclusions: The Amygdala
Given the above caveats and though there is much work to be done to understand even the first implication of functional amygdala differences in youth AB, some initial conclusions can be drawn. As a whole, six studies (Jones et al., 2009; Kalnin et al., 2011; Marsh et al., 2008; Passamonti et al., 2010; Sterzer et al., 2005; White, Marsh, et al., 2012) demonstrated reduced amygdala reactivity in AB youth, two studies (Decety et al., 2009) showed increased amygdala reactivity, one showed no effect (White, Williams, et al., 2012), and two studies (Sebastian et al., 2012; Viding, Sebastian, et al., 2012) demonstrated divergent effects depending on the level of CU traits. However, this counting of studies belies the important details. Of the studies examining CU traits in AB youth, all but one (White, Williams, et al., 2012) found lowered amygdala reactivity to emotional faces, with the majority examining fear faces. Thus we can likely conclude that AB+CU+ youth demonstrate less amygdala reactivity to fearful faces than non-AB youth. The study by Passamonti adds pause as to how specific to fear faces this effect is, but there is little other work to evaluate different types of faces in relation to each other. Moreover, the two studies by White and colleagues (2012) underscore the idea that these effects in AB+CU+ youth, may emerge most robustly only for relatively simple tasks. Finally, the Passamonti and Viding studies are at odds with how specific this effect is, and whether CU traits are driving these associations. Because most of these studies have used groups extreme on both CU and AB, it is difficult to know if these effects are specific to the AB+CU+ group or to all AB youth.
For studies focused only on AB, there are mixed results with three studies (Kalnin et al., 2011; Passamonti et al., 2010; Sterzer et al., 2005) finding amygdala hypoactivation to a variety of tasks and two studies demonstrating amygdala hyperactivation to a different set of tasks (Decety et al., 2009; Herpertz et al., 2008). Though these conflicting findings could be driven by the wide variety of tasks used, the study by Viding and colleagues (2012) offers a possible explanation: youth high on AB but low on CU traits may show increased amygdala reactivity to emotional faces due to their increased emotional dysregulation (Cappadocia, Desrocher, Pepler, & Schroeder, 2009), whereas those high on AB and CU traits may show the opposite effect (see also the striking overlap of neural reactivity patterns of AB+CU− with those seen in depression: Davidson et al., 2002). Thus in studies that only measured AB, conflicting findings may emerge due to different distributions of unmeasured CU traits in the AB group. This hypothesis of divergent relationships (AB+CU+ with low amygdala reactivity; AB+CU− with high amygdala reactivity) fits well with observations of greater proactive and reactive aggression in AB+CU+ youth but only reactive aggression in AB+CU− youth (Bezdjian et al., 2011; Cornell et al., 1996; Frick, Cornell, Barry, et al., 2003) and the implied neural correlates to reactive (and emotional dysregulated) versus proactive (and non-emotional) behaviors. Thus, we put forward the tentative hypothesis that individuals high on CU traits and AB may show divergent relationships with biological outcomes in comparison to those high on AB but low on CU: AB+CU− youth appear to be overly sensitive to and emotionally deregulated by threat (and mostly reactive in their aggression), whereas AB+CU+ youth appear to be under-aroused by threat and emotion (and both proactive and reactive in their aggression) (Cappadocia et al., 2009; Stadler et al., 2010; Viding, Fontaine, et al., 2012). Certainly some of the reviewed fMRI work suggests this possibility (though not overwhelmingly) and thus we consider evidence in other areas throughout this review to evaluate this hypothesis.
Beyond conclusions about subgroups of youth, there is preliminary evidence that task effects may be large, even when differences between task demands are small. This point is vital in evaluating the remaining empirical literature in this area as it spans a very wide array of tasks. Though there is preliminary evidence that though some task demands (or youth attention to aspects of the task, such as the eyes) may moderate amygdala differences in AB youth (White, Marsh, et al., 2012; White, Williams, et al., 2012), there is also evidence that differences in AB youth may extend beyond fearful versus neutral faces to other face types (Passamonti et al., 2010) and other tasks using highly stimulating or emotional tasks (Decety et al., 2009; Kalnin et al., 2011; Sebastian et al., 2012; Sterzer et al., 2005). Understanding the specificity of these amygdala effects is critical for future research as it will have major implications for intervention (i.e., should interventions specifically target improving eye contact and/or focus on identifying fearful facial expressions?).
In sum, these studies have advanced our understanding of the link between amygdala reactivity and AB in youth, suggesting that youth with both CU traits and AB show less amygdala reactivity than controls to negative versus neutral stimuli (with the most evidence suggesting specificity to fearful faces) and the possibility that AB+CU− youth may show the opposite pattern of amygdala reactivity. However, future studies are needed to address the following limitations of the literature: the use of small samples, dichotomous versus continuous measurement of CU and AB, accounting for co-occurring disorders, lack of attention to youth developmental status, the specificity of effects, the effects of child attention to the task, and lack of attention to other subtyping approaches.
Areas within the Prefrontal Cortex
Though there has been an explosion of studies narrowly focusing on emotion viewing designs and the amygdala, there has also been a wide array of studies aimed at understanding the neural bases of learning, response to reward and punishment, and other deficits seen in AB youth. However, this literature has been more scattered, so we consider each study more briefly with some attempt to highlight which brain areas and which tasks are overlapping across studies.
In a series of articles (Rubia, Halari, et al., 2009; Rubia et al., 2008; Rubia, Smith, et al., 2009), the neural correlates of CD and ADHD were explored to address issues of both comorbidity and shared versus common neural correlates. To address this question, a sample of boys with “pure” CD (who also met criteria for early onset and ODD), a sample of boys (age 9–16) with “pure” ADHD, and a control group low on both CD and ADHD symptoms were assessed using tasks that focus on attention, inhibition, and reward. In the first study that utilized a task tapping attention and inhibition (a Simon task where subjects have to inhibit a dominant response to similar but competing information), both ADHD and CD groups displayed shared reduced activation across some brain areas, including the dlPFC (and activation in this area was correlated with number of CD symptoms), with the ADHD group showing disorder specific decreases in activation in the ventral lateral PFC (vlPFC) (Rubia, Halari, et al., 2009). The authors noted that their data support implications of weak prefrontal functioning that may account for attention and inhibitory deficits in both ADHD and CD and disorder-specific decreases in functioning in those with ADHD in vlPFC. In the second study (Rubia, Smith, et al., 2009), using a rewarded continuous performance task to assess attention and response to reward, the CD group displayed decreased activation in paralimbic regions (insula, hippocampus, ACC) during attention intervals, and OFC hypo-activation during reward components of the task, whereas the ADHD group had specific decreases in activation in vlPFC. Hypo-activation found in the CD group in the OFC are consistent with theory that the OFC integrates signals and then may modulate the activity of areas such as the amygdala through reciprocal connections based on environmental cues such as reward (Blair, 2004; Finger et al., 2011). In the third related study, during a visual tracking stop task used to measure inhibition of motor responses, the CD group was found to have decreased activation in parietal areas during failed trials (Rubia et al., 2008) and both patient groups displayed differences in posterior cingulate functioning during failed trials which may implicate dysfunctional performance monitoring networks in CD.
In a fourth study, groups of boys with CU traits and AB (age 10–17), boys with pure ADHD, and controls were scanned and compared while undergoing a probabilistic reversal learning task in which reward contingencies are periodically changed to measure ability to flexibly adjust to changes in reinforcement (Finger et al., 2008). Using a contrast of trials in which the participant does not change their response to all correct responses, the CU+AB+ group was found to have greater activity than controls and the ADHD group in a ventral portion of the bilateral medial frontal gyrus (BA 10, vmPFC). In a similar study, boys and girls with CU traits and AB (mean age 13–14) were contrasted with controls during a passive avoidance task to examine AB youths’ capability to learn to avoid stimuli that predict punishment (Finger et al., 2011). Youth high on AB and CU traits displayed less activity in the OFC and caudate in response to early stimulus-reinforcement exposures, and in response to rewarded responses, they also demonstrated decreased OFC activity. Moreover, while the task itself did not evoke group differences in the amygdala, the AB+/CU+ group was shown to have decreased amygdala activity across the entire task (perhaps indicating overall differences in level of arousal in the AB group). These results emphasize the possibility that OFC, caudate, and amygdala circuit functioning may be disrupted in those high on AB and CU traits, and this disruption could affect learning of reinforcement across development and explain why these youth often repeat poor decisions (Blair, 2004, 2007a, 2007b).
These studies attempt to disentangle what may be underlying differences between youth with pure ADHD and early starting CD by selecting specific deficits seen behaviorally (i.e., attention and inhibition). Moreover, these studies help researchers address findings from the ADHD literature to determine if these differences are really specific to ADHD or to externalizing behavioral problems more broadly. The preliminary evidence from these studies implicates under-activation specific to CD youth in areas highlighted in other studies (e.g., OFC/vmPFC, limbic structures, possibly caudate) (see also Marsh et al., 2008), under-activation specific to ADHD in vlPFC, and possibly shared differences in activation between both groups (e.g., dlPFC, cingulate regions, although these findings need to be replicated). A recent review describes these results as reflecting dysfunction in “cool” inferior frontostriatal regions in ADHD and dysfunction in “hot” ventromedial orbitofrontal-limbic in AB (Rubia, 2011). More broadly, these results are consistent with behavioral data suggesting that youth with externalizing disorders demonstrate differences in inhibition, attention, and reward learning, but that there may be specific profiles for youth with pure CD or ADHD (see Herpertz et al., 2001). However, it is interesting to note that much of the extant neuroimaging literature highlights differences between these groups even though the comorbidity between these disorders is quite high (Hinshaw & Lee, 2003) and behavioral genetic studies implicate shared genetic liability as explaining this high overlap (Nadder, Rutter, Silberg, Maes, & Eaves, 2002). These studies highlight a useful strategy for examining shared and specific neural correlates by using groups of “pure” ADHD versus CD, but also highlight the difficulty in generalizing beyond “pure” presentations of these disorders based on their high comorbidity. If youth with CD versus ADHD are mostly dissimilar in terms of brain functioning, do the majority of externalizing youth who are high on both CD and ADHD symptoms have a third etiology, or do they share some of the differences seen in these “pure” groups?
Other Brain Areas Involved in Reward and Learning
Though two previously described studies (Finger et al., 2011; Finger et al., 2008) focused on reward via passive avoidance tasks and found PFC correlates, other studies have examined reward with different tasks and have explored correlates in different brain regions including the ACC, caudate, and insula. For example, a study of 12 – 16 year old boys with “externalizing disorders” (almost all diagnosed with both ADHD and CD) compared to controls, used a task in which their behavior was rewarded or no reward was available, with a focus on specific ROIs (caudate and ACC) (Gatzke-Kopp et al., 2009). Though both groups showed activation in the caudate more during reward blocks, when the same behavior was not rewarded, control boys shifted to increased ACC and decreased caudate activity, while the externalizing group continued to have greater caudate reactivity. The authors noted that the pattern of activity in controls was consistent with literature implicating the caudate in reward and the ACC in error monitoring. Thus, the findings imply continuing activation of reward rather than error monitoring networks within externalizing youth, mirroring behavioral studies demonstrating externalizing youth’s tendency to perseverate on responses that were previously, but no longer, rewarded (Fonseca & Yule, 1995).
Examining a different sort of reward, in a study of Antisocial Substance Disorder – boys with comorbid CD and substance abuse (age 14–18) – investigators used a task that included opportunities to make risky versus cautious decisions and receive or lose monetary rewards (Crowley et al., 2010). This study highlighted lesser activation in the OFC, dlPFC, ACC, insula, amygdala, and hippocampus during decision making trials and lesser activation in the ACC during reward in the AB youth. This approach highlights targeting youth comorbid for disorders likely to be linked to neural functioning (substance use and CD). In another study examining reward comparing high versus low sensation seeking adolescents in a community sample, high sensation seeking youth demonstrated greater response in a cluster across the insula and PFC to win versus no-win trials in a rewarded decision making task (Cservenka, Herting, Seghete, Hudson, & Nagel, 2013). Given the high overlap between sensation seeking and AB (Krueger, Markon, Patrick, Benning, & Kramer, 2007; Lahey, Waldman, & McBurnett, 1999; Perez & Torrubia, 1985; Zucker, Heitzeg, & Nigg, 2011), this study highlights that tasks probing reward may be important in understanding youth AB and related risky temperamental profiles and how understanding sensation seeking can inform our understanding of AB (e.g., Shannon et al., 2011).
Finally, a small study examining sharing in a social exchange game found that externalizing youth (compared to “non-externalizing” youth; age 10–16) demonstrated less differential activation in the insula when they were deciding on whether to share or not, and less divergence in response in the caudate and anterior insula when receiving outcome from neutral relatively to mean or kind peers (Sharp, Burton, & Ha, 2011). A study of the same game in healthy youth, suggests that neural response in the dorsal ACC and anterior insula to this game may be modulated by CU traits (White, Brislin, Meffert, Sinclair, & Blair, 2013). As these studies were quite preliminary, they suggest that further work elucidating the response of AB to peer interaction and reward games may be fruitful in understanding their social interactions.
As a whole, these studies implicate that youth with AB may differ in their response to learning and reward paradigms in brain areas associated with learning, error monitoring, and reward (e.g., the OFC, ACC, caudate, insula). In a sense, these studies help to support the observation from behavioral studies that youth with AB struggle with certain learning and reward tasks by showing that differences in activity in brain areas thought to be implicated in these processes do differ in these youth. At the same time, these studies are difficult to compare as they have used a wide variety of different tasks and samples with different comorbidities and different age ranges. Many of these studies highlight a strategy based on probing specific deficits seen in youth with AB by adapting tasks for use in the scanner to explore these deficits. This strategy helps connect specific neural patterns seen in youth AB to behavioral deficits seen in this population and these studies continue to implicate areas such as the amygdala and the PFC (especially the OFC) and expand the list of important brain areas to structures such as the ACC, insula, and caudate. At the same time, these studies all suffer from small sample sizes, contrasts of two groups of youth that may differ on more variables than just AB, heterogeneity in dimensions and persistence of externalizing behavior (e.g., “externalizing” youth versus CD plus substance use disorder), and wide age range within the groups. Although these studies contribute to the literature, idiosyncratic approaches of these studies and the lack of replication of their findings makes it difficult to know if these results would emerge in other samples and how to fit these results into the broader understanding of youth AB. Whereas the group of studies focusing on amygdala reactivity reviewed earlier have led to replicated findings, as most studies in this area each target a unique question within a unique population, the findings do not form a coherent whole but rather highlight several areas of study to be followed up in future research.
Conclusion: Direct Evidence from Youth with Antisocial Behavior
This collection of studies has vastly improved our understanding of some underlying neural correlates of youth AB generally and youth with AB and CU traits more specifically. Several of the findings are consistent with some studies using structural MRI in youth, fMRI in adult psychopaths and other biological approaches to be reviewed below. Moreover, this rapidly growing body of literature is beginning to consistently implicate functioning in some of the same brain areas: the amygdala and prefrontal regions (i.e., OFC), as well as the insula, ACC, caudate and possibly the cingulate and dlPFC. Findings from these reports also connect these brain areas to functioning during tasks related to secondary behavior seen in youth AB, such as abnormal face processing, difficulty with attention and inhibition, abnormal reversal learning and poor passive avoidance performance. These findings highlight several dimensions of behaviors that are disrupted in AB: emotion processing and regulation, empathy and social interaction, learning and attention, and impulsivity and reward, each of which may have distinct neural correlates that predict youth AB in areas such as the amygdala, OFC, insula, and ACC. However, several fundamental questions remain such as whether antisocial youth tend to be more generally hypo- or hyper-reactive to emotional and threatening stimuli, whether CU traits or severity of AB are driving findings in AB+CU+ youth, which brain areas would be expected to show greater versus lesser response and to which types of tasks and stimuli, and whether fMRI may help us uncover distinct subgroups that vary in both behavior and neural responding to specific tasks.
