Abstract
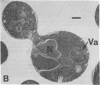
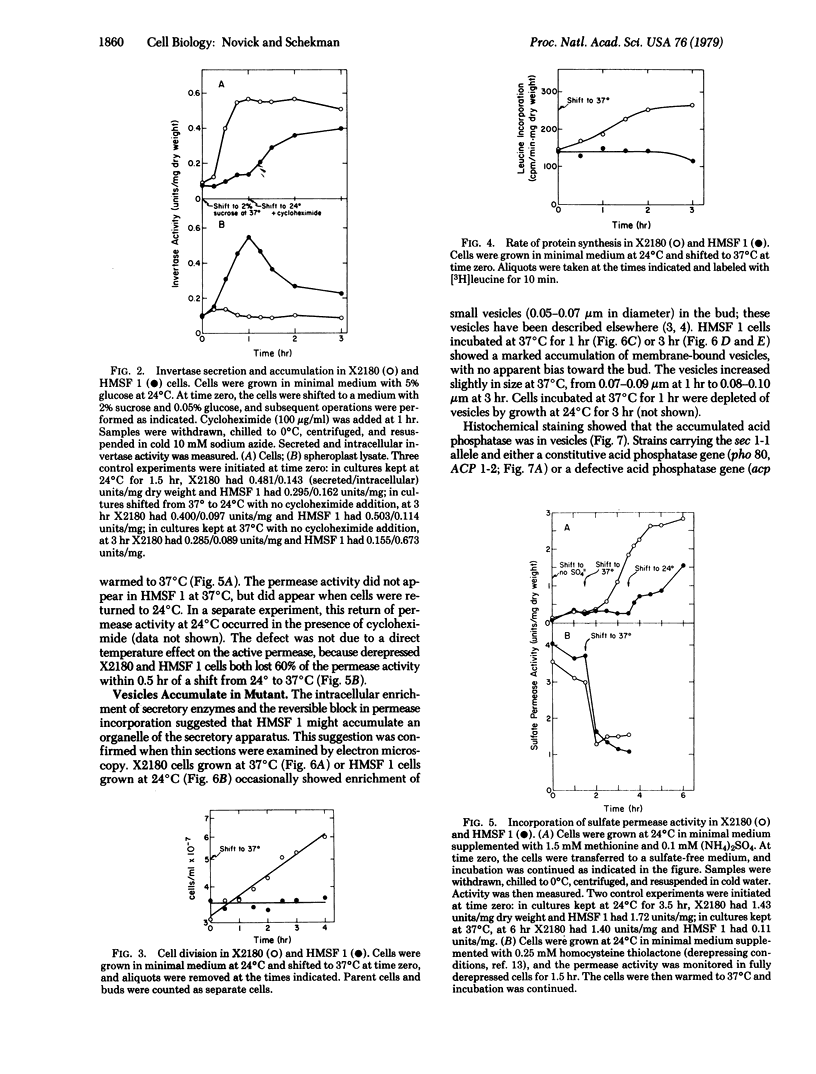
Saccharomyces cerevisiae cells contain a small internal pool of the secretory enzymes invertase and acid phosphatase. This pool increases up to 8-fold at 37 degrees C in a temperature-sensitive, secretion-defective mutant strain (sec 1-1). Cell division and incorporation of a sulfate permease activity stop abruptly at the restrictive temperature, while protein synthesis continues for several hours. Electron microscopy of mutant cells incubated at 37 degrees C reveals a large increase in the number of intracellular membrane-bound vesicles, which are shown by histochemical staining to contain the accumulated acid phosphatase. The vesicles are removed and the accumulated enzymes are secreted when cells are returned to a permissive temperature in the presence or absence of cycloheximide. These results are consistent with a vesicle intermediate in the yeast secretory pathway and suggest that exocytosis may contribute to cell-surface growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breton A., Surdin-Kerjan Y. Sulfate uptake in Saccharomyces cerevisiae: biochemical and genetic study. J Bacteriol. 1977 Oct;132(1):224–232. doi: 10.1128/jb.132.1.224-232.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975 Oct;124(1):511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A., Lampen J. O. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Hansche P. E., Beres V., Lange P. Gene duplication in Saccharomyces cerevisiae. Genetics. 1978 Apr;88(4 Pt 1):673–687. [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E., Kidby D. K. Effects of proteolytic enzymes on invertase secretion in sphaeroplasts of Saccharomyces: inhibition by trypsin. Can J Microbiol. 1977 Feb;23(2):202–208. doi: 10.1139/m77-029. [DOI] [PubMed] [Google Scholar]
- Linnemans W. A., Boer P., Elbers P. F. Localization of acid phosphatase in Saccharomyces cerevisiae: a clue to cell wall formation. J Bacteriol. 1977 Aug;131(2):638–644. doi: 10.1128/jb.131.2.638-644.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P., Cortat M., Wiemken A., Frey-Wyssling A. Isolation of glucanase-containing particles from budding Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1971 Mar;68(3):636–640. doi: 10.1073/pnas.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Matile P. H. Subcellular distribution of yeast invertase isoenzymes. Arch Microbiol. 1975 Mar 12;103(1):51–55. doi: 10.1007/BF00436329. [DOI] [PubMed] [Google Scholar]
- Moor H. Endoplasmic reticulum as the initiator of bud formation in yeast (S. cerevisiae). Arch Mikrobiol. 1967 Jun 6;57(2):135–146. doi: 10.1007/BF00408697. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S. Cell-free activity of a sulfate binding site involved in active transport. Proc Natl Acad Sci U S A. 1966 Jan;55(1):189–191. doi: 10.1073/pnas.55.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Brawley V. Localized deposition of chitin on the yeast cell surface in response to mating pheromone. Proc Natl Acad Sci U S A. 1979 Feb;76(2):645–649. doi: 10.1073/pnas.76.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn H. J., Boer P., Steyn-Parvé E. P. Biosynthesis of acid phosphatase of baker's yeast. Factors influencing its production by protoplasts and characterization of the secreted enzyme. Biochim Biophys Acta. 1972 May 12;268(2):431–441. doi: 10.1016/0005-2744(72)90339-7. [DOI] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- van Rijn H. J., Linnemans W. A., Boer P. Localization of acid phosphatase in protoplasts from Saccharomyces cerevisiae. J Bacteriol. 1975 Sep;123(3):1144–1149. doi: 10.1128/jb.123.3.1144-1149.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]