Abstract
Several drug delivery systems have been proposed to overcome physiological barriers, improving ocular bioavailability. Systemic routes are seldom used due to the blood-ocular barrier. Novel drug delivery systems based on nanotechnology techniques have been developed to overcome ocular physiological barriers. This non-systematic review suggests the utilization of a transitory blood-ocular breakdown to allow the access of drugs by nanotechnology drug delivery systems via the systemic route. We discuss the possible ways to cause the breakdown of the blood-ocular barrier: acute inflammation caused by intraocular surgery, induced ocular hypotony, and the use of inflammatory mediators. The suitability of use of the systemic route and its toxic effects are also discussed in this article.
Keywords: blood-ocular barrier, hypotony, nanotechnology, ocular drug delivery system, ocular inflammatory mediators, systemic route
1. Background
Most ocular diseases are treated with topical drug applications formulated as solutions, suspensions or ointments. Ophthalmic topical formulations bear some drawbacks related to poor ocular bioavailability due to the many anatomical and physiological barriers existing in the eye [1]. The oral and intravenous routes are seldom used in ophthalmology, because the blood ocular barrier impairs the achievement of satisfactory drug concentration in intraocular tissues [2,3].
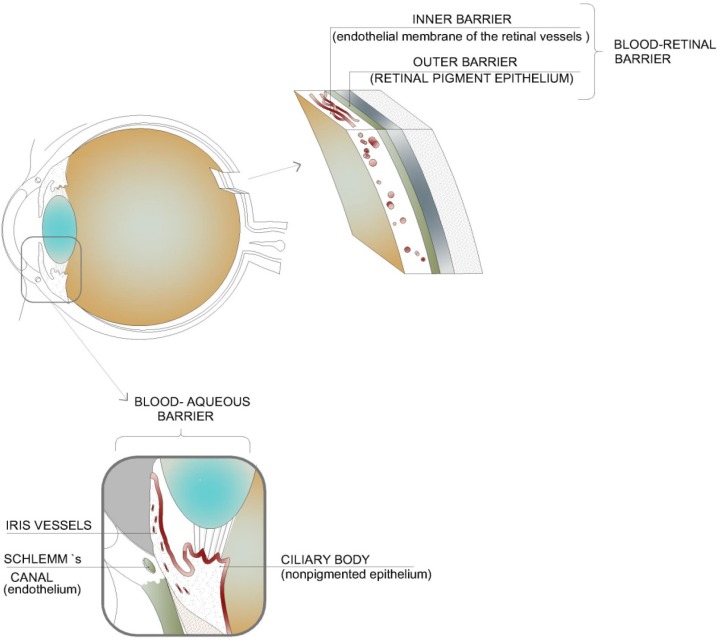
The blood ocular barrier is composed of the blood-aqueous barrier and the blood-retinal barrier, protecting the eye from entry of toxic substances and maintaining the homeostatic control that underpins the ocular physiology [4,5]. The blood-aqueous barrier is formed by the nonpigmented epithelium of the ciliary body, the posterior iris epithelium, the endothelium of the iris vessels with tight junctions of the leaky type, and the endothelium of Schlemm’s canal. The blood-retinal barrier consists of the retinal pigment epithelium (outer barrier) and the endothelial membrane of the retinal vessels (inner barrier), both with tight junctions of the nonleaky type. The two functional barriers restrict the movement of blood elements to the intraocular chambers [6] and explain why drugs administered orally or intravenously can hardly reach therapeutic levels in intraocular tissues (Figure 1).
Figure 1.
Blood-ocular barrier: blood-aqueous barrier and the blood-retinal barrier.
The barrier properties of the blood-brain barrier are similar to the blood-retinal barrier. Small protein tracers like microperoxidase (19 kDa) are capable of entering the perioxonal space but not the retina [6], indicating that the intercellular junctions of the retinal endothelium that are sealed with overabundant amounts of zonulae occludentes contribute to form the bulk of the blood-retinal barrier [7]. The ciliary epithelium of the ciliary body of the rabbit eye does not have as effective tight junctions as those found in the retina and in the brain capillaries [8]. Thus, the blood-aqueous barrier is not as active as the blood-retinal barrier in respect to limiting molecular diffusion. After intravenous injection, a lot of test substances, such as inulin, chloride, sucrose, phosphate, potassium, sodium, urea, proteins and some antibiotics could be found on the anterior side of the vitreous humor resulting from the ciliary circulation, but could not reach the retina due to the blood-retinal barrier [9].
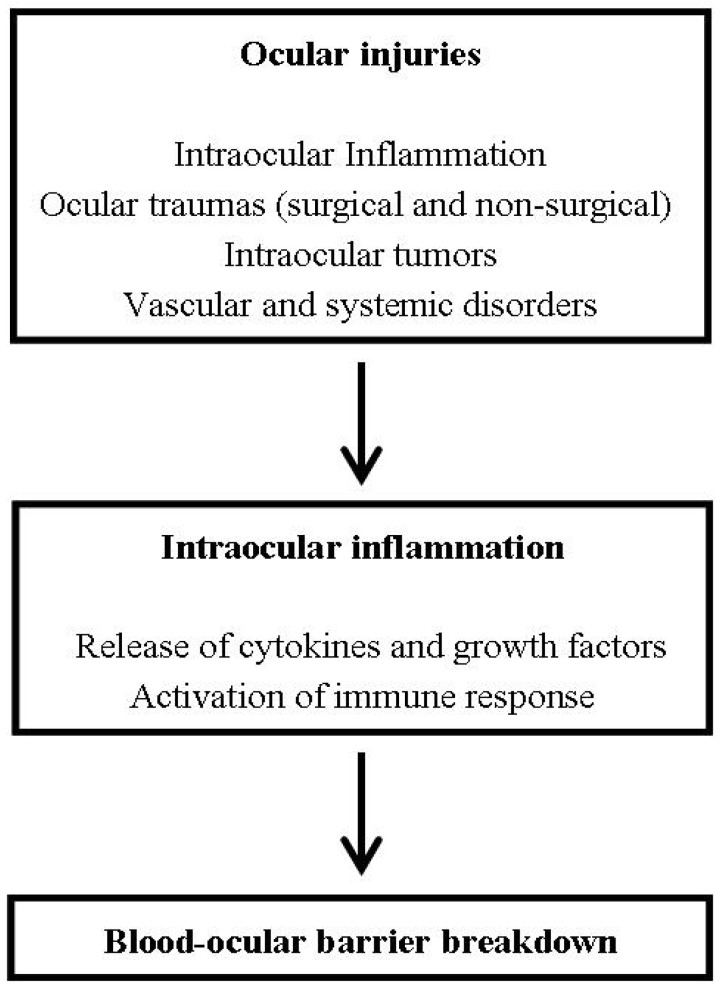
There are some situations when the breakdown of the blood-ocular barrier can occur, such as ocular injuries and ocular hypotonia. Many types of ocular injuries, such as surgical and non-surgical traumas, intraocular inflammation (e.g., uveitis, scleritis), vascular disorders (e.g., M. Coats, M. Eales), systemic disorders (e.g., diabetes) and intraocular tumors (e.g., retinoblastoma, uveal melanoma) can disturb the blood-ocular barrier, resulting in a variable inward movement of inflammatory cells and blood plasma constituents such as proteins, cytokines and growth factors [10] (Scheme 1).
Scheme 1.
Effects of ocular injuries on the blood-ocular barrier.
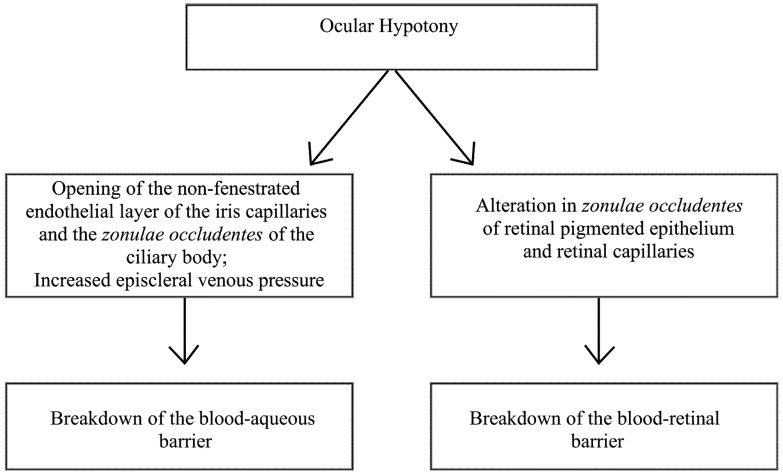
Ocular hypotony can promote the breakdown of the blood-aqueous barrier, permitting the leakage of serum proteins into the anterior and posterior chambers through the opening of the non-fenestrated endothelial layer of the iris capillaries and the zonulae occludentes of the ciliary body [11] (Figure 1). Ocular hypotony may also cause a breakdown of the blood-ocular barrier when intraocular pressure is lower than the episcleral venous pressure, leading to reversion of the flow direction of the ocular fluids, with plasma proteins entering the anterior chamber from the blood-filled Schlemm’s canal [12]. The blood retinal barrier is concomitantly broken down with the lowering of intraocular pressure, which affects the zonulae occludentes of the retinal pigmented epithelium (Verhoeff’s membrane) and the retinal capillaries [13,14,15] (Scheme 2).