Beyond the implications for our current knowledge of the neural responsiveness of antisocial youth to various paradigms, this collection of studies is useful in pointing out both advantages and limitations of the current approaches: First, the wide array of tasks and contrasts demonstrates the trade-offs between more general and more specific tasks and the importance of examining the contrast used within the task. Second, sample selection highlights the difficulty in creating appropriate patient groups and appropriate control groups as well as the limited generalizability of the results. Third, these studies highlight the need for imaging in larger samples where these behaviors and traits can be assessed both dichotomously and continuously, where overlapping behaviors (e.g., AB and CU traits, ADHD) can be parsed, where sample sizes can provide more confidence in the possibility of replicating the findings, and where multiple different tasks can be examined in the same sample. Moreover, as the Jones, Marsh, and Passamonti studies demonstrate, replication and use of relatively standard tasks and stimuli are critical to integrating knowledge across studies. While many of the other studies (i.e., those not focused on the amygdala) reviewed provide intriguing results, without replication it is difficult to conclude as much from their findings. Last, the wide range in the age of youth in these studies makes comparison across studies difficult and complicates our understanding of the development of AB across adolescence when the brain is rapidly changing and new groups of youth with AB may be emerging (Giedd, 2008; Moffitt, 1993a). Clearly programmatic research with larger sample sizes, longitudinal approaches, and attention to the nuances in past studies is needed to address the shortcomings within the current research.
Structural Neuroimaging Findings
Although this review is focused primarily on fMRI findings and evaluating these findings from a developmental psychopathology perspective, we briefly review structural MRI (sMRI) findings in this area, as they highlight many of the same neural structures emphasized in the fMRI literature (underlined throughout) (see also Vloet, Konrad, Huebner, Herpertz, & Herpertz-Dahlmann, 2008; Yang & Raine, 2009).
Gray Matter Differences
Several sMRI studies implicate both temporal and frontal brain areas including the amygdala, PFC, insula and ACC, with studies demonstrating reduced overall volume in the right temporal lobe and reduced right temporal gray matter in youth (age 9 – 21) with early onset CD (Kruesi, Casanova, Mannheim, & Johnson-Bilder, 2004), reduced grey matter in the bilateral anterior insula (correlated with youth empathy) and left amygdala in adolescent CD boys (Sterzer, Stadler, Poustka, & Kleinschmidt, 2007), and reduced gray matter in conduct disordered boys across the amygdala, insula, dmPFC, caudate, and fusiform gyrus, albeit with no differences between early and late starting youth (Fairchild et al., 2011). In a study of incarcerated male adolescents, psychopathic traits were correlated with decreased volumes in the OFC, temporal poles and posterior cingulate (Ermer, Cope, Nyalakanti, Calhoun, & Kiehl, 2013). Decreased OFC/vmPFC volume has also been linked to low impulse control in healthy youth (Boes et al., 2009), and in a sample of healthy youth (boys and girls, age 7–17), the volume of the right ACC was found to correlate with conduct behavior ratings in boys (Boes, Tranel, Anderson, & Nopoulos, 2008; though see Bussing, Grudnik, Mason, Wasiak, & Leonard, 2002). Interestingly, a study of CD in female adolescents found similar divergent relationships to AB based on CU traits using fMRI: reduced bilaterial anterior insula, amygdala and right striatal volume broadly were related to AB, with aggressive CD symptoms negatively correlated to dlPFC volume but CU traits positively correlated with OFC volume (Fairchild et al., 2013).
Interestingly, a recent study found larger cavum septum pellucidum (CSP) volumes in a sample AB youth, which is important because the CSP is a deep, midline, limbic structure and its closing in development is related to the development of structures in the limbic system such as the amygdala and hippocampus (White, Brislin, et al., 2012). Thus differences in this structure may inform the developmental antecedents of differences found in limbic structures, especially the amygdala. Finally, boys high on both CU and conduct problems (age 10 – 13) were found to have increased gray matter concentration in medial OFC and ACC, and increased grey matter concentration and volume in the temporal lobes bilaterally, which the authors suggest may be a marker of delayed cortical maturation in these boys (De Brito et al., 2009; for other evidence of neural immaturity in youth AB see Shannon et al., 2011) and underlines that sMRI findings may vary by the age of the youth studied.
Cortical Thickness and Folding
Differences in cortical thickness and folding have identified many of these same areas: One study found significantly less cortical thickness across the entire brain, with specific deficits in the cingulate, ACC, medial PFC, OFC, insula and cingulate and decreased gray matter density in the OFC and insula in 8 year olds with disruptive behavior disorders (Fahim et al., 2011). Another study found similar deficits in cortical thickness, as well as folding deficits in the insula, vm- and dm-PFC, ACC, temporal lobe, and OFC in a study of adolescents with CD (Hyatt, Haney-Caron, & Stevens, 2012).
Connectivity
Recently several studies have emphasized the importance of examining the connectively between key brain regions in AB (i.e., the OFC and amygdala). Recent studies have used diffusion tension imaging (DTI; measures microstructure of white matter connecting cortical areas) to measure fractional anisotropy (FA; a measure of white matter integrity) values in the uncinate fascicle (UF), a key fiber tract connecting the amygdala and OFC. For example, one study of childhood-onset CD male adolescents (mean age = 18) found increased FA in the UF (Passamonti et al., 2012). Similarly, a study of boys (age 12–19) with CD, found increased FA in the CD group (Sarkar et al., 2013). Interestingly, the authors also found an age by group interaction indicating that controls demonstrated age-related maturation in this tract, but those with CD did not. Additionally, a study of adolescents (age 13–17) with DBDs plus ADHD similarly found decreased FA in the UF, but follow-up analyses indicated that this effect was generally driven by ADHD, rather than CD (Wang et al., 2012). Finally, a recent study of adolescents (mean age = 14 years) with DBDs plus psychopathic traits found no differences in FA in the UF, but did find reduced functional connectivity between the amygdala and ACC, insula, cingulate and superior temporal gyrus during a learning task (Finger et al., 2012) and a similar study found reduced amygdala-OFC functional connectivity during a moral judgments task among AB youth with psychopathic traits (Marsh et al., 2011). Thus, recent connectivity studies have been conflicting in the direction of findings but have helped to emphasize possible differences in the UF, a critical connection between the OFC and amygdala.
The overall pattern of results in examining brain structure is promising in that these studies identify many of the same areas as the fMRI literature. However, it is still unclear what the direct relationship is between brain structure and function, and many of the sMRI studies conflict in the direction of findings. Thus, these structural studies highlight that there is high overlap between areas that differ in function and structure within youth with AB and possibly differences in the fiber tracts connecting these specific areas.
Relevant Research from Other Populations
Studies of Adult Psychopathy and Antisocial Behavior
Studies from adults displaying symptoms of psychopathy (as well as other forms of AB) can help to inform theory and research on youth with AB. The main caveat is that the downward extension of adult psychopathy is likely not to youth AB broadly but to youth with AB and CU traits more specifically, and even then, only a handful of studies have demonstrated links from adolescent CU traits to later adult psychopathy (Blonigen et al., 2006; Burke, Loeber, & Lahey, 2007; Lynam, Caspi, Moffitt, Loeber, & Stouthamer-Loeber, 2007). The important question in regards to this review is: do studies in adults converge with the emerging functional neuroimaging of youth AB literature?
Neuroimaging studies
For the most part neuroimaging studies of AB in adults have focused on criminal psychopaths and these studies can be grouped by the focus on the following brain areas (for more extensive reviews see Gao & Raine, 2010; Glenn & Raine, 2008; Kiehl, 2006; Weber, Habel, Amunts, & Schneider, 2008; Yang, Glenn, & Raine, 2008):
Amygdala
Decreased activity has been noted in the amygdala and broader amygdala-hippocampal formation in adult criminal psychopaths (versus healthy controls) during aversive classical conditioning paradigms (Birbaumer et al., 2005; Schneider et al., 2000; Veit et al., 2002), during an affective lexical task contrasting emotional to neutral phrases (Kiehl et al., 2001), as well as among healthy college students scoring high on a trait measure of psychopathy during an emotional faces paradigm (Gordon, Baird, & End, 2004; Kiehl et al., 2001), a task evoking fear (Marsh & Cardinale, 2012), when cooperation was not reciprocated in interactive games (Osumi et al., 2012; Rilling et al., 2007), and during a moral decision-making task (Glenn, Raine, & Schug, 2009). However, there have been other studies that have failed to show this differential activation in the amygdala even when using paradigms shown to robustly activate limbic areas (Deeley et al., 2006) and some evidence for greater amygdala activity to similar paradigms (Schneider et al., 2000). In contrast, non-psychopathic patient populations with impulsive aggressive behavior, rather than psychopathy, have shown increased amygdala reactivity to emotional face paradigms (Coccaro, McCloskey, Fitzgerald, & Phan, 2007), highlighting the possibility that AB that is impulsive or reactive may differ in terms of neural reactivity from AB that is in the context of psychopathy and callousness. In fact, a recent large study of college students (N = 200) found suppression effects similar to those in youth (Sebastian et al., 2012), in which amygdala reactivity to fearful faces was negatively associated with the interpersonal facet of psychopathy (i.e., like CU traits), whereas reactivity to angry faces was positively associated with the lifestyles facet (e.g., like AB without CU) (Carre, Hyde, Neumann, Viding, & Hariri, 2012) (see also Bobes et al., 2012). Structural MRI studies (see Weber et al., 2008 for a review) also suggest that psychopathy may be associated with lesser amygdala and posterior hippocampal volume (Cope et al., 2012; Laakso et al., 2001). In sum, consistent with some studies on AB and CU in youth, studies of psychopathy in adults implicate decreased reactivity in the amygdala to emotional and learning paradigms, with some suggestion that impulsive, non-CU aggression in adults is linked to increased amygdala reactivity.
Prefrontal Cortex
In many of these same studies, functional differences have been noted in various areas of the PFC (Yang & Raine, 2009). Decreased OFC functioning has been found in psychopaths versus healthy controls and among healthy individuals scoring higher on trait measures of psychopathy during the tasks described above in most studies (Birbaumer et al., 2005; Gordon et al., 2004; Rilling et al., 2007; Veit et al., 2002) though not all (Muller et al., 2003). Psychopathy scores were also correlated to medial PFC activation in a moral decision-making paradigm (Glenn et al., 2009). These findings fit with neuropsychological findings implicating the OFC specifically in psychopathy (Blair, Newman, et al., 2006). Outside of the OFC/medial PFC, two studies have suggested decreased dlPFC functioning among college students high on trait psychopathy (Rilling et al., 2007) and among criminal psychopaths (Veit et al., 2002), while another study demonstrated increased dlPFC functioning in a college sample (Gordon et al., 2004; also see Schneider et al., 2000). Beyond functional studies, psychopathy and other related disorders have been associated with abnormalities in the PFC including reduced grey matter in several studies (de Oliveira-Souza et al., 2008; Gregory et al., 2012; Raine, Lencz, Bihrle, LaCasse, & Colletti, 2000; Yang et al., 2005), though not all (Tiihonen et al., 2008; Yang et al., 2007), abnormal EEG in frontal and temporal regions (Hoptman, 2003), and reduced resting glucose metabolism using PET (Raine, Buchsbaum, & LaCasse, 1997; Raine et al., 1998; Soderstrom, Tullberg, Wikkelso, Ekholm, & Forsman, 2000). A study of connectivity in psychopathy has also emphasized decreased functional and structural connectivity between the OFC and the amygdala (Motzkin, Newman, Kiehl, & Koenigs, 2011) (see also Fulwiler, King, & Zhang, 2012; Pujol et al., 2011). In sum, these studies suggest that in adults, AB broadly, and psychopathy in particular, is associated with decreased functioning of prefrontal regions.
Other regions
In some of the above studies, differences in activation have also been found for other regions including the ACC, insula, and ventral striatum (structural differences see Boccardi et al., 2013; Cope et al., 2012; Ly et al., 2012). Decreased ACC and insula activation has been found in response to conditioning paradigms (Birbaumer et al., 2005; Veit et al., 2002), while both the anterior and posterior cingulate, as well as the ventral striatum displayed decreased activation to an affective lexical task (Kiehl et al., 2001). A recent study found a positive correlation between impulsive-antisocial psychopathy traits in a community sample and dopamine release in the ventral striatum (Buckholtz, Treadway, Cowan, Woodward, Benning, et al., 2010). Psychopathy traits in healthy volunteers have also been correlated to ventral striatum reactivity during a reward task (Bjork, Chen, & Hommer, 2012) and a recent study of college students linked less response in the ventral striatum to positive versus negative reward to the lifestyle (i.e., AB) facet of psychopathy (Carre et al., 2012). Finally, a study of healthy adults demonstrated positive correlations with psychopathy scores and ventral striatum and ACC reactivity during reward anticipation (Bjork et al., 2012).
Summary
The literature from adults broadly converges with neuroimaging on youth AB by pointing to many of the same regions such as the amygdala, OFC, ACC, insula, caudate and the ventral striatum. The adult literature has the advantage of being guided by the extensive theoretical and empirical work on psychopathy starting with behavioral observations (such as differences in learning) that have in turn led to psychophysiological findings, which have then informed imaging studies. By restricting many of these studies to criminal psychopaths, researchers have decreased sample heterogeneity and may be focusing on a more homogenous group (which is often compared to non-psychopath criminals). However, this literature is subject to similar weaknesses, including very small samples sizes (n = 8 per group in several studies), inconsistent findings, and comorbidity (i.e., chronic substance abuse that can damage the brain). Although the findings appear to be convergent, it is unclear how much these studies can be compared to those on youth demonstrating varying levels of AB. Importantly, when adults are chosen based on impulsive aggression rather than psychopathy, the AB participants show greater amygdala reactivity, mirroring findings in youth in non-CU groups.
Normative adolescents
As normative youth generally show an increase in risk taking behaviors during adolescence (Moffitt et al., 2002), emerging imaging studies of this population can inform our understanding of the underlying neural systems involved in the risk-taking behaviors also seen in youth with AB (Steinberg, 2007). Many of the same brain structures already mentioned, including the ACC, PFC, and ventral striatum have already been implicated in these adolescent shifts in behavior. For example, studies of normative adolescents have shown that age-related functional changes in the ACC are associated with differences in error processing (Velanova, Wheeler, & Luna, 2008). Prefrontal areas of the adolescent brain may be less efficient in generating inhibitory responses (Luna et al., 2001), and the activation in these frontal areas may be linked to “disinhibition” (McNamee et al., 2008). Moreover, some authors (e.g., Steinberg, 2007) have suggested that the balance between such early developing areas as the ventral striatum (and broader socioemotional paralimbic areas) and later developing areas, such as the OFC, may underlie risk taking behavior in adolescents because adolescents may be more sensitive to immediate rewards due to these later maturing, top-down, cognitive control systems (Galvan et al., 2006). If there are distinct individual differences in this neural “maturity gap,” it could help explain the emergence of increased rates of AB during adolescence and a recent sMRI study of antisocial youth supports this possibility (De Brito et al., 2009). Studies of normative adolescents can inform knowledge of which brain areas may be linked to impulsive behaviors especially as they implicate mechanisms such as the balance between frontal and reward areas. If these age-related shifts show marked individual differences during adolescence, these processes could help explain late starting patterns of AB (Moffitt, 1993a).
Other influences on neural reactivity and youth AB
Genes, hormones, and neurotransmitters
When functional imaging studies are combined with data from animal and lesion studies, pharmacology, other imaging modalities, and other relevant information (i.e., genetics), a more complete and multi-level theory of youth AB can be specified that eventually should improve intervention methods. As this approach is applied to the study of AB, we may be able to specify models that are informed by multiple domains, including brain structure and function, genes, the environment, and the many connections and interactions among these contributing factors (Hyde, Bogdan, et al., 2011). The promise of such an integrated approach is to advance our understanding of the etiology of AB at multiple levels of analysis, which should inform intervention at multiple levels (Hariri, 2009).
