Abstract
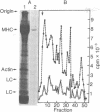
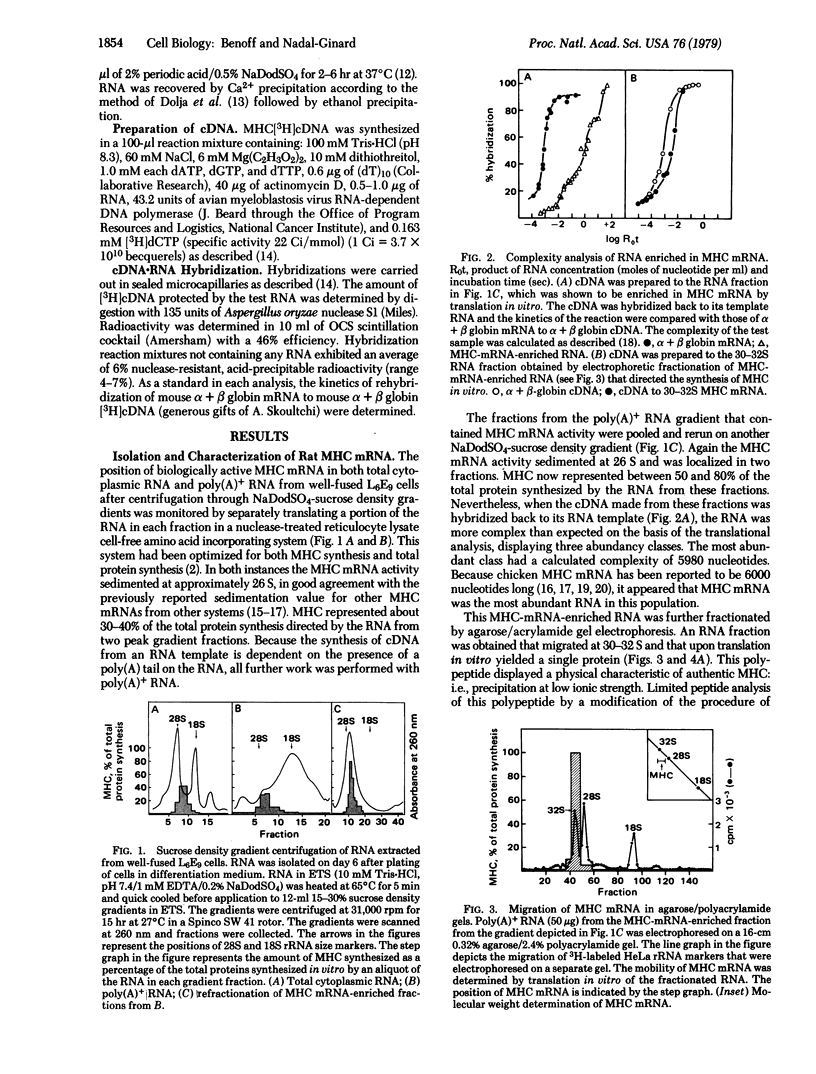
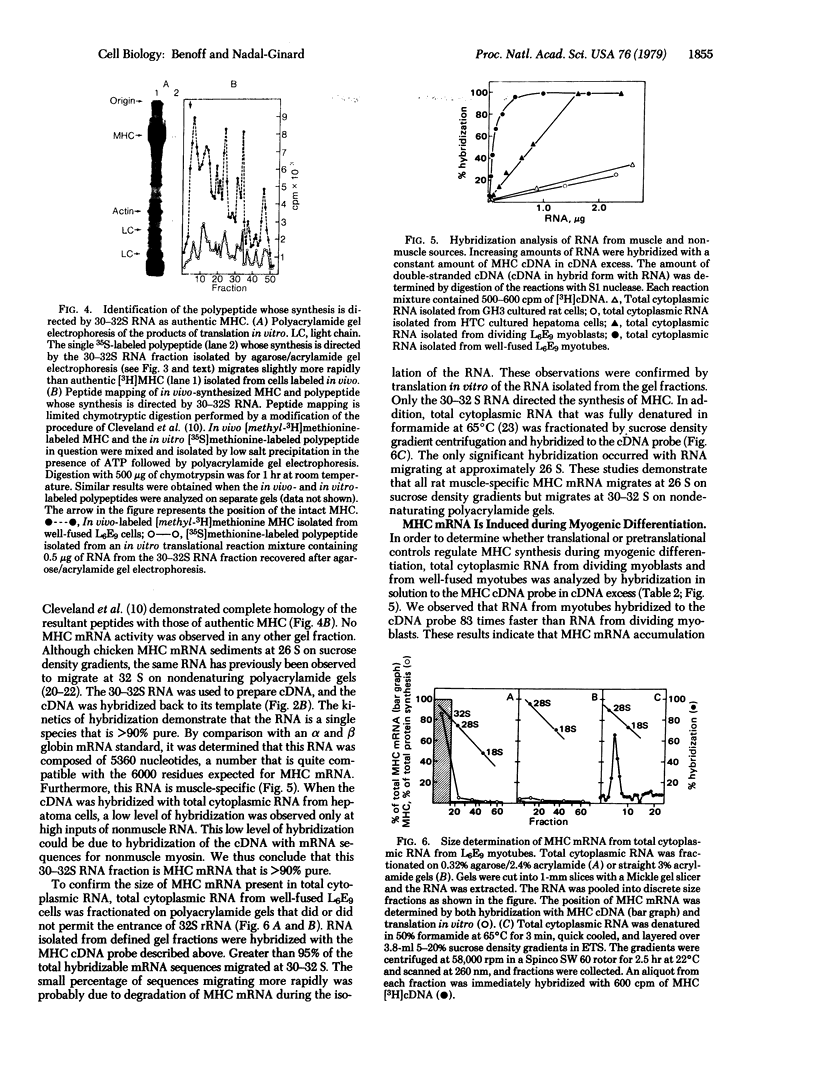
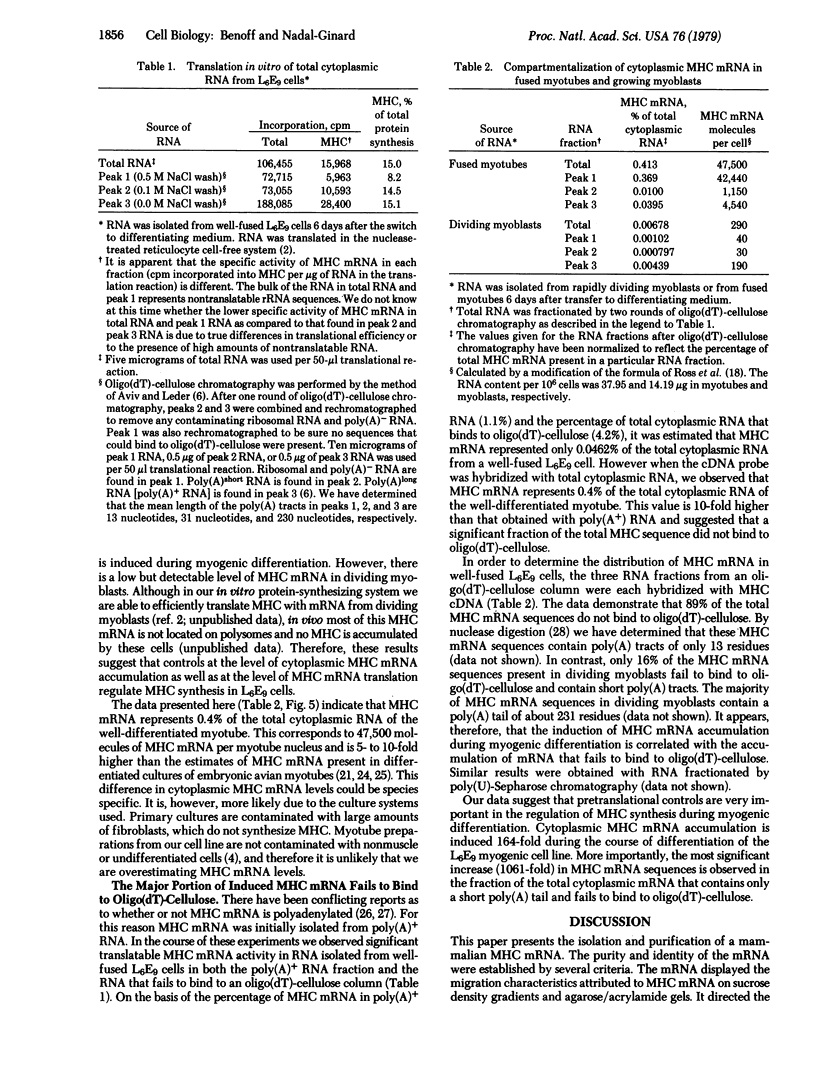
The mRNA for rat muscle myosin heavy chain (MHC) was isolated from L6E9 myotubes by two rounds of sucrose density gradient centrifugation followed by fractionation on an agarose/polyacrylamide gel. The purity of the mRNA isolated was determined by translation in vitro, peptide analysis of the in vitro product and comparison with authentic MHC, analysis of the kinetics of hybridization with cDNA prepared with this RNA, and titration analysis of total cytoplasmic RNA from muscle and nonmuscle sources. By using the MHC cDNA as probe of myogenic differentiation, it was observed that the level of cytoplasmic MHC mRNA increased approximately 200-fold as the dividing myoblast differentiated into the fused myotube. Titration analysis of RNAs fractionated by oligo(dT)-cellulose chromatography indicated that the majority of the increase occurred in that RNA population that failed to bind to an oligo(dT)-cellulose column.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S., Bruce S. A., Skoultchi A. I. Negative control of hemoglobin production in somatic cell hybrids due to heme deficiency. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4354–4358. doi: 10.1073/pnas.75.9.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. N. Cell-free translation of mammalian myosin heavy-chain messenger ribonucleic acid from growing and fused-L6E9 myoblasts. Biochemistry. 1979 Feb 6;18(3):494–500. doi: 10.1021/bi00570a019. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buckingham M. E., Caput D., Cohen A., Whalen R. G., Gros F. The synthesis and stability of cytoplasmic messenger RNA during myoblast differentiation in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1466–1470. doi: 10.1073/pnas.71.