Scheme 2.
Effects of ocular hypotony on the blood-ocular barrier.
The three commonly used ocular routes of drug administration are topical, local and systemic, but they bear limitations and may lead to complications. The topical route is frequently accompanied by toxicity in the cornea, conjunctiva and nasal mucosal [16,17,18,19]. Furthermore, daily topical instillation frequently leads to a lack of compliance [20,21]. Local toxicity can also develop with the use of subconjunctival and sub-Tenon routes. These procedures can cause muscle trauma [22], and inadvertent globe perforation [23,24]. Finally, retinal toxicity and infections may occur after intravitreal injections [25,26,27]. The systemic application of drugs is another method of reaching the posterior segment of the eye, but it is still a challenge due to the blood-ocular barrier. Despite the presence of an extensive vascular network in the choroid, the entry of drugs into the posterior segment of the eye is limited by the outer and inner blood-retinal barriers. In addition to the limitations imposed by the blood-ocular barrier, other facts limit the systemic application, since dilution and degradation of the drug before reaching the target site may occur. However, the systemic route could be a useful alternative, since this route is less invasive and the patients prefer it to the local route in the treatment of some diseases. There is the additional benefit of facilitating out-patient treatment [28].
Innovative techniques of drug delivery are required to overcome the limitations of systemic treatment of ophthalmic disorders. One of these strategies is the use of nanoparticles in drug delivery systems, that can be intravenously administrated and that overcome the physiological barriers.
2. Nanosystem
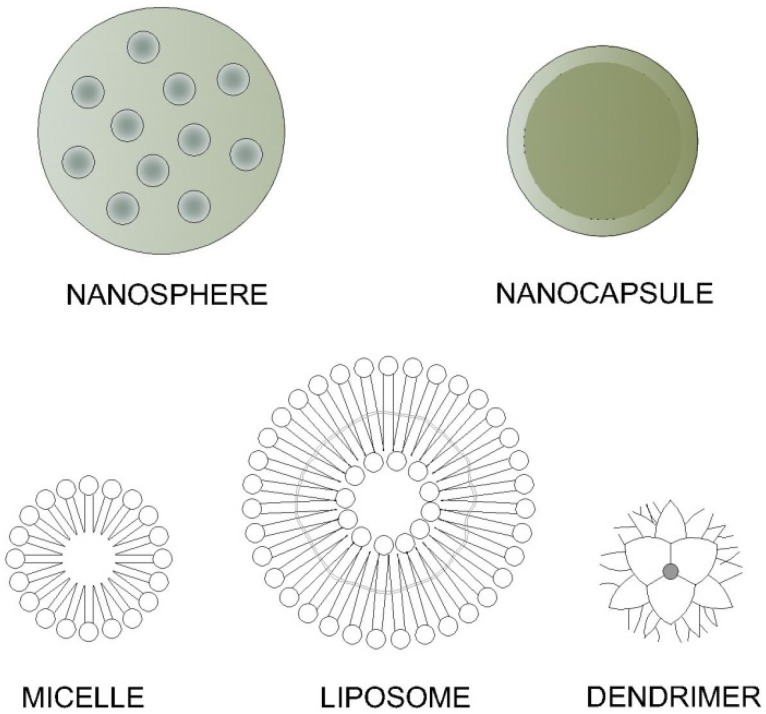
Any physical system that is engineered at nanoscale is defined as a nanosystem. Nanosystems include nanoparticles (nanospheres and nanocapsules), liposomes, dendrimers, niosomes, and soluble macromolecules, amongst others. Some examples of these systems are shown in Figure 2.
Figure 2.
Examples of nanosystems [29].
Nanoparticles are spherical particles varying in size from 10 nm to 1 µm, containing a drug molecule or not. They can be prepared using materials of different chemical classes, for example proteins, lipids, polymers or polysaccharides [30]. The active agent may be encapsulated/entrapped, dissolved or distributed to a nanoparticle matrix, and then can be broadly classified into nanospheres, nanoparticles or nanocapsules.
Nanoparticles can have the ability to overcome physiological barriers, passing even through capillaries, and transporting the drug to specific cells or intracellular compartments by several targeting mechanisms [31,32,33,34]. Singh et al. used arginine-glycide-aspartic acid peptide or dual-functionalized poly-(lactide-co-glycolide) nanoparticles to target delivery of anti-vascular endothelial growth factor (VEGF) intraceptor plasmid to choroidal neovascularization lesions in rats. They demonstrated that nanoparticles enable targeted gene delivery to the neovascular rat eye but not the control eye [35].
Liposomes are membrane-like vesicles that consist of one or more concentric phospholipid or cholesterol bilayers designed to carry a drug either into the core or into the bilayer. They can have a positive, negative or neutral surface charge, depending on their chemical composition, and they present different properties, such as binding affinity [36].
Light-targeted liposomal delivery was employed after systemic administration of thermosensitive liposomes for the diagnosis and therapy of chorioretinal neovascularization in age-related macular degeneration, showing the possibility of targeting a particular tissue [37]. In this approach, a liposomal formulation of a light-sensitive delivery system was selectively withheld in neovascular areas of the eye for the treatment of age-related macular degeneration, which demonstrated targeted therapy, even though this therapy was not completely successful in its purpose of destroying abnormal blood vessels without causing damage to normal surrounding tissues.
Dendrimers, macromolecular compounds from which a number of highly branched, tree-like arms originate in a symmetric fashion [38], are considered attractive for biomedical applications due to their unique physicochemical properties. As the dendrimer’s size can be controlled based on the stepwise chemical synthetic processes of their generation, they can become similar in size to a number of biological structures, such as G5 polyamidoamine dendrimers with the size of a hemoglobin molecule [39]
Niosomes are bilayered nonionic surfactant vesicles chemically stable with the ability to carry both lipophilic and hydrophilic drugs. Niosomes are nonimmunogenetic, biodegradable and biocompatible, and show low toxicity due to their nonionic structure [40].
Micelles are aggregates of amphiphilic molecules dispersed in a liquid colloid. Some micelar formulations for topical applications seem to be promising, but one of the major disadvantages of micellar formation is the demicellization that happens as a result of dilution upon injection in vivo [29].
Macromolecules in nanometer dimensions, when present in solution, including virtually any drug, can provide special properties to the drug molecule. Macugen®, a marketed treatment for neovascular age-related macular degeneration, is a nanoconjugate of an oligonucleotide (aptamer) with two polyethylene glycols (PEG) that prolongs the half-life of the drug in the vitreous humor [41].
Our laboratory has pioneered the use of artificially made, solid nanoparticles directed to therapeutic targets, called LDE [42]. This nanoemulsion has a structure similar to that of a low-density lipoprotein (LDL), with a core constituted of cholesteryl esters with trace amounts of triglycerides, surrounded by a phospholipid monolayer; at the surface monolayer there is a small proportion of free cholesterol. When the LDE is injected into the bloodstream, it acquires apo E from the native lipoproteins and binds to LDL receptors that recognize both apo B and apo E [42]. The mechanism behind this drug delivery system lies in the overexpression of cell surface receptors that bind and internalize LDL, as well as the artificial lipid nanoemulsions in tissues undergoing cell proliferation [43,44,45]. This drug-targeting nanoemulsion system can be used not only for cancer treatment, but also to carry drugs directed against atherosclerotic lesions in rabbits [46,47,48]. We also showed that the intense immunogenic-inflammatory processes in rabbits following heart transplantation could be attenuated when using nanoemulsion associated with anti-proliferative drugs [49].