While an entire review of the role of genes, hormones, and neurotransmitters in aggression and AB is beyond the scope of this review (see van Goozen et al., 2007), findings from these areas can help inform (a) which brain areas may be implicated in youth AB and why, (b) the existence of possible biologically important subgroups, and (c) how integrating these findings may help to inform a more detailed understanding of youth AB at multiple biological and environmental levels. Ultimately, to advance our knowledge base about neural differences in youth AB, it is important to understand how differences in neurotransmitters, genes, and environment contribute to these differences (Bogdan et al., 2012; Hariri, 2009; Stadler et al., 2010).
Serotonin genes
Across animal and human studies, lower serotonin (5-HT) levels have been implicated theoretically (Coccaro, 1996; Coccaro & Kavoussi, 1996; Soubrie, 1986; Spoont, 1992) and empirically (for reviews see Higley et al., 1992; Manuck, Kaplan, & Lotrich, 2006; Mehlman et al., 1994; Tuinier, Verhoeven, & Van Praag, 1995) to higher aggression and impulsivity broadly, although studies in youth have been mixed (van Goozen et al., 2007). From a genetic standpoint, within rodent models and human linkage studies, variation in genes coding for 5-HT receptors (1A, 1B, 2A, 3, and 7) and molecules important for the synthesis (tryptophan hydroxylase 1 and 2 - TPH), reuptake (5-HT transporter) and degradation of 5-HT (monoamine oxidase A and B– MAOA and MAOB) have been linked to impulsivity- and aggression-related behaviors (Holmes, 2008; Lesch & Merschdorf, 2000). This literature is important when considering neuroimaging studies of AB because 5-HT is a critical modulator of many neural circuits implicated in AB: 5-HT neurons emanating from the dorsal and median raphe nuclei project to forebrain targets implicated in AB, including the amygdala and PFC (Azmitia & Gannon, 1986; Holmes, 2008; Sterzer & Stadler, 2009). Thus, it is not surprising that 5-HT has been an important component in several neurobiological models of youth and adult AB (Blair, Peschardt, et al., 2006b; Siegel, Bhatt, Bhatt, & Zalcman, 2007; van Goozen et al., 2007).
When linking 5-HT genes to youth AB, a few important studies bear closer examination. First, several gene x environment (GxE) interaction studies have demonstrated links between individual variability in a common variant in the promoter of the MAOA gene (which affects degradation of monoamines including 5-HT) and AB in youth and adults who have experienced maltreatment (e.g., Caspi et al., 2002; Kim-Cohen et al., 2006; Weder et al., 2009) (though see Haberstick et al., 2005; Young et al., 2006). Similarly, variants in genes for TPH and 5-HTT have also been linked to aggression and AB in adults or youth (Beitchman et al., 2006; Beitchman et al., 2003; Cicchetti, Rogosch, & Thibodeau, 2012; Manuck et al., 1999; Sadeh et al., 2010; Young & Leyton, 2002), but sometimes in contradictory directions. Interestingly, some of these same variants (e.g., 5-HTTLRP and MAOA 5′ VNTR) have also been linked to functioning of the amygdala and PFC (Brown et al., 2005; Buckholtz et al., 2008; Hariri et al., 2002; Pezawas et al., 2005). For example, Buckholtz and colleagues have linked the pattern of neural reactivity that was related to variation in MAOA (Buckholtz et al., 2008) to patterns of neural reactivity seen in aggressive and violent populations (Buckholtz & Meyer-Lindenberg, 2008; Meyer-Lindenberg et al., 2006): Those with low expressing MAOA alleles displayed increased functional activity in the left amygdala and decreased response across various cortical areas (e.g., lateral OFC and insula). However, these links are not as straightforward as they seem. Several of the specific alleles linked to AB (e.g., 5-HTTLPR S allele, low expressing MAOA alleles) have been linked to greater reactivity in brain areas, such as the amygdala (Buckholtz et al., 2008; Buckholtz & Meyer-Lindenberg, 2008; Hariri et al., 2002), whereas much of the literature reviewed emphasizes lesser amygdala reactivity in AB (though perhaps only for AB+CU+).
One intriguing hypothesis is that the link between 5-HT genes and behavior may not be the same for all groups of antisocial individuals (Stadler et al., 2010). Similar to neuroimaging studies already reviewed, such associations may differ drastically when comparing subgroups of AB youth (Glenn, 2011), which may help explain contradictory findings (e.g., Caspi et al., 2002; vs. Manuck, Flory, Ferrell, Mann, & Muldoon, 2000). Glenn (2011) noted that although the 5-HTTLPR S allele is thought of as the “risk” allele and has been linked to depression and anxiety, as well as impulsive aggression (and possibly AB when comorbid with internalizing), the L allele has been linked to many intermediate phenotypes (e.g., decreased amygdala reactivity, decreased skin conductance during fear conditioning, deficits in passive avoidance learning) that have also been linked to psychopathy (though see Fowler et al., 2009). The hypothesis linking the L allele to psychopathy has been subsequently supported in a recent study of adults with alcohol dependence (Herman et al., 2011), in a GxE study within a forensic sample (Sadeh, Javdani, & Verona, 2012), and in a GxE study of youth (Sadeh et al., 2010). In this third study, individuals with the S allele evidenced more impulsivity, but those with the L allele and low socioeconomic status had greater CU traits (Sadeh et al., 2010). A similar argument has been made in regards to MAOA: GxE findings (Caspi et al., 2002) and literature on early maltreatment in humans and animals (Kaufman & Charney, 2001; Pollak & Sinha, 2002) suggest that low expressing MAOA alleles, especially in the presence of early maltreatment, could lead to greater amygdala reactivity and later reactive AB (Dannlowski et al., 2012; Hanson et al., 2010; Márquez et al., 2013; McCrory et al., 2013; Tottenham et al., 2011; Viding & Frith, 2006). However, high expressing MAOA alleles could be linked with proactive aggression and CU traits similar to that described for the 5-HTTLPR L allele (Sadeh et al., 2012). Thus, there is continued evidence for subgroups of youth with AB who show divergent patterns of genetic and neuroimaging associations based on the level of CU traits.
Cortisol and the HPA Axis
Studies have also demonstrated two subgroups of children in which lower levels of salivary cortisol in boys are associated with persistent aggression, while higher levels of cortisol are associated with boys with CD and comorbid anxiety. These findings have led some to propose dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis as an etiological factor in youth AB and one that may work in opposite directions depending on the subgroup of youth studied (McBurnett et al., 1991; McBurnett, Lahey, Rathouz, & Loeber, 2000; Stadler et al., 2010; van Goozen et al., 2007). For example, some authors have highlighted that low cortisol levels may lead to punishment insensitivity and later AB (with CU traits/proactive aggression) (Dadds & Salmon, 2003), whereas others have explained pathways through which greater cortisol reactivity could be linked to youth AB through emotional dysregulation (Pardini & Frick, 2013) and reactive antisocial acts (Lopez-Duran, Olson, Hajal, Felt, & Vazquez, 2009). Thus some (van Goozen et al., 2007) have proposed a dual model of youth AB in which high cortisol and low 5-HT lead to reactive aggression (dysregulated affect), whereas low cortisol leads to proactive aggression. Studies linking abnormal (especially high) cortisol are particularly important when considering links between child maltreatment and the development of youth AB (Dodge, Bates, & Pettit, 1990) and between maltreatment and disruption of the HPA axis (particularly dysregulated and higher levels of cortisol) (Cicchetti & Rogosch, 2001), which could suggest that early maltreatment has an influence on youth AB via its disruption of cortisol (and 5-HT) systems (Gowin et al., 2013). This dysregulated HPA axis activity (higher cortisol) may then interact with disrupted neural systems (e.g., OFC, amygdala) to increase risk for reactive AB (Blair, Peschardt, et al., 2006b). Thus, preliminarily studies from both the 5-HT and cortisol literature suggest the possibility of dual pathways leading to youth AB, albeit to different subgroups of youth with proactive versus reactive aggression or combinations of both types of aggression (Lopez-Duran et al., 2009; Stadler et al., 2010), as well as AB comorbid with internalizing.
Other genes, neurotransmitters, and hormones
Many other genes, neurotransmitters and hormones have been linked to human aggression or AB. For example, dopamine has been linked to impulsivity, reward sensitivity, ventral striatum functioning (Buckholtz, Treadway, Cowan, Woodward, Benning, et al., 2010; Forbes et al., 2009) and to substance abuse disorders (Comings & Blum, 2000; Foll, Gallo, Le Strat, Lu, & Gorwood, 2009), all of which are also implicated in youth AB. Moreover, recent studies implicate dopamine release in the ventral striatum and ventral striatum reactivity to reward in community adults with greater psychopathic traits and higher trait impulsivity (Buckholtz, Treadway, Cowan, Woodward, Benning, et al., 2010; Buckholtz, Treadway, Cowan, Woodward, Li, et al., 2010). As the field moves forward, exploring neurotransmitters (e.g., dopamine) and hormones that have been linked to aggression, prosocial behavior, or AB (e.g., androgens, oxytocin, and vasopressin) but have not been the focus within theory and neuroimaging studies of youth AB may help us understand neuroimaging results at multiple biological levels (Cushing, Perry, Musatov, Ogawa, & Papademetriou, 2008; den Heijer et al., 2004; Gregg & Siegel, 2001; Meyer-Lindenberg et al., 2008; Siegel et al., 2007; Walum et al., 2008). From the genetic perspective, although there have been few genome-wide association studies of youth AB (Dick et al., 2011; Viding et al., 2010), these studies could eventually help identify new candidate genes, particularly when these studies examine more homogenous subtypes of AB (e.g., Viding et al., 2010).
Interaction with the environment
Recent research is quickly challenging assumptions that behaviors that are highly heritable are unchangeable (Meaney, 2010). Youth AB has been shown to have a heritable component, particularly for those with CU traits (Viding et al., 2005), but these heritability estimates have been qualified by GxE studies (Caspi et al., 2002). For example, the heritability of youth AB has been found to be much higher among higher versus lower SES families (Tuvblad, Grann, & Lichtenstein, 2006), suggesting that different mechanisms may underlie AB across different environments (Raine, 2002). These types of findings have several critical implications. First, the populations from which neuroimaging studies are drawn are critically important. In the neuroimaging studies of youth reviewed above, very little information was provided about participants’ backgrounds (e.g., SES and race/ethnicity), although these samples appear to have been mostly European-American and middle class. Thus, the generalizability of these findings may be particularly limited based on the higher prevalence of youth AB in both lower SES and ethnic minority populations (Nock et al., 2006; Schonberg & Shaw, 2007). Second, GxE studies suggest that biological and environmental vulnerability must be qualified by their interaction. Studies can address this issue by investigating the biological bases of resilience in harsh environments or conversely, by examining children with biological risk for AB that do not end up offending. Third, epigenetic studies (see Meaney, 2010; van Vliet, Oates, & Whitelaw, 2007) also emphasize that any neural differences seen in antisocial youth are not static or necessarily genetic. It is likely that environmental experiences such as psychiatric treatment can alter these neural pathways (e.g., Lewis et al., 2008; Woltering, Granic, Lamm, & Lewis, 2011) and certainly experiences like trauma are likely to affect this circuitry throughout development (Dannlowski et al., 2012). Fourth, beyond experiential epigenetic effects on gene expression, the environment can also affect brain functioning through direct assault. For example, lead poisoning in young children, toxins (e.g., nicotine, alcohol) during pregnancy, and youth’s use of drugs can all affect brain structure and functioning and are all common exposures in this population of youth. Studies that collect or assay pertinent biological information from saliva (e.g., genes, cortisol) or blood (e.g., toxin levels, hormones) and/or collect neuroimaging early in life before the onset of drug use (Schiffer et al., 2011) can help to uncover how the environment interacts with the genome to influence risk for AB and how this process may affect neural functioning. Fundamentally, many of the differences found in neuroimaging studies of youth AB reviewed could simply be reflecting different exposures to drugs (prescribed or recreational), other environmental toxins, or higher levels of stress in the AB group.
Summary and integration of findings
Although research on the neural correlates of youth AB is still in its infancy, evidence from direct and indirect sources suggests some specific neural areas that are likely to be involved. As discussed above, several integrative theories have been postulated to account for differences in neural activity among related disorders (i.e., adult psychopathy, youth aggression), but these theories also will likely need to be ‘pruned’ and/or expanded as more holes in our understanding become filled in the ensuing years. It is also possible to identify the strengths and weaknesses of the current body of literature on neural functioning and its relation to the emergence of AB.
The amygdala
The amygdala is crucial in understanding many psychopathologies, especially youth AB, because of its roles in connecting subcortical and cortical structures (which allow for important developmental processes such as emotional learning), and in regulating arousal and emotion (Whalen & Phelps, 2009). Studies from both child and adult populations implicate abnormal amygdala functioning in AB. It appears that AB in youth (particularly those with CU traits) and psychopathy in adults may be most strongly related to under-arousal (Shirtcliff et al., 2009), perhaps representing a “paralimbic dysfunction” that would be consistent with theories of general under-arousal to threat and emotion in these disorders. At the same time, there is some suggestion that, for some youth with AB (likely those without CU traits), the amygdala may be over-active to some stimuli (Viding, Sebastian, et al., 2012), leading to a more reactive style of aggression through emotion dysregulation (Cappadocia et al., 2009) and over-reactivity to threatening or neutral stimuli via social information processing mechanisms (Dodge, 1993; Dodge et al., 1990). While much more tenuous, this finding could explain discrepant results from neuroimaging studies of youth, and is in accord with literature on early maltreatment (common among youth with early starting AB) (McCrory et al., 2013), non-psychopathic adults, and other biological approaches (e.g., 5-HT and cortisol studies). Future imaging and behavioral studies of early starting youth examining those both high and low on CU traits could address the possibility that within early starters there are two sub-groups with distinct etiologies.
Prefrontal regions: the OFC
Studies on youth with AB, adult psychopaths, and patients with lesions as well as theories of inhibition and aggression (Anderson et al., 1999; Best et al., 2002; Blair, 2004), have emphasized the potential contribution played by the OFC and related areas (e.g., vmPFC). The OFC has a role in sensory integration, representing affective values of reinforcements, and decision making, and thus has been a prime candidate for helping to understand ABs that are linked to affect, reward, and decision making (Finger et al., 2011; Kringelbach, 2005). Based on the reciprocal connections between the OFC and amygdala (among many other limbic regions), it is likely that the balance between these PFC regions and subcortical structures such as the amygdala may be important. For example, subcortical regions may initiate activation to prefrontal regions, which may then help “qualify” the activation and either suppress or enhance it (Cardinal et al., 2002; Fuster, 2001; Stein et al., 2007). Thus, dysfunction in one region (e.g., the OFC) could lead to dysfunction in another region (e.g., the amygdala) and dysfunction in the connections between regions could be implicated beyond the activation of any one region (e.g., the uncinate fasciculus). This hypothesized pathway is particularly interesting based on findings from the sMRI literature that suggest differences in gray to white matter balance in the PFC among antisocial populations. Future studies that link functional and structural techniques (and techniques such as diffusion tensor imaging) simultaneously may help address these issues (e.g., Li, Mathews, Wang, Dunn, & Kronenberger, 2005).