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. E., Cohen A., Gros F. Cytoplasmic distribution of pulse-labelled poly(A)-containing RNA, particularly 26 S RNA, during myoblast growth and differentiation. J Mol Biol. 1976 May 25;103(3):611–626. doi: 10.1016/0022-2836(76)90220-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dolja V. V., Negruk V. I., Novikov V. K., Atabekov J. G. A simple method for isolating pure RNA preparations after electrophoresis in polyacrylamide gel. Anal Biochem. 1977 Jun;80(2):502–506. doi: 10.1016/0003-2697(77)90673-x. [DOI] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Beckner S. K. Activation of myosin synthesis in fusing and mononucleated myoblasts. J Mol Biol. 1975 Apr 25;93(4):431–447. doi: 10.1016/0022-2836(75)90238-7. [DOI] [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S. Purification of myosin translational control RNA and its interaction with myosin messenger RNA. Biochemistry. 1976 Jul 27;15(15):3314–3319. doi: 10.1021/bi00660a023. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- John H. A., Patrinou-Georgoulas M., Jones K. W. Detection of myosin heavy chain mRNA during myogenesis in tissue culture by in vitro and in situ hybridization. Cell. 1977 Oct;12(2):501–508. doi: 10.1016/0092-8674(77)90126-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann Y., Milcarek C., Berissi H., Penman S. HeLa cell poly(A)- mRNA codes for a subset of poly(A)+ mRNA-directed proteins with an actin as a major product. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4801–4805. doi: 10.1073/pnas.74.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mondal H., Sutton A., Chen V. J., Sarkar S. Highly purified mRNA for myosin heavy chain: size and polyadenylic acid content. Biochem Biophys Res Commun. 1974 Feb 27;56(4):988–996. doi: 10.1016/s0006-291x(74)80286-x. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Patrinou-Georgoulas M., John H. A. The genes and mRNA coding for the heavy chains of chick embryonic skeletal myosin. Cell. 1977 Oct;12(2):491–499. doi: 10.1016/0092-8674(77)90125-8. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Przybyla A., Strohman R. C. Myosin heavy chain messenger RNA from myogenic cell cultures. Proc Natl Acad Sci U S A. 1974 Mar;71(3):662–666. doi: 10.1073/pnas.71.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Heywood S. M. Preparation and characterization of myosin copy DNA. Biochem Biophys Res Commun. 1976 Feb 9;68(3):918–926. doi: 10.1016/0006-291x(76)91233-x. [DOI] [PubMed] [Google Scholar]
- Robbins J., Heywood S. M. Quantification of myosin heavy-chain mRNA during myogenesis. Eur J Biochem. 1978 Jan 16;82(2):601–608. doi: 10.1111/j.1432-1033.1978.tb12056.x. [DOI] [PubMed] [Google Scholar]
- Ross J., Gielen J., Packman S., Ikawa Y., Leder P. Globin gene expression in cultured erythroleukemic cells. J Mol Biol. 1974 Aug 25;87(4):697–714. doi: 10.1016/0022-2836(74)90079-5. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Mukherjee S. P. Isolation of messenger ribonucleic acid for myosin heavy chain. Prep Biochem. 1973;3(6):583–604. doi: 10.1080/00327487308061540. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Gros F. The majority of calf muscle cell messenger RNAs contain poly(A). Biochim Biophys Acta. 1978 Jul 24;519(2):372–382. doi: 10.1016/0005-2787(78)90090-4. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Sawicki S. G., White P. A., Darnell J. E., Jr A correlation between the rate of poly(A) shortening and half-life of messenger RNA in adenovirus transformed cells. J Mol Biol. 1978 Nov 25;126(1):23–36. doi: 10.1016/0022-2836(78)90277-2. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. B., Goll D. E., Stromer M. H. Isolation of myosin-synthesizing polysomes from cultures of embryonic chicken myoblasts before fusion. Dev Biol. 1975 Nov;47(1):123–135. doi: 10.1016/0012-1606(75)90268-7. [DOI] [PubMed] [Google Scholar]