Applications of Nanosystems in Ophthalmology
Constant efforts have been made toward the development of ocular drug delivery systems with various pharmacological properties, such as increased bioavailability, prolonged drug release, compatibility with ocular tissues, ease of use in the form of eye drops causing no blurred vision or irritation and fewer instillations to obtain the intended therapeutic effect.
There are some interesting studies about topical applications of nanotechnological drug delivery systems for ophthalmic treatments. Adibkia et al. showed that piroxicam nanoparticles of Eudragit RS 100 lead to a better control of inflammatory symptoms compared with a microsuspension of piroxicam alone in rabbits with endotoxin-induced uveitis [50].
De La Fuente et al. have shown that a hyaluronic acid-chitosan nanoparticles system was successfull in transfection experiments for ocular gene therapy. The results demonstrated that bioadhesive polysaccharides, hyaluronic acid and chitosan nanoparticles were able to provide high transfection levels in cornea and conjunctiva, without affecting cell viability [51].
Ciclosporin-loaded nanoparticles are also used for the treatment of extraocular disorders, such as keratoconjuctivitis sicca. In vivo experiments showed that, following topical instillation of cyclosporin-A-loaded chitosan nanoparticles into rabbits, it was possible to achieve therapeutic concentrations in external ocular tissues (i.e., cornea and conjunctiva) during at least 48 h while maintaining negligible cyclosporin-A levels in inner ocular structures. Additionally, the chitosan nanoparticles levels were significantly higher than those obtained following instillation of a chitosan solution containing cyclosporin A and an aqueous cyclosporin suspension [52]. Table 1 shows some other nanostructured drug delivery systems used in ophthalmology.
Table 1.
Examples of nanotechnology based ocular drug delivery systems.
| Active Agent | Drug Delivery System | References |
|---|---|---|
| Ibuprofen
Ciclosporin Diclofenac sodium |
Solid lipid nanoparticles | [53,54] |
| Ciclosporin
Dexamethasone Pilocarpine |
Nanoemulsion | [55,56] |
| Dexamethasone | Micelles | [57] |
| Flurbiprofen | Nanosuspension | [58] |
| Timolol maleate | Discosomes | [59] |
| Pilocarpine nitrate
Dexamethasone |
2-hydroxypropyl-â-cyclodextrin | [60,61] |
| Acetazolamide
Inulin Oligonucleotides Pilocarpine hydrochloride Diclofenac sodium |
Liposomes | [62,63] |
| Tropicamide
Pilocarpine nitrate |
Dendrimers | [64] |
| Cyclopentolate | Niosomes | [65] |
Since studies about the use of nanosystems applied systemically for ocular permeation are still scarce, further studies on the development of nanosystems are necessary to accomplish the purpose of ocular delivery of the desired drug in an efficient and safe way.
3. Systemic Administration
Despite the limitations of the systemic route to ocular drug delivery, researchers have made continuous efforts to develop drug delivery systems to administer drugs systemically in order to clear these barriers.
Among the challenges of effective systemic nanodelivery into tissues, the limited penetration across the vascular endothelium and the uptake by the reticuloendothelial system are considerable factors. The current nanodelivery systems depend on transvascular exchange and tissue accumulation, which require high dosages to create large concentration gradients to drive nanoparticles passively across the blood-tissue interface [66]. Nevertheless, passive accumulation allows only a fractional dosage of nanoparticles penetrating into target tissues, which diminishes therapeutic efficacy and aggravates potential side effects. Therefore, active delivery of targeted nanoparticles across the vascular endothelium could increase the desired therapeutic index with fewer side effects [66]. In addition, other facts limit the systemic application, since dilution and degradation of the drug before reaching the target site can occur. Furthermore, drug-drug interactions in patients being treated for coexisting diseases may also influence the administration of systemic drugs [66].
The use of nanoparticles that have a higher selectivity for the target tissue is a challenge to be achieved to improve systemic administration. Hence, specific targeting systems are needed to transport molecules to deeper layers of the eye, overcoming the blood-ocular barrier. Recent studies have shown promising results with systemic administration of nanoparticles. Kim et al. have demonstrated that 20 nm gold nanoparticles, administered intravenously, were able to pass through the blood-retinal barrier and were distributed into all retinal layers [67]. The absence of toxicity was noted in retinal endothelial cells, astrocytes and retinoblastoma cells [67]. Another recent study demonstrates that transferrin, arginine-glycine-aspartic acid peptide, or dual-functionalized poly-(lactide-co-glycolide) nanoparticles carrying anti-VEGF intraceptor plasmid, administrated intravenously, were able to reach choroidal neovascularization lesions, probably due to the broken blood-retinal barrier as a result of choroidal neovascularization in the laser-treated rat eye [39].
The similarities between the blood-brain and the blood-retinal barriers allow the proposal of the hypothesis that there is the possibility of systemically administered nanoparticles also gaining the ability to pass through the ocular barrier. Dalargin, a hexapeptide analog of leucine-enkephalin containing D-alanine, which produces central nervous system analgesia, can cross the blood-brain barrier when conjugated with poly(butylcyanoacrylate) nanoparticles and accumulate in the brain of mice, but not when it administrated without nanoparticles [68]. Furthermore, these studies on blood-brain barriers suggest the future application of systemically administered nanosystems for ophthalmic purposes.
Kinetics of Systemically Administered Nanoparticles
The systemic use of nanoparticles in order to reach ocular tissues is attractive, but more accurate kinetics studies are needed to show the real possibility of their safety and effectiveness.
In vitro experiments should have some attributes to be accurate, such as the ability to measure pathological situations separately (e.g., oxidative stress and inflammation), and the possibility to represent cell types targeted by nanoparticles through common routes of exposure (e.g., macrophages and other elements of the reticuloendothelial system) [69,70].
In vivo biodistribution of nanoparticles is a new and wide field of research. Several studies have been published recently describing the nanoparticle distribution, but, even though the attempts to modify their features, such as size, shape, surface coating and dosing, aiming to enhance treatment effectiveness by extending the blood circulation, nonspecific distribution has thus far been unavoidable. Excretion of nanoparticles is a concern that needs further long-term studies, since a recent study showed the variation in the levels of nanoparticle excretion dependent on time. Lastly, there is no standardized characterization of possible interaction of nanoparticles with proteins and immune cells [29].
A list of some examples of pharmacokinetic experimental and clinical studies using nanoparticles is summarized in Table 2.
Table 2.
Examples of pharmacokinetic nanoparticle in vitro and in vivo studies.
| Author | Study Approach | Reference |
|---|---|---|
| Shokeen et al. | Radiolabeled nanoparticles with positron emitting radionuclides along in vitro and in vivo protocols. | [71] |
| Almeida et al. | Comprehensive review of in vivo biodistribution of nanoparticles, including structure, toxicity and in vitro comparison. | [72] |
| Khlebtsov et al. | Comprehensive review of in vitro and in vivo biodistribution of nanoparticles, with nanoparticle parameters, used doses and toxicity. | [73] |
| Pires et al. | Treatment of breast cancer using paclitaxel associated to an artificial lipid nanoemulsion, emphasizing the improved pharmacokinetic parameters and the reduced toxicity of the drug. | [74] |
| Maranhão et al. | Plasma kinetics and biodistribution of an artificial lipid nanoemulsion in patients with acute leukemia. | [43] |
| Azevedo et al. | Plasma kinetics and biodistribution of an artificial lipid nanoemulsion associated to etoposide oleate in patients with ovarian carcinoma. | [44] |
| Saha et al. | Review of preparation, characterization, biodistribution and pharmacokinetic of nanosystems for cancer chemotherapy. | [75] |
4. Toxicity of Nanoparticle Systems
The evaluation of systemic nanosystem toxicity is a complex task, since the drug-delivery systems have to take into account the toxicity of the polymer used, the drug, and entire system after administration in vivo. Moreover, systemic use of nanoparticles can induce changes in every organ of the human body, which may cause alterations in blood clinical chemistry, liver function, kidney function, blood cell counts, and other unknown side effects.
The establishment of a complete risk-benefit ratio of the use of nanoparticles by systemic routes depends on the system configuration, specific materials used, dosage, and frequency of administration, besides the individual patient factors. Any change in these elements can affect the immunological response, cellular uptake, excretion, and, by consequence, the toxic effects elicited by the nanoparticle.