Other emerging areas of interest: dlPFC, ACC, caudate, and the ventral striatum
Another important prefrontal area, the ACC, has been implicated in several studies involving both children and adults assessing both functional and structural facets of neural function in relation to AB (e.g., Boes et al., 2008; Kiehl et al., 2001; Sterzer et al., 2005). These findings are not surprising based on data indicating that the ACC may play a large role in error detection and correction, as well as regulation of both cognitive and emotional processing (Bush et al., 2000). Beyond the OFC and ACC, there is some suggestion that the dlPFC may be functioning differently in both child and adult populations with AB. The dlPFC does not connect directly to the amygdala but does have connections through the vmPFC (Vuilleumier, 2009). Interestingly, differences in the amygdala and related areas (OFC) are seen mostly in affective tasks, while dlPFC and other prefrontal areas appear to show functional differences in attention, learning and inhibition tasks (e.g., Crowley et al., 2010; Rubia, Halari, et al., 2009; Veit et al., 2002). Perhaps these two separate lines of work are uncovering two highly connected but different circuits involved in two different aspects of AB: affect and inhibition. However, the evidence in youth of dlPFC differences in AB is still quite limited and needs to be replicated. Finally, emerging evidence suggests that the ventral striatum, caudate, and insula are also likely to be implicated in AB due to their respective roles in reward/motivation and interoception (see Table 1 & 2), although more research is needed linking these structures to youth AB.
Table 2.
Multi-method evidence for brain areas possibly implicated in youth AB
| Brain area and function | Type of evidence | Implication for functioning | Task/stimuli/contrast | Studies |
|---|---|---|---|---|
|
| ||||
|
Amygdala Emotional learning, fear response and classical conditioning, memory consolidation, general arousal. |
fMRI in youth | Decreased activation | Angry & sad faces | Passamonti et al., 2010[LWH1] |
| Fearful faces | Marsh et al., 2008; Jones et al., 2009 | |||
| Emotional words in stroop task | Kalnin et al., 2011 | |||
| Animations of ToM tasks | Sebastian et al., 2012 | |||
| Masked fear faces (CU+ boys) | Viding et al., 2012 | |||
| Fear faces (low attentional low) | White, Marsh et al., 2012 | |||
| Increased activation | Negative pictures | Sterzer et al., 2005; Herpertz et al., 2008 | ||
| Animations of pain in others | Decety et al., 2008 | |||
| Masked fear faces (CU− boys) | Viding et al., 2012; Posner et al., 2011 | |||
| No difference | Cued eye gaze (fear faces) | White, Williams et al., 2012 | ||
| Fear faces (high attentional load) | White, Marsh et al., 2012 | |||
|
| ||||
| sMRI in youth | Decreased grey matter volume | Sterzer et al., 2007; Fairchild et al., 2011; Fairchild et al., 2012; | ||
| Kruesi et al., 2004 (broad temporal lobe) | ||||
|
| ||||
| fMRI in adult psychopaths or those rated higher on psychopathy measures | Decreased activation | Aversive conditioning | Veit et al., 2002; Birbaumer et al., 2005 | |
| Affective lexical task | Kiehl et al., 2001 | |||
| Emotional faces | Gordon et al., 2004 | |||
| Decision making games | Rilling et al., 2007; Osumi et al., 2012 | |||
| Moral decision making | Glenn et al., 2009 | |||
| Fear faces (interpersonal/CU facet) | Carre et al., 2012 | |||
| Judgments about fear evoking statements | Marsh & Cardinale, 2012 | |||
| Increased activation | Aversive conditioning (odor) | Schneider et al., 200 | ||
| Anger faces (lifestyle/AB facet) | Carre et al., 2012 | |||
|
| ||||
| Lesion studies | Impair ability to interpret social cues | Adolphs et al., 1998; Scott et al., 1997 | ||
|
| ||||
| MAOA studies | Low expressing variant linked with both increased activity in the amygdala, decreased grey matter in the amygdala, and antisocial behavior | Lesch & Merchdorf, 2000; Buckholtz et al., 2008; Buckholtz & Meyer-Lindenberg, 2008; Meyer-Lindenberg et al., 2006 | ||
|
| ||||
| PET 5-HT studies | Decreased 5-HT1A binding in the amygdala correlated with aggression | Parsey et al., 2002 | ||
|
| ||||
|
Orbito-Frontal Cortex (OFC) and related areas such as vmPFC Sensory integration, representing affective values of reinforcers, decision making, executive function |
fMRI in youth | Decreased functional connectivity between amygdala and vmPFC | Fearful faces | Marsh et al., 2008 |
| Decreased activation | Reward in continuous performance task | Smith, Rubia, et al., 2009 | ||
| Early stimulus-reinforcement and rewards during passive avoidance task | Finger et al., 2011 | |||
| Decision making | Crowley et al., 2010 | |||
| Angry & sad faces | Passamonti et al., 2010 | |||
| Increased activation | Reversal learning task | Finger et al., 2008 | ||
|
| ||||
| sMRI in youth | Increased grey matter concentration | De Brito et al., 2009; Ermer, et al. 2013; Fairchild et al., 2012 (specific to CU) | ||
| Decreased grey matter volume | Boes et al., 2009 (low impulse control youth) | |||
| Decreased cortical thickness & decreased gray matter density/folding | Fahim et al., 2011; Hyatt et al., 2012 | |||
|
| ||||
| fMRI in adult psychopaths or those rated higher on psychopathy measures | Decreased activation | Aversive conditioning | Veit et al., 2002; Birbaumer et al., 2005 | |
| Prisoner’s dilemma game | Rilling et al., 2007 | |||
| Emotional faces | Gordon et al., 2004 | |||
|
| ||||
| PET in adult psychopaths | Decreased resting blood flow | Soderstrom et al., 2002 | ||
|
| ||||
| Lesion studies | Irresponsibility, lack of concern over future, increased aggression | Anderson et al., 1999; Damasio, 1994 | ||
|
| ||||
| MAOA studies | Decreased response in OFC and reduced coupling with the amygdala; reduction in grey matter | Low expressing variant | Buckholtz et al., 2008; Lesch & Merschdorf, 2000; Meyer-Lindenberg et al., 2006 | |
|
| ||||
| fMRI in normative adolescents | Lesser balance between ventral striatum and OFC linked to reward sensitivity | Galvan et al., 2006 | ||
|
| ||||
|
Anterior Cingulate Cortex (ACC) and related areas Error detection and correction, regulation of cognitive and emotional processing |
fMRI studies in youth | Decreased activation | Negative pictures | Sterzer et al., 2005 |
| Attention | Rubia, Smith et al., 2009 | |||
| Emotional words in Stroop task | Kalnin et al., 2011 | |||
| During non-rewarded trials | Gatzke-Kopp et al., 2009 | |||
|
| ||||
| sMRI in youth | Decreased volume | Boes et al., 2008 | ||
| Increased grey matter concentration | De Brito et al., 2009 | |||
| Decreased cortical thickness/folding | Fahim et al., 2011; Hyatt et al., 2012 | |||
|
| ||||
| fMRI in adult psychopaths | Decreased activation | Aversive conditioning | Veit et al., 2002; Birbaumer et al., 2005 | |
| Affective lexical task | Kiehl et al., 2001 | |||
| Increased activation | Reward anticipation | Bjork et al., 2012 | ||
|
| ||||
| Lesion studies | Callousness and shallow affect | Kiehl, 2006 | ||
|
| ||||
| fMRI in normative adolescents | Age related functional changes associated with differences in error processing | Velanova et al., 2008 | ||
|
| ||||
|
Caudate Learning and memory, feedback processing, voluntary movement |
fMRI studies in youth | Decreased activation | Early stimulus-reinforcement exposures | Finger et al., 2011 |
| Neutral vs. kind/mean partner interaction | Sharp et al., 2011 | |||
| Increased activation | Shift away from reward | Gatzke-Kopp et al., 2009 | ||
| Animations of pain in others | Decety et al., 2009 | |||
|
| ||||
| sMRI in youth | Reduced grey matter concentration | Fairchild et al., 2011 | ||
|
| ||||
|
Dorsolateral Prefrontal Cortex (DLPFC) Executive function, motor planning, and working memory |
fMRI in youth | Decreased activation | Animations of pain on purpose | Decety et al., 2008 |
| Attention | Rubia, Halari, et al., 2009 | |||
|
| ||||
| sMRI in youth | Decreased volume | Fairchild et al., 2012 | ||
|
| ||||
| fMRI in adult psychopaths or those rated higher on psychopathy measures | Decreased activation | Prisoner’s dilemma game | Rilling et al., 2007 | |
| Aversive conditioning | Veit et al., 2002 | |||
| Increased activation | Emotional faces | Gordon et al, 2004 | ||
|
| ||||
|
Insula Integration of interoceptive (bodily) states particularly disgust, conscious feelings, decision in risk and reward (Naqvi & Bechara, 2009) |
fMRI in youth | Decreased activation | Attention | Rubia, Smith et al., 2009 |
| Angry faces | Passamonti et al., 2010 | |||
| Affective ToM task | Sebastian, et al., 2012 | |||
| Increased activation | Animations of pain in others | Decety et al., 2008 | ||
| Decisions to share with a peer | Sharp et al., 2011 | |||
| Response to reward | Cservenka et al., 2012 (high sensation seeking youth) | |||
|
| ||||
| sMRI in youth | Reduced grey matter | Sterzer et al., 2007; Fairchild et al., 2011; Fairchild et al., 2012; | ||
| Decreased cortical thickness & reduced gray matter concentration/folding | Fahim et al., 2011; Hyatt et al., 2012 | |||
|
| ||||
| Adult psychopaths | Decreased activation | Aversive conditioning | Veit et al., 2002; Birbaumer et al., 2005 | |
|
| ||||
| MAOA studies | Decreased grey matter and decreased fMRI reactivity | Low expressing variant | Lesch & Merschdorf, 2000; Meyer-Lindenberg et al., 2006 | |
|
| ||||
|
Ventral Striatum (Nucleus Acumbens) Motivational salience of stimuli, reward-dependent behaviors (Berridge & Robinson, 2003; Olds & Milner, 1954) |
fMRI in youth | Decreased reactivity | Animations of pain in others | Decety et al., 2008 – differences were broadly in the caudate/striatum |
|
| ||||
| fMRI in adult psychopaths | Decreased activation | Affective lexical task | Kiehl et al., 2001 | |
| Positive versus negative feedback (Lifestyle facet) | Carre et al., 2012 | |||
| Pharmacological PET and fMRI in community adults | Increased activation | Reward anticipation | Bjork et al., 2012 | |
| Increased dopamine release & increased VS reactivity correlated with impulsive antisociality | Amphetamine challenge & Reward anticipation within a reward task | Buckholtz et al., 2010a | ||
|
| ||||
| fMRI studies of impulsivity and reward | Ventral Striatal functioning linked to reward sensitivity | Reward tasks | See Forbes et al., 2009 | |
| Pharmacological PET | Amphetamine induced dopamine release in the VS | Amphetamine challenge | Buckholtz et al., 2010b | |
|
| ||||
| fMRI of normative adolescents | Lesser balance between ventral striatum and OFC linked to reward sensitivity | Galvan et al., 2006 | ||
The aforementioned list represents the field’s most promising brain regions linked to AB and specifically youth AB (see Table 2). However, as the more extensive research on the amygdala demonstrates, it is unlikely that the broad category of youth AB will be linked to simply greater or lesser functioning in one or two brain areas, for two reasons. First, results from any one study on functioning in a brain area must be qualified by the task being undertaken, the stimuli used, and the specific population studied. The amygdala may show differential functioning for emotional tasks but not reward tasks (or for tasks with low attentional demands but not high attentional demands), whereas the ventral striatum may show differential functioning for reward tasks but not emotional tasks. Second, as data from the OFC and amygdala demonstrate, functioning in just one area is not as informative as how functioning is linked across brain areas. Studies employing combining task related fMRI with more sophisticated methods, such as connectivity analyses, are much more informative as to how brain areas are interacting (e.g., do differences in the UF mediate differences seen in amygdala and OFC function to specific tasks?). Understanding how these areas communicate effectively or ineffectively may help inform the understanding of behavior more than just understanding changes in blood flow within any one brain area in response to a certain task.
Implications for Intervention
Understanding the neural correlates of youth AB can help inform intervention efforts in several ways. First, neuroscience research can help us to understand mechanisms linking risk to AB, which can inform new targets for intervention. Similarly, this type of research can help to understand how existing treatments work. For example, a recent study of AB and ADHD showed that stimulant medication treatment normalized amygdala reactivity differences in the AB group (Posner et al., 2011), indicating that one possible mechanism through which stimulants may be effective is through their effect on amygdala reactivity to threat. Second, in combinations with environmental approaches, this work can help to identify risk factors (i.e., behaviors, neurocognitive profiles) that emerge before AB becomes severe and deeply entrenched and thus targeted in prevention programs. Third, neuroscience research can help to identify subgroups of youth who may have distinct etiologies. This approach may help to inform new interventions or tailor existing interventions to each subgroup to make interventions more effective (e.g., CU versus non-CU) (Viding & McCrory, 2012).
In line with the promise of neuroscience research, in a thought-provoking article, Dadds and Rhodes (2008) apply much of the literature reviewed above to understand the etiology of different forms of AB and link these issues to implications for developmental processes and possibilities for intervention. For example, they put forward evidence to support a theory that serotonergic dysregulation decreases thresholds for explosive (reactive) violence while decreased cortisol relates to predatory (proactive) violence for youth with a low capacity for fear and punishment sensitivity (Dadds & Rhodes, 2008). Moreover, as there is some evidence that parenting treatments could be improved in their effectiveness for antisocial children with CU traits (Hawes & Dadds, 2005) (though see Hyde et al., 2012; Waller et al., 2013), the authors suggest that simple changes in parenting interventions may increase effectiveness across subtypes of this behavior. For example, children with more reactive subtypes of AB may respond better to the use of timeouts so they can be removed from stimulation and eye contact with caregivers to decrease threat-related physiological reactivity and learn to better regulate their dysregulated emotions (Pardini & Frick, 2013). Alternatively, children who show more proactive types of AB (and CU traits) appear less sensitive to threat, less emotionally dysregulated, and less able to process others’ pain and emotions (i.e., some have eye-gaze deficits: Dadds, Allen, et al., 2012; Dadds et al., 2011) might benefit from timeouts in which they are instructed to look into the caregiver’s eyes or from strategies focused more on reward. This modification would be consistent with some research suggesting that instructions can help overcome eye-gaze deficits and augment prior learning (Dadds et al., 2006). These ideas emphasize the importance of how understanding the neural basis of youth AB can help to identify subgroups with different etiologies and understand mechanisms within each etiology that may inform personalized treatment. As biological measures (i.e., neuroimaging, molecular genetics) continue to inform our understanding of these subtypes and mechanisms, they could potentially be used to inform the development of more tailored interventions (e.g., Dadds, Cauchi, Wimalaweera, Hawes, & Brennan, 2012; DeRubeis, Siegle, & Hollon, 2008; Willard & Ginsburg, 2009). Finally, it should be noted that interventions studies can also help inform our basic understanding of the neural correlates of AB (Brody, Beach, Philibert, Chen, & Murry, 2009) by allowing for more causal inference. For example, if these interventions can change neural functioning and behavior (e.g., changing eye gaze resulting in changes in amygdala reactivity and decreases in proactive aggression), then we can focus more on cause than correlation in understanding the links from experience to brain to behavior (Jaffee, Strait, & Odgers, 2012).
Future Directions
Several suggestions for future studies follow from this review, particularly as we evaluate this literature from a developmental psychopathology perspective. First, the neuroimaging of youth AB literature has a strong foundation in identifying and addressing subgroups of youth using a person-centered approach (e.g., CU vs. non-CU youth) that may have a distinct etiology (e.g., Viding, Sebastian, et al., 2012) and some studies have begun to address issues of comorbidity (e.g., Marsh et al., 2008). Moreover, very recent work may be elucidating equifinality in neural pathways of AB (Viding, Sebastian, et al., 2012). However, the behavioral literature of youth AB highlights multiple other diagnostic (e.g., proactive versus reactive aggression) and behavioral constructs (e.g., social information processing) that have not been embraced within the neuroimaging literature. Most of the literature has focused on CU traits to the neglect of these other important constructs. Much of the literature has also ignored developmental considerations such as the age of participants, the age range of studies (thus including heterogeneous set of youth at different biological and socioemotional stages), and developmental stage of the participants and developmental pathways to AB through repeated neuroimaging or the use of developmental behavior trajectories (all compounded by small sample sizes where development/age cannot be explored as a moderator of effects),. Basic research that examines dimensions such as amygdala reactivity and OFC development and their behavioral correlates over time could help to address questions of heterotypic continuity (e.g., for youth with low amygdala reactivity to threat, what does their behavior look like at age 5, 10, and 15?). Moreover, both neuroimaging and behavioral studies that focus on elucidating mechanisms of heterotypic continuity can help inform our understanding of findings that may differ by developmental stage. As noted above, there has also been little work examining normative development (e.g., marked changes in adolescent brain development during adolescence), as it may inform understanding the development of youth AB. Additionally, results in related areas (e.g., adult psychopathy, normative adolescence) support much of the focus on the amygdala and PFC in youth with AB. However, the comparison to these populations highlight how much behavioral, diagnostic and longitudinal work is needed to explore the extent to which these comparisons are valid (e.g., do CU traits predict later psychopathy? Are antisocial youth simply less mature than their peers at behavioral and neural levels?).