New methods and biological models are necessary to increase the research in nanotoxicology. Recent toxicology studies seem to be promising to reach new evaluation standards. Kong et al. have shown the most important factors affecting nanoparticle cytotoxicity assays due to the current lack of standardized protocols, in order to improve the method that could evaluate the toxicity without false-negative or false-positive misinterpretations [76].
Almeida et al. have recently published an extensive review about nanoparticle biodistribution and toxicity [72]. The authors pointed out that nanoparticle modifications can be made to prolong blood circulation and enhance treatment efficacy, but simultaneous nonspecific distribution is unavoidable, as well as the need for long-term in vivo studies to properly investigate the innate and adaptive immune response, excretion over time and toxicity of nanoparticles. Another important point of this study is the need for more systematic in vitro approaches to improve the correlation with in vivo work, and to clarify the real nanoparticle therapeutic action and side effects [72].
Stensberg et al. have shown the activity, transport and fate of silver nanoparticles at the cellular and organismic level, in conjunction with the available methods of nanoparticle characterization [77]. They proposed several mechanisms of cytotoxicity based on such studies, as well new opportunities for investigating the uptake and fate of silver nanoparticles in living systems [77].
Maranhão et al. have shown that the association of chemotherapeutic agents, such as carmustine, etoposide, and paclitaxel, with artificial lipid nanoemulsion (LDE) pronouncedly reduces the toxicity of those drugs while preserving or even increasing the antineoplastic activity, as shown in tumor-implanted rats and mice [78,79] and in trials enrolling patients with advanced cancers [43,44,45,74].
There are recent reports regarding the toxicity concerns of the use of nanoparticles for ophthalmic purposes. Escobar et al. have shown that budesonide-PLA microparticles administered periocularly in rabbits remained at the periocular site of injection for a long time without causing local side effects, such as elevation in intraocular pressure, lens opacity or blood chemistry changes [80]. In addition, Amrite et al. have demonstrated that periocular administered celecoxib-PLGA particles were useful sustained drug delivery systems for inhibiting diabetes-induced elevation in PGE2 and VEGF without inducing fibrotic reactions at the site of administration or damage to the retina [81].
The systemic use of nanoparticle drug delivery systems for ophthalmic treatments is rare, equally scarce are the corresponding toxicological studies regarding this route of administration.
5. Hypothesis
The breakdown of the blood-ocular barrier is an attractive manner to allow the penetration of systemically applied drugs into the eye. We hypothesize that the breakdown of the blood-ocular barrier—that may occur in acute inflammation following intraocular surgeries, induced ocular hypotony and controlled use of inflammatory mediators—could allow the use of drugs carried in targeting nanosystems administered by the systemic route for ophthalmic purposes.
6. Blood-Ocular Barrier Breakdown
Blood-ocular barrier breakdown can occur in some situation, such as acute inflammation caused by intraocular surgery, induced ocular hypotony, and the use of inflammatory mediators.
6.1. Ocular Surgeries
The majority of the intraocular surgical procedures may cause a breakdown in the blood-ocular barrier, leading to increased protein content in the aqueous humor and edema of the sensory retina, due to the induced inflammation [82]. The spectacular advances in intraocular microsurgery of both the anterior and posterior segment in recent years allow surgeons to perform minimally traumatic procedures. However, every surgical manipulation of the eye causes ocular inflammation and may lead to many adverse effects [83], if not inhibited by the use of anti-inflammatory medication.
The sequence of events that follow ocular trauma includes vasodilatation, increased blood flow and hyperemia, increased permeability of blood vessels, edema, increased tissue pressure (disrupted blood-ocular barrier), and the presence of inflammatory cells [82].
Almost all morphological, physiological and metabolic responses to trauma may be attributed to the synthesis and release of endogenous mediators, such as prostaglandins [84], leukotriens, and various cytokines such as interleukins, tumor necrosis factor α and VEGF [85], causing a breakdown of the blood-ocular barrier. The critical phase of inflammation caused by surgical trauma begins at the conclusion of surgery and continues up to the first postoperative week [83,86]. The subconjunctival corticosteroid injection at the end of the surgical procedure is the current practice [87]. Thus, theoretically, the correct time for the use of systemic medication, as an alternative to reduce ocular inflammation, would be at the conclusion of surgery, taking advantage of the breakdown of the blood-ocular barrier.
6.2. Induced Ocular Hypotony
The mean intraocular pressure is described statistically to be around 15.9 ± 2.9 mmHg [88]. Therefore, hypotony could be defined as an intraocular pressure below two standard deviations from the mean. In recent years, most authors [89,90,91,92] have been considering an intraocular pressure of ≤5 mmHg as clinically relevant. However, using a single cut-off value to define hypotony may be inadequate, since low intraocular pressure can be completely asymptomatic, without causing any irreversible functional damage [93].
Transient hypotonia, when it is resolved in fifteen to thirty days, is relatively common in clinical practice and usually presents no functional sequelae [94]. On the other hand, acute hypotonia can cause structural changes, depending on various factors such as the degree of hypotony, its duration and individual eye conditions, although most of the time it is anatomically and functionally reversible [95].
Currently, the only possible way to induce transient ocular hypotony is by performing a paracentesis. Paracentesis is widely used in ophthalmic microsurgery [96] and may also be performed at the slit lamp as an outpatient procedure. Some authors have shown the feasibility and safety of performing an immediate anterior chamber paracentesis, combined with antiglaucomatous drugs, to relief the symptoms of acute primary angle-closure glaucoma [97,98,99,100].
Induced ocular hypotony could only be considered if the paracentesis could be performed in a controlled way, with a minimal duration and extent. The leakage of aqueous through corneal paracentesis should be quick enough to decrease the intraocular pressure without causing the anterior chamber to collapse.
The only justification to perform a paracentesis to induce a transitory ocular hypotony, causing the breakdown of the blood-ocular barrier, would be the availability of an effective drug that would reach long-term therapeutic levels, requiring maybe one or two procedures per year. Drugs with a short life could not be used in this scenario, since repetitive paracentesis could be associated with an unacceptable risk of infection and other potential complications.
Controlled experiments investigating hypotony are scarce. Nevertheless, the occurrence of the breakdown of the ocular-blood barrier could be achieved by the induction of an iatrogenic, surgically controlled hypotony. Thus, the hypotony became a target ocular pressure through an induced condition to allow the opening of the natural vascular fenestrations into the eye. The risk of infection from a paracentesis is possibly lower than that of an intravitreal injection that penetrates into the back of the eye. However, this invasive procedure limits its use, unless one or few intravascular injections are enough to achieve the intended therapeutic response.
Concomitantly, there are no current available hypotensive topical drugs that permit a potent decrease of the intraocular pressure in order to avoid a surgical procedure. Furthermore, the available systemic hypotensive drugs are nonselective and the aggressive side effects limit their current use. A model of chronic hypotony in an animal model has already been described [101]. The development of models of acute hypotony, associated with rigorous pharmacokinetic and anatomopathological studies, could clarify our questions about the viability of this hypothesis. Further studies on the induction of acute hypotony are necessary to establish the safety of the method, although the several studies on transient ocular hypotony have shown that, if there are no associated comorbidities, the ocular anatomy and functions are gradually normalized from the point in time that the intraocular pressure is corrected [102].
6.3. Inflammatory Mediators
Inflammatory mediators have been studied mainly in experimental uveitis models [103,104,105,106,107], which cause the breakdown of the blood-ocular barrier. Several inflammatory mediators have been identified during intraocular inflammation, such as prostaglandins, leukotrienes and hydroxieicosatetraenoic acids, all chemical mediators from the arachidonic acid cascade. Other identified active mediators of the immune and inflammatory response include platelet-activating factor, interleukin-1, and tumor necrosis factor [108,109], while others still remain unidentified.
Foxman et al. have demonstrated the inflammatory mediators during T-cell mediated ocular inflammation, such as chemokines and their receptors and cytokines, besides indicating the cytokines as potential therapeutic targets [110]. The accumulation of these mediators increases the vascular permeability, leading to the breakdown of the blood-ocular barrier and to the consequent onset of pathophysiological responses [82]. Inflammatory mediators such as the tumor necrosis factor-alpha (TNFα) have been identified as an underlying factor causing the late blood-retinal barrier breakdown in diabetic retinopathy [111]. Huang et al. have shown that TNFα could be a therapeutic target for the prevention of the progressive blood-retinal barrier breakdown, retinal leukostasis, and apoptosis associated with diabetic retinopathy [112]. Although some authors have demonstrated the capacity of inflammatory mediators to induce acute inflammatory reactions in vivo, few studies have evaluated the morphological and functional responses to inflammatory mediators in the eye [113].