Second, smaller scale studies must be very careful about how participants are recruited and compared in regards to comorbidity and the phenotypic structure of AB and psychopathology. As evidenced by several of the studies of youth with AB, different levels of CU traits and comorbid symptoms could drastically affect the outcome of the study. For example, a study on youth with AB that are low on CU traits and high on unmeasured depressive symptomatology may have increased amygdala reactivity due to differences related to the etiology of depression rather than factors specific to AB. Smaller studies that identify samples based on selective group membership (e.g., Viding, Sebastian, et al., 2012) have the potential to make much needed contrasts, such as among AB+CU+, AB+CU−, AB−CU+, and AB−CU− youth, which would improve our current understanding of the contribution of CU traits to neural functioning among youth with AB.
Third, studies that are much larger and use dimensional measures of these behaviors and traits (e.g., AB, CU traits) are likely to improve upon our current understanding of the extant literature in regard to both neuroimaging and behavioral studies of youth AB. As research mounts supporting a dimensional and hierarchical structure to externalizing behaviors (Krueger & Markon, 2011; Krueger et al., 2007; Markon & Krueger, 2005), studies that incorporate these models of externalizing with neuroimaging can help to determine the general versus specific nature of neural correlates on broad versus narrow definitions of AB (Hicks, Krueger, Iacono, McGue, & Patrick, 2004; Ofrat & Krueger, 2012), and help to assess heterotypic continuity across time by elucidating the extent to which underlying latent factors (e.g., amygdala reactivity) drive the appearance of heterotypic behaviors across time (e.g., do early temper tantrums, childhood lying and adolescent aggression share similar underlying biological liabilities?). Moreover, neuroimaging research that approaches different, developmentally appropriate manifestations of AB at different developmental stages can help to inform our understanding of AB across the lifespan (Sampson & Laub, 1992).
With the cost of neuroimaging decreasing, conducting large longitudinal neuroimaging studies may become more feasible. Moreover, when these studies are of youth that have already been followed longitudinally (e.g. Aguilar et al., 2000; Caspi, Moffitt, Newman, & Silva, 1996; Deater-Deckard, Dodge, Bates, & Pettit, 1998; Dodge, Greenberg, & Malone, 2008; Hipwell et al., 2007; Pardini, Obradovic, & Loeber, 2006; Shaw et al., 2012), the richness of behavioral data collected can be applied to the imaging data to link earlier experience with current brain functioning. Employing cutting edge imaging techniques is weakened when behavioral phenotypes are not well-measured. Ideally, future longitudinal studies would begin following youth in early childhood (Shaw & Gross, 2008) with more appropriate neural measures (e.g., ERP, EEG, sMRI), followed by fMRI and sMRI across multiple points (and before onset of AB and drug use) with measures of observational and self-report behavioral data.
Fourth, behavioral and imaging studies should be genetically informed and/or take other measures of underlying biology (e.g., measures of circulating hormones, heart rate variability, cortisol levels). Although some of these techniques are invasive, they help address the need for understanding a complex cascade of biological processes that lead to and result from neural differences seen at the systems level via fMRI. This approach can help to understand ABs at multiple levels of analysis. Moreover, many of the most important effects of the environment on biology are likely developmentally stage-dependent and thus, if biology is not measured at multiple levels and at multiple times, important pieces to this complex puzzle are lost. To have a realistic and complex understanding of youth AB, cross-discipline collaboration is needed to create larger studies that approach AB from multiple vantage-points.
Fifth, advances are needed within each area of study (genes, brain, behavior) and studies are needed that combine across areas (Bogdan et al., 2012). Much of the literature concerning the neuroimaging of youth AB has focused on single brain areas to a single contrast. Resting state fMRI can help identify circuits that may differ systematically in these youth (Shannon et al., 2011) and novel approaches to neuroimaging analysis (e.g, pattern classification) can uncover novel brain areas linked to AB (Sato et al., 2011). Moreover, novel integration of genes, brain and behavior studies may provide the richest understanding of the interaction of biology and experience (Sameroff, 1995, 2010) at multiple levels of analysis (e.g., neighborhoods to families to children to neural function) in the development of youth AB through probing the interaction of genes and environment as they predict neural differences (i.e., Imaging Gene by Environment (IGxE) interaction studies) associated with AB (Bronfenbrenner & Ceci, 1994; Hyde, Bogdan, et al., 2011), especially if they are applied in experimental or treatment settings (Brody et al., 2009; Jaffee et al., 2012). These studies can also help to determine in what contexts or with what biology, children are likely to be at most risk (or most resilient under high risk). Future research may highlight neural profiles of youth that are particularly resilient or most likely to desist from early problem behaviors. This type of interaction-focused work can help to take the youth AB neuroimaging literature beyond simple correlations between brain and behavior and identify multifinality in pathways involving differences in brain function as moderated by various factors.
In conclusion, research probing the neural correlates of youth AB is just beginning but has great potential. Initial studies have supported some existing theories and provided information about potential areas of the brain that may be different across samples of youth who vary on biological and environmental risk factors associated with AB. However, these initial studies have also demonstrated the advantages and disadvantages of present approaches in a way that should inform future studies. Although the reviewed studies highlight a wide variety of approaches that leave many questions to be answered, they have also helped identify important brain areas for understanding youth AB (e.g., the amygdala, OFC). Moreover, when behavioral or neuroimaging literature is combined with studies from similar populations (e.g., adult psychopaths, normative adolescents) or from other areas of science (e.g., molecular genetics, psychophysiology), a more nuanced understanding of the brain’s role in youth AB emerges. Future studies that address these limitations and take a thoughtful cross-discipline approach while merging literature from the disparate domains of neuroscience, molecular genetics, and developmental psychopathology are likely to yield results that lead to a better understanding of the etiology, prevention, and treatment of youth AB. These studies will likely require individuals to have training across disciplines and for researchers to collaborate across disciplines. However, it is only through these collaborative and integrative approaches that we can even begin to understand such a complex problem.
Highlights.
We evaluate neuroimaging studies of youth antisocial behavior (AB)
We examine these studies from a developmental psychopathology standpoint
Differences in amygdala and prefrontal cortex functioning are correlated with AB
Details of studies (e.g., fMRI task, type of AB measured) impact results
There is some evidence for two subgroups of youth based on neural reactivity
Acknowledgments
The work was supported by grants T32-GM081760 (first author), K05-DA025630 and R01-DA026222 (second author), and R01-DA031579 (third author). We wish to thank the following people who provided comments on previous drafts of this review: Susan B. Campbell, Patrick M. Fisher, Stephen B. Manuck and Essi Viding.
Footnotes
Note we have excluded one paper from the text of this review because its methods and results were not described in sufficient detail to evaluate or present (e.g., no contrasts were used in the statistical model, no table was provided with results and statistics, coordinates, or cluster size) (Qiao, Xie, & Du, 2012).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Luke W. Hyde, University of Michigan.
Daniel S. Shaw, University of Pittsburgh
Ahmad R. Hariri, Duke University
References
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Aguilar B, Sroufe LA, Egeland B, Carlson E. Distinguishing the early-onset/persistent and adolescence-onset antisocial behavior types: from birth to 16 years. Development and Psychopathology. 2000;12:109–132. doi: 10.1017/s0954579400002017. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Azmitia E, Gannon P. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Advances in Neurology. 1986;43:407–468. [PubMed] [Google Scholar]
- Banaschewski T, Hollis C, Oosterlaan J, Roeyers H, Rubia K, Willcutt E, et al. Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Developmental Science. 2005;8:132–140. doi: 10.1111/j.1467-7687.2005.00400.x. [DOI] [PubMed] [Google Scholar]
- Beitchman JH, Baldassarra L, Mik H, De Luca V, King N, Bender D, et al. Serotonin transporter polymorphisms and persistent, pervasive childhood aggression. American Journal of Psychiatry. 2006;163:1103. doi: 10.1176/ajp.2006.163.6.1103. [DOI] [PubMed] [Google Scholar]
- Beitchman JH, Davidge KM, Kennedy JL, Atkinson L, Lee V, Shapiro S, et al. The serotonin transporter gene in aggressive children with and without ADHD and nonaggressive matched controls. Ann NY Acad Sci. 2003;1008:248–251. doi: 10.1196/annals.1301.025. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proceedings of the National Academy of Sciences USA. 2002;99:8448–8453. doi: 10.1073/pnas.112604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdjian S, Tuvblad C, Raine A, Baker LA. The genetic and environmental covariation among psychopathic personality traits and reactive and proactive aggression in childhood. Child Development. 2011;82:1267–1281. doi: 10.1111/j.1467-8624.2011.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Hommer DW. Psychopathic tendencies and mesolimbic recruitment by cues for instrumental and passively obtained rewards. Biological Psychology. 2012;89:408–415. doi: 10.1016/j.biopsycho.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Newman C, Mitchell DG, Richell RA, Leonard A, Morton J, et al. Differentiating among prefrontal substrates in psychopathy: neuropsychological test findings. Neuropsychology. 2006;20:153–165. doi: 10.1037/0894-4105.20.2.153. [DOI] [PubMed] [Google Scholar]
- Blair R. Responsiveness to distress cues in the child with psychopathic tendencies. Personality and individual differences. 1999;27:135–145. [Google Scholar]
- Blair R, Colledge E, Murray L, Mitchell D. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001;29:491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blair R, Mitchell D, Leonard A, Budhani S, Peschardt K, Newman C. Passive avoidance learning in individuals with psychopathy: modulation by reward but not by punishment. Personality and individual differences. 2004;37:1179–1192. [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Pine DS. The development of psychopathy. Journal of Child Psychology and Psychiatry. 2006a;47:262–276. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Pine DS. Neurobiology of aggression in children. In: Nelson RJ, editor. Biology of aggression. New York: Oxford U.P; 2006b. [Google Scholar]
- Blair RJR. Neurobiological basis of psychopathy. British Journal of Psychiatry. 2003;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in cognitive sciences. 2007a;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Dysfunctions of medial and lateral orbitofrontal cortex in psychopathy. Annals of the New York Academy of Sciences. 2007b;1121:461–479. doi: 10.1196/annals.1401.017. [DOI] [PubMed] [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Continuity and change in psychopathic traits as measured via normal-range personality: a longitudinal-biometric study. Journal of Abnormal Psychology. 2006;115:85–95. doi: 10.1037/0021-843X.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobes MA, Ostrosky F, Diaz K, Romero C, Borja K, Santos Y, et al. Linkage of functional and structural anomalies in the left amygdala of reactive-aggressive men. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss1101. epub online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M, Bocchetta M, Aronen HJ, Repo-Tiihonen E, Vaurio O, Thompson PM, et al. Atypical nucleus accumbens morphology in psychopathy: Another limbic piece in the puzzle. International Journal of Law and Psychiatry. 2013 doi: 10.1016/j.ijlp.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Social Cognitive and Affective Neuroscience. 2009;4:1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, Tranel D, Anderson SW, Nopoulos P. Right anterior cingulate: a neuroanatomical correlate of aggression and defiance in boys. Behavioral Neuroscience. 2008;122:677–684. doi: 10.1037/0735-7044.122.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Hyde L, Hariri A. A neurogenetics approach to understanding individual differences in brain, behavior, and risk for psychopathology. Molecular Psychiatry. 2012;18:288–299. doi: 10.1038/mp.2012.35. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SRH, Philibert RA, Chen Y, Murry VMB. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: Gene× Environment hypotheses tested via a randomized prevention design. Child Development. 2009;80:645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature-nuture reconceptualized in developmental perspective: A bioecological model. Psychological Review. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, et al. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Molecular Psychiatry. 2005;10:884–888. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, et al. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Molecular Psychiatry. 2008;13:313–324. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends in Neurosciences. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience. 2010:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari M, et al. Dopaminergic Network Differences in Human Impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB. Adolescent conduct disorder and interpersonal callousness as predictors of psychopathy in young adults. Journal of Clinical Child & Adolescent Psychology. 2007;36:334–346. doi: 10.1080/15374410701444223. [DOI] [PubMed] [Google Scholar]
- Burt SA. How do we optimally conceptualize the heterogeneity within antisocial behavior? An argument for aggressive versus non-aggressive behavioral dimensions. Clinical Psychology Review. 2012;32:263–279. doi: 10.1016/j.cpr.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Burt SA, Donnellan MB, Iacono WG, McGue M. Age-of-onset or behavioral sub-types? A prospective comparison of two approaches to characterizing the heterogeneity within antisocial behavior. Journal of Abnormal Child Psychology. 2011;39:633–644. doi: 10.1007/s10802-011-9491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biological Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Bussing R, Grudnik J, Mason D, Wasiak M, Leonard C. ADHD and conduct disorder: an MRI study in a community sample. World Journal of Biological Psychiatry. 2002;3:216–220. doi: 10.3109/15622970209150624. [DOI] [PubMed] [Google Scholar]
- Cappadocia MC, Desrocher M, Pepler D, Schroeder JH. Contextualizing the neurobiology of conduct disorder in an emotion dysregulation framework. Clinical Psychology Review. 2009;29:506–518. doi: 10.1016/j.cpr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carré JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Social Cognitive and Affective Neuroscience. 2012;7:213–221. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signature of distinct psychopathic traits. Social Neuroscience. 2012;8:122–135. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Science. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders: Longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. Developmental psychopathology: Reactions, reflections, projections. Developmental Review. 1993;13:471–502. [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8:587–600. [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Thibodeau EL. The effects of child maltreatment on early signs of antisocial behavior: Genetic moderation by tryptophan hydroxylase, serotonin transporter, and monoamine oxidase A genes. Development and Psychopathology. 2012;24:907–928. doi: 10.1017/S0954579412000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF. Neurotransmitter correlates of impulsive aggression in humans. Annals of the New York Academy of Sciences. 1996;794:82–89. doi: 10.1111/j.1749-6632.1996.tb32511.x. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ. Neurotransmitter correlates of impulsive aggression. In: Stoff DM, Cairns RB, editors. Aggression and violence: Genetic, neurobiological and biological perspectives. Mahwah, NJ: Erlbaum; 1996. pp. 67–85. [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Cole DA, Carpentieri S. Social status and the comorbidity of child depression and conduct disorder. Journal of Consulting and Clinical Psychology. 1990;58:748–757. doi: 10.1037//0022-006x.58.6.748. [DOI] [PubMed] [Google Scholar]
- Cole SW. Social regulation of human gene expression. Current Directions in Psychological Science. 2009;18:132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman I, Murray J, Abbott RA, Maughan B, Kuh D, Croudace TJ, et al. Outcomes of conduct problems in adolescence: 40 year follow-up of national cohort. British Medical Journal. 2009;338:a2981. doi: 10.1136/bmj.a2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Cope LM, Shane MS, Segall JM, Nyalakanti PK, Stevens MC, Pearlson GD, et al. Examining the effect of psychopathic traits on gray matter volume in a community substance abuse sample. Psychiatry Research: Neuroimaging. 2012;204:91–100. doi: 10.1016/j.pscychresns.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell DG, Warren J, Hawk G, Stafford E, Oram G, Pine D. Psychopathy in instrumental and reactive violent offenders. Journal of Consulting and Clinical Psychology. 1996;64:783–790. doi: 10.1037//0022-006x.64.4.783. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Foley DL, Angold A. 10-year research update review: the epidemiology of child and adolescent psychiatric disorders: II. Developmental epidemiology. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:8–25. doi: 10.1097/01.chi.0000184929.41423.c0. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? the anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crowe SL, Blair RJR. The development of antisocial behavior: what can we learn from functional neuroimaging studies? Development and Psychopathology. 2008;20:1145–1159. doi: 10.1017/S0954579408000540. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, et al. Risky Decisions and Their Consequences: Neural Processing by Boys with Antisocial Substance Disorder. PLoS One. 2010;5:e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Seghete KLM, Hudson KA, Nagel BJ. High and low sensation seeking adolescents show distinct patterns of brain activity during reward processing. Neuroimage. 2013;66:184–193. doi: 10.1016/j.neuroimage.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings EM, Davies PT, Campbell SB. Developmental psychopathology and family process: Theory, research, and clinical implications. New York, NY: Guilford Press; 2000. [Google Scholar]
- Cushing BS, Perry A, Musatov S, Ogawa S, Papademetriou E. Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. Journal of Neuroscience. 2008;28:10399–10403. doi: 10.1523/JNEUROSCI.1928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Rathouz PJ, Lahey BB. The importance of understanding gene-environment correlations in the development of antisocial behavior. In: Kendler KS, Jaffee SR, Romer D, editors. The dynamic genome and mental health. New York: Oxford; 2011. [Google Scholar]
- Dabbs JM, Morris R. Testosterone, social class, and antisocial behavior in a sample of 4,462 men. Psychological Science. 1990;1:209–211. [Google Scholar]
- Dadds MR, Allen JL, Oliver BR, Faulkner N, Legge K, Moul C, et al. Love, eye contact and the developmental origins of empathy v. psychopathy. The British Journal of Psychiatry. 2012;200:191–196. doi: 10.1192/bjp.bp.110.085720. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Cauchi AJ, Wimalaweera S, Hawes DJ, Brennan J. Outcomes, moderators, and mediators of empathic-emotion recognition training for complex conduct problems in childhood. Psychiatry Research. 2012;199:201–207. doi: 10.1016/j.psychres.2012.04.033. [DOI] [PubMed] [Google Scholar]
- Dadds MR, El Masry Y, Wimalaweera S, Guastella AJ. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:455–463. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Jambrak J, Pasalich D, Hawes DJ, Brennan J. Impaired attention to the eyes of attachment figures and the developmental origins of psychopathy. Journal of Child Psychology and Psychiatry. 2011;52:238–245. doi: 10.1111/j.1469-7610.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, et al. Attention to the eyes and fear-recognition deficits in child psychopathy. British Journal of Psychiatry. 2006;189:280–281. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Rhodes T. Aggression in young children with concurrent callous-unemotional traits: can the neurosciences inform progress and innovation in treatment approaches? Philosophical Transactions of the Royal Society of London - B Biological Sciences. 2008;363:2567–2576. doi: 10.1098/rstb.2008.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, Salmon K. Punishment insensitivity and parenting: temperament and learning as interacting risks for antisocial behavior. Clinical Child and Family Psychology Review. 2003;6:69–86. doi: 10.1023/a:1023762009877. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping. 2012 doi: 10.1002/hbm.22112. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, et al. 5-HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology. 2007;33:418–424. doi: 10.1038/sj.npp.1301411. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ. Regional response differences across the human amygdaloid complex during social conditioning. Cerebral Cortex. 2010;20:612–621. doi: 10.1093/cercor/bhp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, et al. Size matters: Increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Azevedo Ignacio F, Tovar-Moll F, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Dodge KA, Bates JE, Pettit GS. Multiple risk factors in the development of externalizing behavior problems: Group and individual differences. Development and Psychopathology. 1998;10:469–493. doi: 10.1017/s0954579498001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. The Neurodevelopment of Empathy in Humans. Developmental Neuroscience. 2010;32:257–276. doi: 10.1159/000317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Jackson PL. A social-neuroscience perspective on empathy. Current Directions in Psychological Science. 2006;15:54–58. [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley Q, Daly E, Surguladze S, Tunstall N, Mezey G, Beer D, et al. Facial emotion processing in criminal psychopathy. Preliminary functional magnetic resonance imaging study. British Journal of Psychiatry. 2006;189:533–539. doi: 10.1192/bjp.bp.106.021410. [DOI] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Ryan SL, Davis FC, Whalen PJ. Human amygdala sensitivity to the pupil size of others. Cerebral Cortex. 2008;18:2729. doi: 10.1093/cercor/bhn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, Schuit SC, Pols HA, van Meurs JB, Hofman A, Koudstaal PJ, et al. Variations in estrogen receptor alpha gene and risk of dementia, and brain volumes on MRI. Molecular Psychiatry. 2004;9:1129–1135. doi: 10.1038/sj.mp.4001553. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nature Reviews Neuroscience. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dick D, Aliev F, Krueger R, Edwards A, Agrawal A, Lynskey M, et al. Genome-wide association study of conduct disorder symptomatology. Molecular Psychiatry. 2011;16:800–808. doi: 10.1038/mp.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Patterson GR. The development and ecology of antisocial behavior. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. Vol. 3: Risk, disorder and adaptations. New York: Wiley; 2006. pp. 503–541. [Google Scholar]
- Dishion TJ, Patterson GR, Stoolmiller M, Skinner ML. Family, school, and behavioral antecedents to early adolescent involvement wtih antisocial peers. Developmental Psychology. 1991;27:172–180. [Google Scholar]
- Dodge KA. Social-cognitive mechanisms in the development of conduct disorder and depression. Annual Review of Psychology. 1993;44:559–584. doi: 10.1146/annurev.ps.44.020193.003015. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Greenberg MT, Malone PS. Testing an idealized dynamic cascade model of the development of serious violence in adolescence. Child Development. 2008;79:1907–1927. doi: 10.1111/j.1467-8624.2008.01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Lochman JE, Harnish JD, Bates JE, Pettit GS. Reactive and proactive aggression in school children and psychiatrically impaired chronically assaultive youth. Journal of Abnormal Psychology. 1997;106:37–51. doi: 10.1037//0021-843x.106.1.37. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Schwartz D. Social information processing mechanisms in aggressive behavior. In: Stoff DM, Breiling J, editors. Handbook of antisocial behavior. New York: Wiley; 1997. pp. 171–180. [Google Scholar]
- Dolan RJ. Neuroimaging of cognition: past, present, and future. Neuron. 2008;60:496–502. doi: 10.1016/j.neuron.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S. A review of the biological bases of ADHD: what have we learned from imaging studies? Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Differential susceptibility to the environment: Toward an understanding of sensitivity to developmental experiences and context. Development and Psychopathology. 2011;23:1–5. doi: 10.1017/S095457941000060X. [DOI] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:94–103. doi: 10.1016/j.jaac.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim C, He Y, Yoon U, Chen J, Evans A, Pérusse D. Neuroanatomy of childhood disruptive behavior disorders. Aggressive Behavior. 2011;37:1–12. doi: 10.1002/ab.20396. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry. 2013;54:86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EAH, van Goozen SHM, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. American Journal of Psychiatry. 2011 doi: 10.1176/appi.ajp.2010.10081184. epub online. 10.1176/ajp. 2010.10081184. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Goodyer IM. Fear conditioning and affective modulation of the startle reflex in male adolescents with early-onset or adolescence-onset conduct disorder and healthy control subjects. Biological Psychiatry. 2008;63:279–285. doi: 10.1016/j.biopsych.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Van Goozen SHM, Calder AJ, Stollery SJ, Goodyer IM. Deficits in facial expression recognition in male adolescents with early onset or adolescence onset conduct disorder. Journal of Child Psychology and Psychiatry. 2009;50:627–636. doi: 10.1111/j.1469-7610.2008.02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SHM, Stollery SJ, Aitken MRF, Savage J, Moore SC, et al. Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biological Psychiatry. 2009;66:162–168. doi: 10.1016/j.biopsych.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SHM, Stollery SJ, Brown J, Gardiner J, Herbert J, et al. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biological Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M, et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Archives of General Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannin N, Dabbs JM. Testosterone and the work of firefighters: Fighting fires and delivering medical care. Journal of Research in Personality. 2003;37:107–115. [Google Scholar]
- Feilhauer J, Cima M, Korebrits A, Kunert HJ. Differential associations between psychopathy dimensions, types of aggression, and response inhibition. Aggressive Behavior. 2011;38:77–88. doi: 10.1002/ab.20415. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2009;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, Gupta K, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Research: Neuroimaging. 2012;202:239–244. doi: 10.1016/j.pscychresns.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry. 2011;168:152–162. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of General Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foll BL, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behavioral Pharmacology. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Fonseca A, Yule W. Personality and antisocial behavior in children and adolescents: An enquiry into Eysenck’s and Gray’s theories. Journal of Abnormal Child Psychology. 1995;23:767–781. doi: 10.1007/BF01447476. [DOI] [PubMed] [Google Scholar]
- Fontaine NM, McCrory EJP, Boivin M, Moffitt TE, Viding E. Predictors and outcomes of joint trajectories of callous–unemotional traits and conduct problems in childhood. Journal of Abnormal Psychology. 2011;120:730–742. doi: 10.1037/a0022620. [DOI] [PubMed] [Google Scholar]
- Fontaine NMG, Rijsdijk FV, McCrory EJP, Viding E. Etiology of different developmental trajectories of callous-unemotional traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:656–664. doi: 10.1016/j.jaac.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annual Review of Neuroscience. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T, Langley K, Rice F, van den Bree M, Ross K, Wilkinson LS, et al. Psychopathy trait scores in adolescents with childhood ADHD: the contribution of genotypes affecting MAOA, 5HTT and COMT activity. Psychiatric Genetics. 2009;19:312–319. doi: 10.1097/YPG.0b013e3283328df4. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. Journal of Abnormal Child Psychology. 2003;31:457–470. doi: 10.1023/a:1023899703866. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT, Loney BR. Callous-unemotional traits and developmental pathways to severe conduct problems. Developmental Psychology. 2003;39:246–260. doi: 10.1037//0012-1649.39.2.246. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ellis M. Callous-unemotional traits and subtypes of conduct disorder. Clinical Child and Family Psychology Review. 1999;2:149–168. doi: 10.1023/a:1021803005547. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Kimonis ER, Dandreaux DM, Farell JM. The 4 year stability of psychopathic traits in non-referred youth. Behavioral Sciences and the Law. 2003;21:713–736. doi: 10.1002/bsl.568. [DOI] [PubMed] [Google Scholar]
- Frick PJ, White SF. Research review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences USA. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, King JA, Zhang N. Amygdala–orbitofrontal resting-state functional connectivity is associated with trait anger. Neuroreport. 2012;23:606–610. doi: 10.1097/WNR.0b013e3283551cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. San Diego: Academic Press; 2008. [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Raine A. Successful and unsuccessful psychopaths: A neurobiological model. Behavioral Sciences and the Law. 2010;28:194–210. doi: 10.1002/bsl.924. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Beauchaine TP, Shannon KE, Chipman J, Fleming AP, Crowell SE, et al. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. Journal of Abnormal Psychology. 2009;118:203–213. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. Journal of Adolescent Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey B, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6:551–559. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Glenn AL. The other allele: Exploring the long allele of the serotonin transporter gene as a potential risk factor for psychopathy: A review of the parallels in findings. Neuroscience & Biobehavioral Reviews. 2011;35:612–620. doi: 10.1016/j.neubiorev.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn AL, Raine A. The neurobiology of psychopathy. Psychiatric Clinics of North America. 2008;31:463–475. doi: 10.1016/j.psc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2009;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Gopal A, Clark E, Allgair A, D’Amato C, Furman M, Gansler DA, et al. Dorsal/ventral parcellation of the amygdala: Relevance to impulsivity and aggression. Psychiatry Research: Neuroimaging. 2013;211:24–30. doi: 10.1016/j.pscychresns.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56:516–521. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Green CE, Alcorn J, Swann AC, Moeller FG, Lane SD. The role of cortisol and psychopathy in the cycle of violence. Psychopharmacology. 2013 doi: 10.1007/s00213-00013-02992-00211. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Progress in Neuropsychopharmacology and Biological Psychiatry. 2001;25:91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- Gregory S, Simmons A, Kumari V, Howard M, Hodgins S, Blackwood N. The antisocial brain: psychopathy matters: a structural MRI investigation of antisocial male violent offenders. Archives of General Psychiatry. 2012;69:962–972. doi: 10.1001/archgenpsychiatry.2012.222. [DOI] [PubMed] [Google Scholar]
- Guo G. Gene-environment interactoins for delinquency. In: Dodge KA, Rutter M, editors. Gene-Environment Interactions in Developmental Psychopathology. New York: Guilford; 2011. pp. 121–139. [Google Scholar]
- Haberstick BC, Lessem JM, Hopfer CJ, Smolen A, Ehringer MA, Timberlake D, et al. Monoamine oxidase A (MAOA) and antisocial behaviors in the presence of childhood and adolescent maltreatment. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;135:59–64. doi: 10.1002/ajmg.b.30176. [DOI] [PubMed] [Google Scholar]
- Han T, Alders GL, Greening SG, Neufeld RWJ, Mitchell DGV. Do fearful eyes activate empathy-related brain regions in individuals with callous traits? Social Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsr1068. epub online, 10/22/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin B, Nederhof E, Oppenheimer C, Jenness J, Young J, Abela J, et al. Differential susceptibility in youth: evidence that 5-HTTLPR x positive parenting is associated with positive affect ‘for better and worse’. Translational Psychiatry. 2011;1 doi: 10.1038/tp.2011.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, et al. Early stress is associated with alterations in the Orbitofrontal Cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey B. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annual Review of Neuroscience. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Whalen PJ. Face to face with the emotional brain. F1000 Biol Reports. 2011;3 doi: 10.3410/B3-2. published online: http://f1000.com/reports/b/1003/1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes DJ, Dadds MR. The treatment of conduct problems in children with callous-unemotional traits. Journal of Consulting and Clinical Psychology. 2005;73:737–741. doi: 10.1037/0022-006X.73.4.737. [DOI] [PubMed] [Google Scholar]
- Herman AI, Conner TS, Anton RF, Gelernter J, Kranzler HR, Covault J. Variation in the gene encoding the serotonin transporter is associated with a measure of sociopathy in alcoholics. Addiction Biology. 2011;16:124–132. doi: 10.1111/j.1369-1600.2009.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, et al. Emotional processing in male adolescents with childhood-onset conduct disorder. Journal of Child Psychology and Psychiatry. 2008;49:781–791. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Wenning B, Mueller B, Qunaibi M, Sass H, Herpertz-Dahlmann B. Psychophysiological responses in ADHD boys with and without conduct disorder: implications for adult antisocial behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1222–1230. doi: 10.1097/00004583-200110000-00017. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman P, Taub D, Higley S, Suomi SJ, Linnoila M, et al. Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Archives of General Psychiatry. 1992;49:436. doi: 10.1001/archpsyc.1992.01820060016002. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Lee SS. Conduct and oppositional defiant disorders. In: Mash EJ, Barkley RA, editors. Child psychopathology. 2. New York: Guilford Press; 2003. pp. 144–198. [Google Scholar]
- Hipwell AE, Pardini DA, Loeber R, Sembower M, Keenan K, Stouthamer-Loeber M. Callous-unemotional behaviors in young girls: Shared and unique effects relative to conduct problems. Journal of Clinical Child & Adolescent Psychology. 2007;36:293–304. doi: 10.1080/15374410701444165. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neuroscience and Biobehavioral Reviews. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ. Neuroimaging studies of violence and antisocial behavior. Journal of Psychiatric Practice. 2003;9:265–278. doi: 10.1097/00131746-200307000-00002. [DOI] [PubMed] [Google Scholar]