Interestingly, ocular inflammation has also been experimentally induced through the use of synthetic mediators. The complement component C5a, N-formylmethionyl-leucyl-phenylananine (FMLP) and chemotactic mediators were injected into the rabbit cornea, vitreous, and skin in order to induce a reaction resembling the “Arthus phenomenon” [114]. Miyake et al. have demonstrated that topically used epinephrine can induce the partial disruption of the blood-ocular barrier due to the release of prostaglandins and other cyclooxygenase products [115]. Elliot et al. have demonstrated that bradykinin agonist RMP-7 could, experimentally, enhance the permeability of the blood-ocular barrier [116]. This class of drug may be used as a useful adjunct to achieve an enhanced delivery of therapeutics to the eye under conditions in which blood-ocular barriers limit treatment.
Inflammatory mediators are not the only way to promote the breakdown of the blood-ocular barrier. Puerarin is an isoflavonoid derived from Radix puerariae that has been investigated as a self-microemulsifying drug system. Deng et al. have shown that puerarin can penetrate the blood-ocular barrier accessing aqueous humor and vitreous humor after systemic administration [117]. This pharmacokinetic study can provide a principle basis for treating eye diseases with puerarin by systemic administration.
In the same way, recent studies have also demonstrated the possibility of rapid suppression of ocular inflammation, leading to restoration of the blood-ocular barrier. Copland et al. have shown that Fingolimod (FTY720), a drug used in FDA phase III human multiple sclerosis trials, prevents T-cell migration to inflammatory sites by decreasing expression of the sphingosine-1 phosphate receptor generally required for egression from secondary lymphoid tissue [118]. Fingolimod seems to be a promising anti-inflammatory drug for the treatment of ocular immune-mediated inflammation, where even a single-dose treatment was effective, and repeated doses did not affect the vascular integrity of the blood-ocular barrier [118]. Thus, the induction of a transient breakdown of the blood-ocular barrier could be immediately followed by its reversal, as soon as the intended therapeutic effect is achieved.
Therefore, the breakdown of the blood-ocular barrier could also be obtained by deliberate utilization of inflammatory mediators. However, the deliberate use of specific inflammatory mediators would only be justified based on this hypothesis, if the topical route was utilized to promote the transitory breakdown of the blood-ocular barrier. After the topical application of the inflammatory drug, the systemic administration of the specific nanosystem would be held, followed by the topical pharmacological neutralization of the inflammatory mediator previously applied, as soon as the delivery rate time of the systemic drug was concluded in order to yield therapeutic drug levels in ocular tissues.
6.4. Measuring the Blood-Ocular Barrier Breakdown
When the aqueous-blood barrier is broken down, there is an inward movement of plasma constituents and cells to the anterior chamber. Since 1988, it is possible to quantify, in vivo, the aqueous flare by measuring the scatter of a laser beam that is scanned into the anterior chamber. This reproducible and noninvasive procedure designed to measure the integrity of the blood-aqueous barrier is performed with a flare meter and is called tyndallometry [119,120,121,122,123,124].
Several methods of examination are currently used to evaluate the function of the blood-retinal barrier [125]. Vitreous fluorophotometry [126,127] is a technique to quantify blood-retinal barrier permeability. Optical coherence tomography is a widespread method that allows good quality image acquisition in order to evaluate blood-ocular barriers [128]. Fluorescein angiography is method to obtain excellent qualitative images of the retina, but carries some restrictions because iodinated contrast may cause severe allergic reactions and needs to be applied by an intravenous injection [129]. Contrast-enhanced magnetic-imaging is a modern and sophisticated method to measure the aqueous and vitreous humor protein concentration due to vascular leakage [130,131], but also requires an intravenous administration of gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA) or other contrast agents, which limits its use in everyday practice. A recent development, the Retinal Leakage Analyzer [132], maps blood-retinal barrier changes by a multimodal macula mapping, and probably will offer an interesting additional tool to register the blood-ocular barrier dynamics.
Thus, according to our proposition, the breakdown of the blood-ocular barrier could be monitored by the quantification of the aqueous flare and/or vitreous flare, which would help to indicate the correct time of the administration of the desired systemic medication.
6.5. Future Perspectives of the Possibility of Taking Advantage of Blood-Ocular Barrier Breakdown to Access Intraocular Tissues
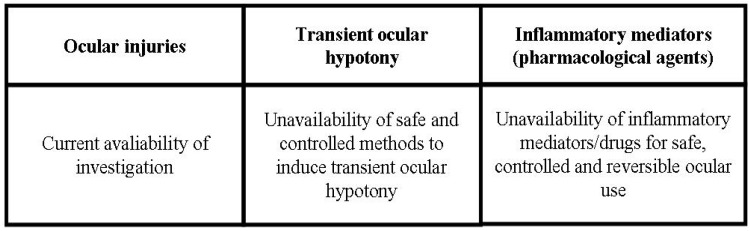
In summary, there are several variables that can occur in the three types of breakdown of ocular barriers, namely, in eye diseases or ocular surgeries—the intensity of inflammatory reaction; in ocular hypotony—its intensity and duration; and in the use of inflammatory mediators—the dose and specific pharmacological characteristics of the drugs. Thus, it is not currently possible to designate a preferred potential method of breakdown of the blood-ocular barrier, since the intensity and duration of the breakdown of the blood-ocular barrier are parameters highly dynamic due to the wide range of pathophysiological variables. The current absence of reliable and safe methods to induce the rupture of the blood-ocular barrier is also another important concern to establish dependable means to achieve this hypothetical goal. The current status of use or investigation of each types of breakdown of ocular barriers is summarized in Scheme 3.
Scheme 3.
Status of investigaton of the natural and induced ways to the breakdown of blood-ocular barriers.
7. Conclusions
The proposition presented in this article refers to the possibility of taking advantage of the breakdown of the blood-ocular barrier to use the systemic route as an alternative to access intraocular tissues. Monitoring this breakdown is currently possible, indicating the correct time to use intravascular medication. Besides the natural enhanced permeability of the blood-ocular barrier in ophthalmic surgeries, we suggest two possible ways to induce a transient, controlled breakdown of the blood-ocular barrier, both with limitations that need to be overcame.
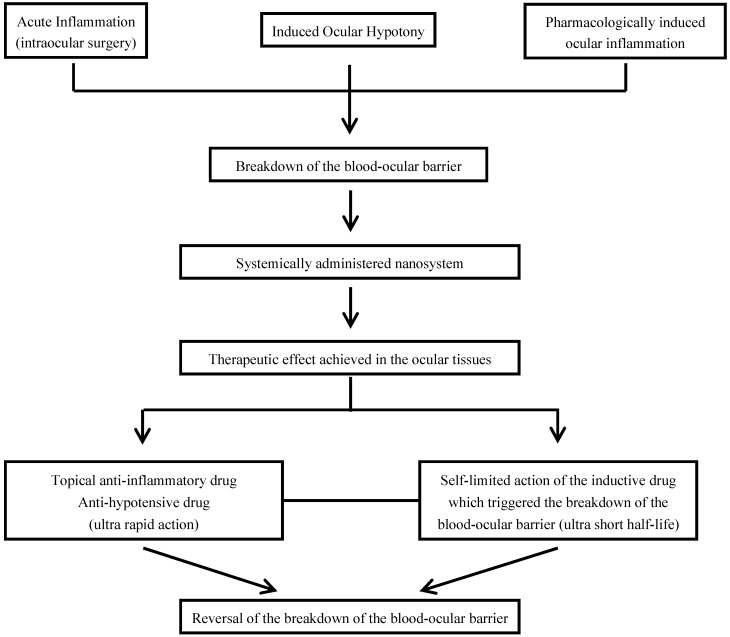
The induced breakdown of the blood-ocular barrier would be an interesting and acceptable option to facilitate the ocular access of the systemically administered nanosystem, if a topical route is chosen to administer pharmacological agents that induce it, and likewise, other topical agents are used to reverse it after the therapeutic effect is achieved. Apart from the invasive fact of the intravenous administration of the nanosystem, only non-invasive additional topical methods would be plausible to be applied concurrently. Moreover, the reversion of the breakdown of the blood-ocular barrier, when induced, would be achieved with the use of topical anti-inflammatory or anti-hypotensive drugs. The utilization of short half-life inductive drugs would be essential to carry out a self-limiting process. Scheme 4 summarizes the hypothetical pathways that are taken advantage of the breakdown of the blood-ocular barrier in order to allow the systemically administered nanosystems to reach the ocular tissues.