- Hyatt CJ, Haney-Caron E, Stevens MC. Cortical thickness and folding deficits in conduct-disordered adolescents. Biological Psychiatry. 2012;72:207–214. doi: 10.1016/j.biopsych.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene-environment interactions. Trends in Cognitive Sciences. 2011;15:417–427. doi: 10.1016/j.tics.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Manuck SB, Hariri AR. Social support moderates the link between amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49:651–656. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Gardner F, Cheong JW, Dishion TJ, Wilson M. Dimensions of callousness in early childhood: Links to problem behavior and family intervention effectiveness. Development and Psychopathology. 2012;25:347–363. doi: 10.1017/S0954579412001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Rainville P, Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006;125:5–9. doi: 10.1016/j.pain.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Jaffee SR. Genotype-environment correlations: Definitions, methods of measurement, and implications for research on adolescent pscyhopathology. In: Kendler KS, Jaffee SR, Romer D, editors. The dynamic genome and mental health. New York, NY: Oxford; 2011. pp. 79–102. [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Dodge KA, Rutter M, Taylor A, et al. Nature X nurture: genetic vulnerabilities interact with physical maltreatment to promote conduct problems. Development and Psychopathology. 2005;17:67–84. doi: 10.1017/s0954579405050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Strait LB, Odgers CL. From correlates to causes: Can quasi-experimental studies and statistical innovations bring us closer to identifying the causes of antisocial behavior? Psychological Bulletin. 2012;138:272–295. doi: 10.1037/a0026020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Happé FG, Gilbert F, Burnett S, Viding E. Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2010;51:1188–1197. doi: 10.1111/j.1469-7610.2010.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Gareth JB, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnin AJ, Edwards CR, Wang Y, Kronenberger WG, Hummer TA, Mosier KM, et al. The interacting role of media violence exposure and aggressive-disruptive behavior in adolescent brain activation during an emotional Stroop task. Psychiatry Research: Neuroimaging. 2011;192:12–19. doi: 10.1016/j.pscychresns.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Development and Psychopathology. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- Kendler KS. A conceptual overview of gene-environment interaction and correlation in a developmental context. In: Kendler KS, Jaffee SR, Romer D, editors. The dynamic genome and mental health. New York, NY: Oxford; 2011a. [Google Scholar]
- Kendler KS. Peer ground deviance, conduct disorder, and alcohol intake. In: Kendler KS, Jaffee SR, Romer D, editors. The dynamic genome and mental health. New York: Oxford; 2011b. [Google Scholar]
- Kendler KS, Aggen SH, Patrick CJ. Familial Influences on Conduct Disorder Reflect 2 Genetic Factors and 1 Shared Environmental FactorGenetic/Environmental Factors for Conduct Disorder. JAMA Psychiatry (Archives of General Psychiatry) 2013;70:78–86. doi: 10.1001/jamapsychiatry.2013.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, et al. Limbic abnormalities in affective processing by criminal psychpaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene–environment interaction predicting children’s mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kim S, Nordling JK, Yoon JE, Boldt LJ, Kochanska G. Effortful Control in “Hot” and “Cool” Tasks Differentially Predicts Children’s Behavior Problems and Academic Performance. Journal of Abnormal Child Psychology. 2013;41:43–56. doi: 10.1007/s10802-012-9661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Baskin-Sommers A, Zeier J, Newman JP. Investigating the neural correlates of psychopathy: a critical review. Molecular Psychiatry. 2011;16:792–799. doi: 10.1038/mp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews: Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kronenberger WG, Mathews VP, Dunn DW, Wang Y, Wood EA, Giauque AL, et al. Media violence exposure and executive functioning in aggressive and control adolescents. Journal of Clinical Psychology. 2005;61:725–737. doi: 10.1002/jclp.20022. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual review of clinical psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. A Dimensional-Spectrum Model of Psychopathology: Progress and Opportunities. Archives of General Psychiatry. 2011;68:10. doi: 10.1001/archgenpsychiatry.2010.188. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Research - Neuroimaging. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Koivisto E, Savolainen L, Eronen M, Aronen HJ, et al. Psychopathy and the posterior hippocampus. Behavourial Brain Research. 2001;118:187–193. doi: 10.1016/s0166-4328(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Singh AL, Waldman ID, Rathouz PJ. Higher-Order Genetic and Environmental Structure of Prevalent Forms of Child and Adolescent Psychopathology. Archives of General Psychiatry. 2011;68:181–189. doi: 10.1001/archgenpsychiatry.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Waldman ID, McBurnett K. Annotation: The development of antisocial behavior: An integrative causal model. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40:669–682. [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 2007;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sciller D. The human amygdala: Insights from other animals. In: Whalen PJ, Phelps EA, editors. The human amygdala. New York: Guilford Press; 2009. pp. 43–60. [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behavioral Sciences and the Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Granic I, Lamm C, Zelazo PD, Stieben J, Todd RM, et al. Changes in the neural bases of emotion regulation associated with clinical improvement in children with behavior problems. Development and Psychopathology. 2008;20:913–939. doi: 10.1017/S0954579408000448. [DOI] [PubMed] [Google Scholar]
- Li T, Mathews V, Wang Y, Dunn D, Kronenberger W. Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. Annals of the New York Academy of Sciences. 2005;1064:184–192. doi: 10.1196/annals.1340.034. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words. Psychological Science. 2007;18:421. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Loeber R. The stability of antisocial and delinquent child behavior: a review. Child Development. 1982;53:1431–1446. [PubMed] [Google Scholar]
- Loeber R, Burke JD, Lahey BB. What are adolescent antecedents to antisocial personality disorder? Criminal Behaviour in Mental Health. 2002;12:24–36. doi: 10.1002/cbm.484. [DOI] [PubMed] [Google Scholar]
- Loeber R, Dishion TJ. Early predictors of male delinquency: A review. Psychological Bulletin. 1983;94:68–99. [PubMed] [Google Scholar]
- Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annual Review of Psychology. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Loeber R, Stouthamer-Loeber M. Development of juvenile aggression and violence. Some common misconceptions and controversies. American Psychologist. 1998;53:242–259. doi: 10.1037//0003-066x.53.2.242. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Magnetic Resonance Imaging. 2004;22:1517–1531. doi: 10.1016/j.mri.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Olson SL, Hajal NJ, Felt BT, Vazquez DM. Hypothalamic pituitary adrenal axis functioning in reactive and proactive aggression in children. Journal of Abnormal Child Psychology. 2009;37:169–182. doi: 10.1007/s10802-008-9263-3. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Ly M, Motzkin JC, Philippi CL, Kirk GR, Newman JP, Kiehl KA, et al. Cortical thinning in psychopathy. American Journal of Psychiatry. 2012;169:743–749. doi: 10.1176/appi.ajp.2012.11111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynam DR, Caspi A, Moffitt TE, Loeber R, Stouthamer-Loeber M. Longitudinal evidence that psychopathy scores in early adolescence predict adult psychopathy. Journal of abnormal psychology. 2007;116:155–165. doi: 10.1037/0021-843X.116.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1:173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB. The reaction norm in gene× environment interaction. Molecular Psychiatry. 2009;15:881–882. doi: 10.1038/mp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biological Psychiatry. 1999;45:603–614. doi: 10.1016/s0006-3223(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Research. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Lotrich FE. Brain serotonin and aggressive disposition in humans and nonhuman primates. In: Nelson RJ, editor. Biology of aggression. New York: Oxford U.P; 2006. pp. 65–113. [Google Scholar]
- Markon KE, Krueger RF. Categorical and continuous models of liability to externalizing disorders: A direct comparison in NESARC. Archives of General Psychiatry. 2005;62:1352–1359. doi: 10.1001/archpsyc.62.12.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez C, Poirier G, Cordero M, Larsen M, Groner A, Marquis J, et al. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Translational Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. 210.1038/tp.2012.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Blair RJR. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neuroscience and Biobehavioral Reviews. 2008;32:454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Cardinale EM. When psychopathy impairs moral judgments: neural responses during judgments about causing fear. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss1097. epub online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz ITN, Schechter JC, Yu HH, et al. Reduced amygdala–orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research: Neuroimaging. 2011;194 doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Masten AS. Ordinary magic. Resilience processes in development. American Psychologist. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- Masten AS, Coatsworth JD. The development of competence in favorable and unfavorable environments. Lessons from research on successful children. American Psychologist. 1998;53:205–220. doi: 10.1037//0003-066x.53.2.205. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, et al. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:192–196. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. The British Journal of Psychiatry. 2013 doi: 10.1192/bjp.bp.1112.116624. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- McNamee RL, Dunfee KL, Luna B, Clark DB, Eddy WF, Tarter RE. Brain activation, response inhibition, and increased risk for substance use disorder. Alcoholism: Clinical and Experimental Research. 2008;32:405–413. doi: 10.1111/j.1530-0277.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene× environment interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Salloway S, Malloy P. The limbic system: an anatomic, phylogenetic, and clinical perspective. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:315–330. doi: 10.1176/jnp.9.3.315. [DOI] [PubMed] [Google Scholar]
- Mehlman P, Higley J, Faucher I, Lilly A, Taub D, Vickers J, et al. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. American Journal of Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, ARH, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, et al. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Molecular Psychiatry. 2008 doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993a;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE. The neuropsychology of conduct disorder. Development and Psychopathology. 1993b;5 [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Jaffee SR, Kim Cohen J, Koenen KC, Odgers CL, et al. Research Review: DSM V conduct disorder: research needs for an evidence base. Journal of Child Psychology and Psychiatry. 2008;49:3–33. doi: 10.1111/j.1469-7610.2007.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Dickson N, Silva P, Stanton W. Childhood-onset versus adolescent-onset antisocial conduct problems in males: natural history from ages 3 to 18 years. Development and Psychopathology. 1996;8:399–424. [Google Scholar]
- Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: follow-up at age 26 years. Development and Psychopathology. 2002;14:179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J, DeBonis M, Dolan R. Human amygdala responses to fearful eyes. Neuroimage. 2002;17:214–222. doi: 10.1006/nimg.2002.1220. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. The Journal of Neuroscience. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moul C, Killcross S, Dadds MR. A Model of Differential Amygdala Activation in Psychopathy. Psychological Review. 2012;119:789–806. doi: 10.1037/a0029342. [DOI] [PubMed] [Google Scholar]
- Muller JL, Sommer M, Wagner V, Lange K, Taschler H, Roder CH, et al. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry. 2003;54:152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- Nadder TS, Rutter M, Silberg J, Maes H, Eaves L. Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (ODD/CD) symptomatologies across informant and occasion of measurement. Psychological Medicine. 2002;32:39–53. doi: 10.1017/s0033291701004792. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychological Methods. 2001;6:18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Baskin-Sommers AR. Early Selective Attention Abnormalities in Psychopathy. In: Posner MI, editor. Cognitive neuroscience of attention, 2nd edition. New York: Guilford Press; 2011. pp. 421–440. [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E, Kessler RC. Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychological Medicine. 2006;36:699–710. doi: 10.1017/S0033291706007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Rutter M. Risk mechanisms in development: Some conceptual and methodological considerations. Developmental Psychology. 1996;32:787–795. [Google Scholar]
- Odgers CL, Caspi A, Broadbent JM, Dickson N, Hancox RJ, Harrington HL, et al. Prediction of differential adult health burden by conduct problem subtypes in males. Archives of General Psychiatry. 2007;64:476. doi: 10.1001/archpsyc.64.4.476. [DOI] [PubMed] [Google Scholar]
- Ofrat S, Krueger RF. How research on the meta-structure of psychopathology aids in understanding biological correlates of mood and anxiety disorders. Biology of Mood & Anxiety Disorders. 2012;2:13. doi: 10.1186/2045-5380-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Orobio de Castro B, Veerman JW, Koops W, Bosch JD, Monshouwer HJ. Hostile attribution of intent and aggressive behavior: a meta-analysis. Child Development. 2002;73:916–934. doi: 10.1111/1467-8624.00447. [DOI] [PubMed] [Google Scholar]
- Osumi T, Nakao T, Kasuya Y, Shinoda J, Yamada J, Ohira H. Amygdala dysfunction attenuates frustration-induced aggression in psychopathic individuals in a non-criminal population. Journal of Affective Disorders. 2012;142:331–338. doi: 10.1016/j.jad.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Pardini D, Frick PJ. Multiple developmental pathways to conduct disorder: Current conceptualizations and clinical implications. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2013;22:20–25. [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Frick PJ, Moffitt TE. Building an Evidence Base for DSM–5 Conceptualizations of Oppositional Defiant Disorder and Conduct Disorder: Introduction to the Special Section. Journal of Abnormal Psychology. 2010;119:683–688. doi: 10.1037/a0021441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Obradovic J, Loeber R. Interpersonal callousness, hyperactivity/impulsivity, inattention, and conduct problems as precursors to delinquency persistence in boys: A comparison of three grade-based cohorts. Journal of Clinical Child & Adolescent Psychology. 2006;35:46–59. doi: 10.1207/s15374424jccp3501_5. [DOI] [PubMed] [Google Scholar]
- Pardini DA, Phillips M. Neural responses to emotional and neutral facial expressions in chronically violent men. Journal of Psychiatry & Neuroscience. 2010;35:390–398. doi: 10.1503/jpn.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Fornito A, Goodyer IM, Nimmo-Smith I, Hagan CC, et al. Abnormal Anatomical Connectivity between the Amygdala and Orbitofrontal Cortex in Conduct Disorder. PloS one. 2012;7:e48789. doi: 10.1371/journal.pone.0048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Archives of General Psychiatry. 2010;67:729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GR, Reid JB, Dishion TJ. Antisocial boys. Eugene, OR: Castalia; 1992. [Google Scholar]
- Paulhus DL, Robins RW, Trzesniewski KH, Tracy JL. Two replicable suppressor situations in personality research. Multivariate Behavioral Research. 2004;39:303–328. doi: 10.1207/s15327906mbr3902_7. [DOI] [PubMed] [Google Scholar]
- Perez J, Torrubia R. Sensation seeking and antisocial behaviour in a student sample. Personality and Individual Differences. 1985;6:401–403. [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neuroscience & Biobehavioral Reviews. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology. 2002;38:784–791. doi: 10.1037//0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112:323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Vardi S, Putzer Bechner AM, Curtin JJ. Physically abused children’s regulation of attention in response to hostility. Child Development. 2005;76:968–977. doi: 10.1111/j.1467-8624.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- Posner J, Nagel BJ, Maia TV, Mechling A, Oh M, Wang Z, et al. Abnormal Amygdalar Activation and Connectivity in Adolescents With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:828–837. doi: 10.1016/j.jaac.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Batalla I, Contreras-Rodríguez O, Harrison BJ, Pera V, Hernández-Ribas R, et al. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Social Cognitive and Affective Neuroscience. 2011;7:917–923. doi: 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Xie B, Du X. Abnormal response to emotional stimulus in male adolescents with violent behavior in China. European Child and Adolescent Psychiatry. 2012;21:193–198. doi: 10.1007/s00787-012-0252-2. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by positron emission tomography. Biological Psychiatry. 1997;42:495–508. doi: 10.1016/S0006-3223(96)00362-9. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Raine A, Meloy JR, Bihrle S, Stoddard J, LaCasse L, Buchsbaum MS. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behavioral Sciences and the Law. 1998;16:319–332. doi: 10.1002/(sici)1099-0798(199822)16:3<319::aid-bsl311>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social Cognitive and Affective Neuroscience. 2006;1:203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D. The interplay between genotype and family relationships: Reframing concepts of development and prevention. Current Directions in Psychological Science. 2005;14:139–143. [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61:1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biological Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith AB, Mohammad M, Scott S, Brammer MJ. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. Journal of Child Psychology and Psychiatry. 2009;50:669–678. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith AB, Mohammed M, Scott S, Giampietro V, et al. Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. American Journal of Psychiatry. 2008;165:889–897. doi: 10.1176/appi.ajp.2008.07071084. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. American Journal of Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Rutter M. Psychosocial influences: Critiques, findings, and research needs. Development and Psychopathology. 2000;12:375–405. doi: 10.1017/s0954579400003072. [DOI] [PubMed] [Google Scholar]