Scheme 4.
Hypothetical ways that are taken advantage of the breakdown of the blood-ocular barrier for systemically administered nanosystems.
The pharmacological evaluation of drugs injected into the bloodstream aiming to permeate the intraocular tissues is necessary to establish their safety, especially if formulated with nanocarriers that still require additional pharmacodynamic and pharmacokinetic studies [66,133]. The future development of methods enabling a better understanding of the complete physicochemical properties of the nanomaterials and the availability of new nanotoxicological standards will allow the real evolution in this field, making it feasible to use nanosystems via systemic route for ocular drug delivery.
In summary, the availability of new effective and safe ocular delivery systems remains a major challenge for pharmaceutical researchers. The development of methods to induce a controlled, safe and transient breakdown of the blood-ocular barrier could be a novel approach to allow the access of systemically injected drugs to the internal parts of the human eye. The ideal nanoparticle system for systemic use in order to reach the intraocular tissues is not presently available, nor is there any evidence of the facilitation of access of these nanosystems with the transient breakdown of blood-ocular barriers. However, previous studies and pathophysiological events mentioned in this article encourage us to propose this hypothesis with the aim to conceive the basis for the possible development of a viable system, allying nanoparticles properties with the facilitated permeation to intraocular tissues by taking advantage of a natural or induced breakdown of the blood-ocular barrier.
The intravenous route could be reconsidered as a good alternative to complement the ophthalmic therapeutics currently in use. As the ability to deliver drugs and other compounds across the blood-ocular barrier via the systemic route for therapeutic purposes is not reached, further studies using nanoengineered technologies and focusing on the development of nanosystems with minimal toxicity and enhanced efficacy are necessary in order to expand the knowledge of the ways to selectively and efficiently overcome the blood-ocular barrier. Moreover, more detailed studies on the normal physiological and pathological conditions of the eye are essential to yield a consistent understanding of nanosystem characteristics under different circumstances in the target organ. In addition, methods to take advantage of the natural or induced breakdown of blood-ocular barriers can complement the nanotechnological properties to get easier access to nanomedicines administered systemically and targeted to the hitherto almost impervious areas of the human eye.
Acknowledgments
Maranhao has a Research Award from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Brasília, Brazil.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Gunda S., Hariharan S., Mandava N., Mitra A.K. Barriers in Ocular Drug Delivery. In: Tombran-Tink J., Barnstable C.J., editors. Ocular Transporters in Ophthalmic Diseases and Drug Delivery. Humana Press; Totowa, NJ, USA: 2008. p. 399. Chapter 21. [Google Scholar]
- 2.Cunha-Vaz J.G. The blood-ocular barriers. Doc. Ophthalmol. 1976;41:287–327. doi: 10.1007/BF00146764. [DOI] [PubMed] [Google Scholar]
- 3.Worakul N., Robinson J.R. Ocular pharmacokinetics/pharmacodynamics. Eur. J. Pharm. Biopharm. 1997;44:71–83. [Google Scholar]
- 4.Cunha-Vaz J.G. The blood-ocular barriers. Surv. Ophthalmol. 1979;23:279–296. doi: 10.1016/0039-6257(79)90158-9. [DOI] [PubMed] [Google Scholar]
- 5.Muh-Shy C., Hou P.K., Tong-Yuan T., Lin B.J. Blood-ocular barriers. Tzu Chi Med. J. 2008;20:25–34. [Google Scholar]
- 6.Smith R.S., Rudt L.A. Ocular vascular and epitelial barriers to microperoxidase. Invest. Ophthalmol. 1975;14:556–560. [PubMed] [Google Scholar]
- 7.Peyman G.A., Bok D. Peroxidase diffusion in the normal and laser-coagulated primate retina. Invest. Ophthalmol. 1972;11:35–45. [PubMed] [Google Scholar]
- 8.Cunha-Vaz J.G. The blood-retinal barriers. Doc. Ophthalmol. 1976;41:287–327. doi: 10.1007/BF00146764. [DOI] [PubMed] [Google Scholar]
- 9.Green K., Pedersno J.E. Effect of 1-tetrahydrocannabinol on aqueous dynamics and ciliary body permeability in the rabbit. Exp. Eye Res. 1973;15:499–507. doi: 10.1016/0014-4835(73)90142-5. [DOI] [PubMed] [Google Scholar]
- 10.Schmack I., Völcker H.E., Grossniklaus H.E. Phthisi bulbi. In: Levin L.A., Albert D.M., editors. Ocular Disease Mechanisms and Management. Saunders Elsevier; Maryland Heights, MO, USA: 2010. p. 420. Chapter 54. [Google Scholar]
- 11.Völcker H.E., Naumann G.O.H. Morphology of uveal and retinal edemas in acute and persisting hypotony. Mod. Probl. Ophthalmol. 1979;20:34–41. [PubMed] [Google Scholar]
- 12.Raviola G. The structural basis of the blood-ocular barriers. Exp. Eye Res. 1997;25:27–63. doi: 10.1016/s0014-4835(77)80009-2. [DOI] [PubMed] [Google Scholar]
- 13.Schubert H.D. Postsurgical hypotony: Relationship to fistulization, inflammation, chorioretinal lesions, and the vitreous. Surv. Ophthalmol. 1996;41:97–125. doi: 10.1016/S0039-6257(96)80001-4. [DOI] [PubMed] [Google Scholar]
- 14.Bill A., Philips C. Uveoscleral drainage of aqueous humor in human eyes. Exp. Eye Res. 1971;12:275–281. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- 15.Salminen L., Chioralia G., Scheiber S. Effect of acute ocular hypotony on the blood-ocular barrier. Trans. Ophthalmol. Soc. (UK) 1977;97:621–622. [PubMed] [Google Scholar]
- 16.Servatt J.J., Bernardino C.R. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011;28:267–282. doi: 10.2165/11588830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Baudouin C. Detrimental effect of preservatives in eyedrops: Implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86:716–726. doi: 10.1111/j.1755-3768.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 18.Baudouin C., Labbé A., Liang H., Pauly A., Brignole-Baudouin F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010;29:312–334. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Cho J.H., Kwun Y.S., Jang H.S., Kang J.M., Won Y.S., Yoon H.R. Long-term use of preservatives on rat nasal respiratory mucosa: Effects of benzalkonium chloride and potassium sorbate. Laryngoscope. 2000;110:312–317. doi: 10.1097/00005537-200002010-00025. [DOI] [PubMed] [Google Scholar]
- 20.Reardon G., Kotak S., Schwartz G.F. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: A systematic review. Patient Prefer Adherence. 2011;5:441–463. doi: 10.2147/PPA.S23780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reardon G., Schwartz G.F., Mozaffari E. Patient persistency with topical ocular hypotensive therapy in a managed care population. Am. J. Ophthalmol. 2004;137:S3–S12. doi: 10.1016/j.ajo.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Jaycock P.D., Mather C.M., Ferris J.D., Kirkpatrick J.N. Rectus muscle trauma complicating sub-Tenon’s local anaesthesia. Eye (Lond) 2001;15:583–586. doi: 10.1038/eye.2001.189. [DOI] [PubMed] [Google Scholar]
- 23.Faure C., Faure L., Billotte C. Globe perforation following no-needle sub-Tenon anesthesia. J. Cataract Refract. Surg. 2009;35:1471–1472. doi: 10.1016/j.jcrs.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Kumar C.M., Eid H., Dodds C. Sub-Tenon’s anaesthesia: Complications and their prevention. Eye (Lond) 2011;25:694–703. doi: 10.1038/eye.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penha F.M., Rodrigues E.B., Maia M., Furlani B.A., Regatieri C., Melo G.B., Magalhães O., Manzano R., Farah M.E. Retinal and ocular toxicity in ocular application of drugs and chemicals—Part II: Retinal toxicity of current and new drugs. Ophthalmic Res. 2010;44:205–224. doi: 10.1159/000316695. [DOI] [PubMed] [Google Scholar]