- Rutter M, Dodge KA. Gene-Environment interactions: the state of science. In: Dodge KA, Rutter M, editors. Gene-Environment interaction in developmental psychopathology. New York: Guilford; 2011. pp. 87–101. [Google Scholar]
- Rutter M, Dunn J, Plomin R, Simonoff E, Pickles A, Maughan B, et al. Integrating nature and nurture: Implications of person-environment correlations and interactions for developmental psychopathology. Development and Psychopathology. 1997;9:335–364. doi: 10.1017/s0954579497002083. [DOI] [PubMed] [Google Scholar]
- Rutter ML. Nature-nurture integration: The example of antisocial behavior. American Psychologist. 1997;52:390–398. [Google Scholar]
- Sadeh N, Javdani S, Jackson JJ, Reynolds EK, Potenza MN, Gelernter J, et al. Serotonin transporter gene associations with psychopathic traits in youth vary as a function of socioeconomic resources. Journal of Abnormal Psychology. 2010;119:604–609. doi: 10.1037/a0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Javdani S, Verona E. Analysis of Monoaminergic Genes, Childhood Abuse, and Dimensions of Psychopathy. Journal of Abnormal Psychology. 2012 doi: 10.1037/a0029866. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. General systems theories and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Vol. 1: Theory and methods. Vol. 1. Oxford, England: John Wiley and Sons; 1995. pp. 659–695. [Google Scholar]
- Sameroff AJ. Developmental systems and psychopathology. Development and Psychopathology. 2000;12:297–312. doi: 10.1017/s0954579400003035. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. A unified theory of development: A dialectic integration of nature and nurture. Child Development. 2010;81:6–22. doi: 10.1111/j.1467-8624.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Laub JH. Crime and deviance in the life course. Annual Review of Sociology. 1992;18:63–84. [Google Scholar]
- Sarkar S, Craig M, Catani M, Dell’Acqua F, Fahy T, Deeley Q, et al. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychological Medicine. 2013;43:401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Sato JR, de Oliveira-Souza R, Thomaz CE, Basílio R, Bramati IE, Amaro E, Jr, et al. Identification of psychopathic individuals using pattern classification of MRI images. Social neuroscience. 2011;14:1–13. doi: 10.1080/17470919.2011.562687. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Muller BW, Scherbaum N, Hodgins S, Forsting M, Wiltfang J, et al. Disentangling Structural Brain Alterations Associated With Violent Behavior From Those Associated With Substance Use Disorders. Archives of General Psychiatry. 2011;68:1039–1049. doi: 10.1001/archgenpsychiatry.2011.61. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Kessler C, Posse S, Grodd W, Muller-Gartner HW. Functional imaging of conditioned aversive emotional responses in antisocial personality disorder. Neuropsychobiology. 2000;42:192–201. doi: 10.1159/000026693. [DOI] [PubMed] [Google Scholar]
- Schonberg MA, Shaw DS. Do the predictors of child conduct problems vary by high- and low- levels of socioeconomic and neighborhood risk? Clinical Child and Family Psychology Review. 2007;10:101–136. doi: 10.1007/s10567-007-0018-4. [DOI] [PubMed] [Google Scholar]
- Scott S, Knapp M, Henderson J, Maughan B. Financial cost of social exclusion: follow up study of antisocial children into adulthood. BMJ. 2001;323:1–5. doi: 10.1136/bmj.323.7306.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJP, Cecil CAM, Lockwood PL, De Brito SA, Fontaine NMG, et al. Neural Responses to Affective and Cognitive Theory of Mind in Children With Conduct Problems and Varying Levels of Callous-Unemotional Traits. Archives of General Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, et al. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proceedings of the National Academy of Sciences. 2011;108:11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C, Burton PC, Ha C. “Better the devil you know”: a preliminary study of the differential modulating effects of reputation on reward processing for boys with and without externalizing behavior problems. European Child and Adolescent Psychiatry. 2011;20:581–592. doi: 10.1007/s00787-011-0225-x. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Bell RQ, Gilliom M. A truly early starter model of antisocial behavior revisited. Clinical Child and Family Psychology Review. 2000;3:155–172. doi: 10.1023/a:1009599208790. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Gross H. Early childhood and the development of delinquency: What we have learned from recent longitudinal research. In: Lieberman A, editor. The long view of crime: A synthesis of longitudinal research. New York: Springer; 2008. pp. 79–127. [Google Scholar]
- Shaw DS, Hyde LW, Brennan LM. Predictors of Boys’ Antisocial Trajectories from Toddlerhood through Adolescence. Development and Psychopathology. 2012;24:871–888. doi: 10.1017/S0954579412000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: Implications for the development of antisocial behavior. Behavioral Sciences and the Law. 2009;27:137–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A, Bhatt S, Bhatt R, Zalcman SS. The neurobiological bases for development of pharmacological treatments of aggressive disorders. Current Neuropharmacology. 2007;5:135–147. doi: 10.2174/157015907780866929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom H, Tullberg M, Wikkelso C, Ekholm S, Forsman A. Reduced regional cerebral blood flow in non-psychotic violent offenders. Psychiatry Research - Neuroimaging. 2000;98:29–41. doi: 10.1016/s0925-4927(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Fani N, McClure-Tone EB. Behavioral and neural representation of emotional facial expressions across the lifespan. Developmental Neuropsychology. 2011;36:408–428. doi: 10.1080/87565641.2010.549865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrie P. Reconciling the role of central serotonin neurons in human and animal behavior. Behavioral and Brain Sciences. 1986;9:319–335. [Google Scholar]
- Spence SA, Hunter MD, Farrow TF, Green RD, Leung DH, Hughes CJ, et al. A cognitive neurobiological account of deception: evidence from functional neuroimaging. Philosophical Transactions of the Royal Society of London - B Biological Sciences. 2004;359:1755–1762. doi: 10.1098/rstb.2004.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoont MR. Modulatory role of serotonin in neural information processing: implications for human psychopathology. Psychological Bulletin. 1992;112:330–350. doi: 10.1037/0033-2909.112.2.330. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Rutter M. The domain of developmental psychopathology. Child Development. 1984;55:17–29. [PubMed] [Google Scholar]
- Stadler C, Poustka F, Sterzer P. The heterogeneity of disruptive behavior disorders–implications for neurobiological research and treatment. Frontiers in Psychiatry. 2010;1:1–14. doi: 10.3389/fpsyt.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler C, Sterzer P, Schmeck K, Krebs A, Kleinschmidt A, Poustka F. Reduced anterior cingulate activation in aggressive children and adolescents during affective stimulation: association with temperament traits. Journal of Psychiatric Research. 2007;41:410–417. doi: 10.1016/j.jpsychires.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence. Current Directions in Psychological Science. 2007;16:55–59. [Google Scholar]
- Sterzer P, Stadler C. Neuroimaging of aggressive and violent behaviour in children and adolescents. Frontiers in Behavioral Neuroscience. 2009;3 doi: 10.3389/neuro.3308.3035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Stickle TR, Kirkpatrick NM, Brush LN. Callous-Unemotional Traits and Social Information Processing: Multiple Risk-Factor Models for Understanding Aggressive Behavior in Antisocial Youth. Law and Human Behavior. 2009 doi: 10.1007/s10979-008-9171-7. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Burmeister M. Recent progress in psychiatric genetics—some hope but no hype. Human Molecular Genetics. 2000;9:927–935. doi: 10.1093/hmg/9.6.927. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. In: Co-planar stereotaxic atlas of the human brain. Rayport M, translator. New York: Thieme Medical Publishers, INC; 1988. [Google Scholar]
- Tiihonen J, Rossi R, Laakso MP, Hodgins S, Testa C, Perez J, et al. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Research. 2008;163:201–212. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare T, Millner A, Gilhooly T, Zevin J, Casey B. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.3309.3068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay RE, Japel C, Perusse D, McDuff P, Boivin M, Zoccolillo M, et al. The search for the age of ‘onset’ of physical aggression: Rousseau and Bandura revisited. Criminal Behaviour and Mental Health. 1999;9:8–23. [Google Scholar]
- Trentacosta CJ, Hyde LW, Shaw DS, Cheong JW. Adolescent dispositions for antisocial behavior in context: The roles of neighborhood dangerousness and parental knowledge. Journal of Abnormal Psychology. 2009;118:564–575. doi: 10.1037/a0016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinier S, Verhoeven W, Van Praag H. Cerebrospinal fluid 5-hydroxyindolacetic acid and aggression: a critical reappraisal of the clinical data. International clinical psychopharmacology. 1995;10:147–156. doi: 10.1097/00004850-199510030-00003. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Grann M, Lichtenstein P. Heritability for adolescent antisocial behavior differs with socioeconomic status: gene-environment interaction. Journal of Child Psychology and Psychiatry. 2006;47:734–743. doi: 10.1111/j.1469-7610.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJLG. Testosterone reduces conscious detection of signals serving social correction implications for antisocial behavior. Psychological Science. 2007;18:663–667. doi: 10.1111/j.1467-9280.2007.01955.x. [DOI] [PubMed] [Google Scholar]
- van Vliet J, Oates NA, Whitelaw E. Epigenetic mechanisms in the context of complex diseases. Cellular and Molecular Life Sciences. 2007;64:1531–1538. doi: 10.1007/s00018-007-6526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnäs K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Human Brain Mapping. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, et al. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Blair RJR, Moffitt TE, Plomin R. Evidence for substantial genetic risk for psychopathy in 7-year-olds. Journal of Child Psychology and Psychiatry. 2005;46:592–597. doi: 10.1111/j.1469-7610.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- Viding E, Fontaine NMG, McCrory EJ. Antisocial behaviour in children with and without callous-unemotional traits. Journal of the Royal Society of Medicine. 2012;105:195–200. doi: 10.1258/jrsm.2011.110223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Frith U. Genes for susceptibility to violence lurk in the brain. Proceedings of the National Academy of Sciences USA. 2006;103:6085–6086. doi: 10.1073/pnas.0601350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Hanscombe KB, Curtis CJC, Davis OSP, Meaburn EL, Plomin R. In search of genes associated with risk for psychopathic tendencies in children: a two-stage genome-wide association study of pooled DNA. Journal of Child Psychology and Psychiatry. 2010;51:780–788. doi: 10.1111/j.1469-7610.2010.02236.x. [DOI] [PubMed] [Google Scholar]
- Viding E, Jones AP, Paul JF, Moffitt TE, Plomin R. Heritability of antisocial behaviour at 9: do callous unemotional traits matter? Developmental Science. 2008;11:17–22. doi: 10.1111/j.1467-7687.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- Viding E, McCrory EJ. Genetic and neurocognitive contributions to the development of psychopathy. Development and Psychopathology. 2012;24:969–983. doi: 10.1017/S095457941200048X. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CAM, De Brito SA, et al. Amygdala Response to Preattentive Masked Fear in Children With Conduct Problems: The Role of Callous-Unemotional Traits. American Journal of Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Brendgen M, Barker ED. Subtypes of aggressive behaviors: A developmental perspective. International Journal of Behavioral Development. 2006;30:12–19. [Google Scholar]
- Vitaro F, Gendreau PL, Tremblay RE, Oligny P. Reactive and proactive aggression differentially predict later conduct problems. Journal of Child Psychology and Psychiatry. 1998;39:377–385. [PubMed] [Google Scholar]
- Vloet TD, Konrad K, Huebner T, Herpertz S, Herpertz-Dahlmann B. Structural and functional MRI-findings in children and adolescents with antisocial behavior. Behavioral Sciences and the Law. 2008;26:99–111. doi: 10.1002/bsl.794. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. The role of the human amygdala in perception and attention. In: Whalen PJ, Phelps EA, editors. The human amygdala. New York: Guilford Press; 2009. pp. 220–249. [Google Scholar]
- Waller R, Gardner F, Hyde LW. What are the associations between parenting, callous-unemotional traits, and antisocial behavior in youth? A systematic review of evidence. Clinical Psychology Review. 2013;33:593–608. doi: 10.1016/j.cpr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Walters G, Ruscio J. Trajectories of Youthful Antisocial Behavior: Categories or Continua? Journal of Abnormal Child Psychology. 2012 doi: 10.1007/s10802-10012-19700-10801. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proceedings of the National Academy of Sciences USA. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Horst KK, Kronenberger WG, Hummer TA, Mosier KM, Kalnin AJ, et al. White matter abnormalities associated with disruptive behavior disorder in adolescents with and without attention-deficit/hyperactivity disorder. Psychiatry Research: Neuroimaging. 2012;202:245–251. doi: 10.1016/j.pscychresns.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Weber S, Habel U, Amunts K, Schneider F. Structural brain abnormalities in psychopaths-a review. Behavorial Sciences and the Law. 2008;26:7–28. doi: 10.1002/bsl.802. [DOI] [PubMed] [Google Scholar]
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, et al. MAOA genotype, maltreatment, and aggressive behavior: The changing impact of genotype at varying levels of trauma. Biological Psychiatry. 2009;65:417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Phelps EA, editors. The human amygdala. New York: Guilford Press; 2009. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion; Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- White SF, Brislin S, Sinclair S, Fowler KA, Pope K, Blair RJR. The relationship between large cavum septum pellucidum and antisocial behavior, callous-unemotional traits and psychopathy in adolescents. Journal of Child Psychology and Psychiatry. 2012 doi: 10.1111/j.1469-7610.2012.02603.x. epub online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Brislin SJ, Meffert H, Sinclair S, Blair RJR. Callous-unemotional traits modulate the neural response associated with punishing another individual during social exchange: A preliminary investigation. Journal of Personality Disorders. 2013;27:99–112. doi: 10.1521/pedi.2013.27.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, et al. Reduced Amygdala Response in Youths With Disruptive Behavior Disorders and Psychopathic Traits: Decreased Emotional Response Versus Increased Top-Down Attention to Nonemotional Features. American Journal of Psychiatry. 2012;169:750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Williams WC, Brislin SJ, Sinclair S, Blair KS, Fowler KA, et al. Reduced activity within the dorsal endogenous orienting of attention network to fearful expressions in youth with disruptive behavior disorders and psychopathic traits. Development and Psychopathology. 2012;24:1105–1116. doi: 10.1017/S0954579412000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard HF, Ginsburg GS. Essentials of genomic and personalized medicine. San Diego: Academic Press; 2009. [Google Scholar]
- Woltering S, Granic I, Lamm C, Lewis MD. Neural changes associated with treatment outcome in children with externalizing problems. Biological Psychiatry. 2011;70:873–879. doi: 10.1016/j.biopsych.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nature Reviews Neuroscience. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behavioral Sciences and the Law. 2008;26:65–83. doi: 10.1002/bsl.788. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A. Functional neuroanatomy of psychopathy. Psychiatry. 2008;7:133–136. [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Research. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biological Psychiatry. 2005;57:1103–1108. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Lencz T, LaCasse L, Colletti P, et al. Localisation of increased prefrontal white matter in pathological liars. British Journal of Psychiatry. 2007;190:174–175. doi: 10.1192/bjp.bp.I06.025056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Smolen A, Hewitt JK, Haberstick BC, Stallings MC, Corley RP, et al. Interaction between MAO-A genotype and maltreatment in the risk for conduct disorder: failure to confirm in adolescent patients. American Journal of Psychiatry. 2006;163:1019. doi: 10.1176/ajp.2006.163.6.1019. [DOI] [PubMed] [Google Scholar]
- Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacology, Biochemistry and Behavior. 2002;71:857–865. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol–disinhibition pathway to substance use disorders: A multilevel developmental problem. Child development perspectives. 2011;5:248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]