- 26.Jager R.D., Aiello L.P., Patel S.C., Cunningham E.T., Jr. Risks of intravitreous injection: A comprehensive review. Retina. 2004;24:676–698. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Sampat K.M., Garg S.J. Complications of intravitreal injections. Curr. Opin. Opthalmol. 2010;21:178–183. doi: 10.1097/ICU.0b013e328338679a. [DOI] [PubMed] [Google Scholar]
- 28.de Smet P.A., Denneboom W., Kramers C., Grol R. A composite screening tool for medication reviews of outpatients: general issues with specific examples. Drug Aging. 2007;24:733–760. doi: 10.2165/00002512-200724090-00003. [DOI] [PubMed] [Google Scholar]
- 29.Raghava S., Goel G., Kompella U.B. Ophthalmic Applications of Nanotechnology. In: Tombran-Tink J., Barnstable C.J., editors. Ocular Transporters in Ophthalmic Diseases and Drug Delivery. Humana Press; Totowa, NJ, USA: 2008. p. 418. Chapter 22. [Google Scholar]
- 30.Amrite A.C., Kompella U.B. In: Nanoparticles for Ocular Drug Delivery. Gupta R.B., Kompella U.B., editors. Taylor and Francis; New York, NY, USA: 2006. [Google Scholar]
- 31.Hamidi M., Azadi A., Rafiei P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Sahoo S.K., Dilnawaz F., Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov. Today. 2008;13:144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Sahoo S.K., Labhasetwar V. Nanotech approaches in drug delivery and imaging. Drug Discov. Today. 2003;8:1112–1120. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 34.Vasir J.K., Reddy M.K., Labhasetwar V. Nanosystems in drug targeting: Opportunities and challenges. Curr. Nanosci. 2005;1:47–64. [Google Scholar]
- 35.Singh S.R., Grossniklaus H.E., Kang S.J., Edelhauser H.F., Ambati B.K., Kompella U.B. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mainardes R.M., Silva L.P. Drug delivery systems: Past, present, and future. Curr. Drug Targets. 2004;5:449–455. doi: 10.2174/1389450043345407. [DOI] [PubMed] [Google Scholar]
- 37.Zeimer R., Goldberg M.F. Novel ophthalmic therapeutic modalities based on noninvasive light-targeted drug delivery to the posterior pole of the eye. Adv. Drug Deliv. Rev. 2001;52:49–61. doi: 10.1016/s0169-409x(01)00194-6. [DOI] [PubMed] [Google Scholar]
- 38.Niederhafner P., Sebestik J., Jezek J. Peptide dendrimers. J. Pept. Sci. 2005;11:757–788. doi: 10.1002/psc.721. [DOI] [PubMed] [Google Scholar]
- 39.D’Emmanuele A., Attwood D. Dendrimer-drug interactions. Adv. Drug Deliv. Rev. 2005;57:2147–2162. doi: 10.1016/j.addr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Perini G., Saettone M.F., Carafa M., Santucci E., Alhaique F. Niosomes as carriers for ophthalmic drugs: In vitro/in vivo evaluation. Boll. Chim. Farm. 1996;135:145–146. [PubMed] [Google Scholar]
- 41.Katz B., Goldbaum M. Macugen (pegaptanib sodium), a novel ocular therapeutic that targets vascular endothelial growth factor (VEGF) Int. Ophthalmol. Clin. 2006;46:141–154. doi: 10.1097/01.iio.0000212130.91136.31. [DOI] [PubMed] [Google Scholar]
- 42.Maranhão R.C., Cesar T.B., Pedroso M.T., Hirata M.H., Mesquita C.H. Metabolic behavior in rats of a nonprotein microemulsion resembling LDL. Lipids. 1993;28:691–696. doi: 10.1007/BF02535988. [DOI] [PubMed] [Google Scholar]
- 43.Maranhão R.C., Garicochea B., Silva E.L., Dorlhiac-Llacer P., Cadena S.M., Coelho I.J., Meneghetti J.C., Pileggi F.J., Chamone D.A. Plasma kinetics and biodistribution of a lipid emulsion resembling low-density lipoprotein in patients with acute leukemia. Cancer Res. 1994;54:4660–4666. [PubMed] [Google Scholar]
- 44.Azevedo C.H., Carvalho J.P., Valduga C.J., Maranhão R.C. Plasma kinetics and uptake by the tumor of a cholesterol-rich microemulsion (LDE) associated to etoposide oleate in patients with ovarian carcinoma. Gynecol. Oncol. 2005;97:178–182. doi: 10.1016/j.ygyno.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Pinheiro K.V., Hungria V.T., Ficker E.S., Valduga C.J., Mesquita C.H., Maranhão R.C. Plasma kinetics of a cholesterol-rich microemulsion (LDE) in patients with Hodgkin’s and non-Hodgkin’s lymphoma and a preliminary study on the toxicity of etoposide associated with LDE. Cancer Chemother. Pharmacol. 2006;57:624–630. doi: 10.1007/s00280-005-0090-8. [DOI] [PubMed] [Google Scholar]
- 46.Maranhão R.C., Tavares E.R., Padoveze A.F., Valduga C.J., Rodrigues D.G., Pereira M.D. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis. 2008;197:959–966. doi: 10.1016/j.atherosclerosis.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 47.Tavares E.R., Freitas F.R., Diament J., Maranhão R.C. Reduction of atherosclerotic lesions in rabbits treated with etoposide associated with cholesterol-rich nanoemulsions. Int. J. Nanomed. 2011;6:2297–2304. doi: 10.2147/IJN.S24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulgarelli A., Dias A.A.M., Caramelli B., Maranhão R.C. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J. Cardiovasc. Pharmacol. 2011;59:308–314. doi: 10.1097/FJC.0b013e318241c385. [DOI] [PubMed] [Google Scholar]
- 49.Lourenço-Filho D.D., Maranhão R.C., Méndez-Contreras C.A., Tavares E.R., Freitas F.R., Stolf N.A. An artificial nanoemulsion carrying paclitaxel decreases the transplant heart vascular disease: A study in a rabbit graft model. J. Thorac. Cardiovasc. Surg. 2011;141:1522–1528. doi: 10.1016/j.jtcvs.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 50.Adbika K., Siani Shadbad M.R., Nokhdochi A., Javadzedeh A., Barzegar-Jalali M., Barar J., Mohammadi G., Omidi Y. Piroxicam nanoparticles for ocular delivery: Physicochemical characterization and implementation in endotoxin-induced uveitis. J. Drug Target. 2007;15:407–416. doi: 10.1080/10611860701453125. [DOI] [PubMed] [Google Scholar]
- 51.de la Fuente M., Seijo B., Alonso M.J. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Invest. Ophthalmol. Vis. Sci. 2008;49:2016–2024. doi: 10.1167/iovs.07-1077. [DOI] [PubMed] [Google Scholar]
- 52.de Campos A.M., Sánchez A., Alonso M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001;224:159–168. doi: 10.1016/S0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- 53.Attama A.A., Reichi S., Muller-Goymann C.C. Diclofenac sodium delivery to the eye: In vitro evaluation of novel solid lipid nanoparticle formulation using human cornea construct. Int. J. Pharm. 2008;355:307–313. doi: 10.1016/j.ijpharm.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Muller R.H., Runge A.S., Ravelli V., Thunemann A.F., Mehnert W., Souto E.B. Cyclosporine-loaded solid lipid nanoparticles (SLN): Drug-lipid physicochemical interations and characterization of drug incorporation. Eur. J. Pharm. Biopharm. 2008;68:535–544. doi: 10.1016/j.ejpb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Fialho S.L., da Silva-Cunha A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004;32:626–632. doi: 10.1111/j.1442-9071.2004.00914.x. [DOI] [PubMed] [Google Scholar]
- 56.Garty N., Lusky M., Zalish M. Pilocarpine in submicron emulsion formulation for treatment of ocular hypertension: A phase II clinical trial. Invest. Ophthalmol. Vis. Sci. 1994;35:2175–2186. [Google Scholar]
- 57.Civiale C., Licciaradi M., Cavallaro G., Giammona G., Mazzone M.G. Polyhydroxyethylaspartamide-based micelles for ocular drug delivery. Int. J. Pharm. 2009;378:177–186. doi: 10.1016/j.ijpharm.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 58.Pignatello R., Bucolo C., Spedalieri G., Maltese A., Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23:3247–3255. doi: 10.1016/s0142-9612(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal D., Garg A., Kaur L.P. Development of a topical niosonaml preparation of acetazolamide: preparation and evaluation. J. Pharm. Pharmacol. 2004;56:1509–1517. doi: 10.1211/0022357044896. [DOI] [PubMed] [Google Scholar]
- 60.Loftsson T., Fririksdóttir H., Thórsdóttir S., Stefánsson E. The effects of hydroxypropyl methylcellulose on the release of dexamethasone from aqueous 2-hydroxypropyl-β-cyclodextrin formulations. Int. J. Pharm. 1994;104:181–184. [Google Scholar]
- 61.Kristinsson J.K., Fririksdóttir H., Thórsdóttir S., Sigurdardottir A.M., Stefánsson E., Loftsson T. Dexamethasone-cyclodextrin-polymer co-complexes in aqueous eye drops. Aqueous humor pharmacokinetics in humans. Invest. Ophthalmol. Vis. Sci. 1996;37:1119–1203. [PubMed] [Google Scholar]
- 62.Bochot A., Fattai E., Gullk A., Couyarraze G., Couvreur P. Liposomes dipersed within a thermosensitive gel: A new dosage form for ocular delivery of oligonucleotides. Pharm. Res. 1998;15:1364–1369. doi: 10.1023/a:1011989202488. [DOI] [PubMed] [Google Scholar]
- 63.Ahmed I., Patton T.F. Disposition of timolol and inulin in the rabbit eye following corneal versus non-corneal absorption. Int. J. Pharm. 1987;38:9–21. [Google Scholar]
- 64.Vandamme T.F., Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release. 2005;102:23–38. doi: 10.1016/j.jconrel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Saettone M.F., Perini G., Carafa M., Santucci E., Alhaique F. Non-ionic surfactant vesicles as ophthalmic carriers for cyclopentolate. A preliminary evaluation. STP Pharma Sci. 1996;6:94–98. [Google Scholar]
- 66.Shah S.S., Denham L.V., Elison J.R., Bhattacharjee P.S., Clement C., Huq T., Hill J.M. Drug delivery to the posterior segment of the eye for pharmacologic therapy. Expert Rev. Ophthalmol. 2010;5:75–93. doi: 10.1586/eop.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J.H., Kim K.W., Kim M.H., Yu Y.S. Intravenously administered gold nanoparticles pass through the blood-retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20 doi: 10.1088/0957-4484/20/50/505101. [DOI] [PubMed] [Google Scholar]
- 68.Alyaudtin R.N., Reichel A., Lobenberg R., Ramge P., Kreuter J., Begley D.J. Interaction of poly(butylcyanoacrylate nanoparticles with the blood-brain barrier in vivo and in vitro. J. Drug Target. 2001;9:209–221. doi: 10.3109/10611860108997929. [DOI] [PubMed] [Google Scholar]
- 69.Holsapple M.P., Farland W.H., Landry T.D., Monteiro-Riviere N.A., Carter J.M., Walker N.J., Thomas K.V. Research strategies for safety evaluation of nanomaterials, part II: Toxicological and safety evaluation of nanomaterials, current challenges and data needs. Toxicol. Sci. 2005;88:12–17. doi: 10.1093/toxsci/kfi293. [DOI] [PubMed] [Google Scholar]
- 70.Nel A., Xia T., Madler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 71.Shokeen M., Fettig N.M., Rossin R. Synthesis in vitro and in vivo evaluation of radiolabed nanoparticles. Q. J. Nucl. Mol. Imaging. 2008;52:267–277. [PubMed] [Google Scholar]
- 72.Almeida J.P., Chen A.L., Foster A., Drezek R. In vivo biodistribution of nanoparticles. Nanomedicine (Lond) 2011;6:815–835. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- 73.Khlebtsov N., Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011;40:1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 74.Pires L.A., Hegg R., Valduga C.J., Graziani S.R., Rodrigues D.G., Maranhão R.C. Use of cholesterol-rich nanoparticles that bind to lipoprotein receptors as a vehicle to paclitaxel in the treatment of breast cancer: Pharmacokinetics, tumor uptake and a pilot clinical study. Cancer Chemother. Pharmacol. 2009;63:281–287. doi: 10.1007/s00280-008-0738-2. [DOI] [PubMed] [Google Scholar]
- 75.Saha R.N., Vasanthakumar S., Bende G., Snehalatha M. Nanoparticulate drug delivery systems for cancer chemotherapy. Mol. Membr. Biol. 2010;27:215–231. doi: 10.3109/09687688.2010.510804. [DOI] [PubMed] [Google Scholar]
- 76.Kong B., Seog J.H., Graham L.M., Lee S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine (Lond) 2011;6:929–941. doi: 10.2217/nnm.11.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stensberg M.C., Wei Q., McLamore E., Porterfield D.M., Wei A., Sepúlveda M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine (Lond) 2011;6:879–898. doi: 10.2217/nnm.11.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maranhão R.C., Graziani S.R., Yamaguchi N., Melo R.F., Latrilha M.C., Rodrigues D.G., Couto R.D., Schreier S., Buzaid A.C. Association of carmustine with a lipid emulsion: In vitro, in vivo and preliminary studies in cancer patients. Cancer Chemother. Pharmacol. 2002;49:487–498. doi: 10.1007/s00280-002-0437-3. [DOI] [PubMed] [Google Scholar]
- 79.Teixeira R.S., Curi R., Maranhão R.C. Effects on Walker 256 tumour of carmustine associated with a cholesterol-rich microemulsion (LDE) J. Pharm. Pharmacol. 2004;56:909–914. doi: 10.1211/0022357023826. [DOI] [PubMed] [Google Scholar]
- 80.Escobar E.R., Cheruvu N.P., Zhan G., Toris C.B., Kompella U.B. Subconjunctival budesonide and budesonide poly-(lactide) microparticles do not elevate intraocular pressure or induce lens opacities in rabbit model. Invest. Ophthalmol. Vis. Sci. 2006;47:4493. [Google Scholar]
- 81.Amrite A.C., Ayalasomayajula S.P., Cheruvu N.P., Kompella U.B. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest. Ophthalmol. Vis. Sci. 2006;47:1149–1160. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Georgoulas S., Dahlmann-Noor A., Brocchini S., Khaw P.T. In: Ocular Disease Mechanism and Management. Levin L.A., Albert D.M., editors. Saunders Elsevier; Maryland Heights, MO, USA: 2010. pp. 214–222. [Google Scholar]
- 83.Naumann G.O.H., Kruse F.E. In: Applied Pathology for Ophthalmic Microsurgeons. Naumann G.O.H., Holbach L., Kruse F.E., editors. Springer; Heildelberg, Germany: 2008. pp. 76–96. [Google Scholar]
- 84.Kosaka T., Mishima H.K., Kiuchi Y., Kataoka K. The effects of prostaglandins on the blood-ocular barrier. Jpn. J. Ophthalmol. 1995;39:368–376. [PubMed] [Google Scholar]
- 85.Cho H., Madu A. Etiology and treatment of the inflammatory causes of cystoid macular edema. J. Inflamm. Res. 2009;2:37–43. doi: 10.2147/jir.s5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang L., Crowston J.G., Cordeiro M.F., Akbar A.N., Khaw P.T. The role of the immune system in conjunctival wound healing after glaucoma surgery. Surv. Ophthalmol. 2000;45:49–68. doi: 10.1016/s0039-6257(00)00135-1. [DOI] [PubMed] [Google Scholar]
- 87.Gaudio P.A. A review of evidence guiding the use of corticosteroids in the treatment of intraocular inflammation. Ocul. Immunol. Inflamm. 2004;12:169–192. doi: 10.1080/092739490500192. [DOI] [PubMed] [Google Scholar]
- 88.Hart W. In: The Glaucomas. Ritch R., Shields M.B., Krupin T., editors. Mosby; St. Louis, MO, USA: 1989. pp. 789–795. [Google Scholar]
- 89.Hodkin M., Goldblatt W., Burgoyne C., Ball S.F., Insler M.S. Early clinical experience with the Baerveldt implant in complicated glaucomas. Am. J. Ophthalmol. 1995;102:32–40. doi: 10.1016/s0002-9394(14)73756-0. [DOI] [PubMed] [Google Scholar]
- 90.Siegner S.W., Netland P.A., Urban R.C., Williams A.S., Richards D.W., Latina M.A., Brandt J.D. Clinical experience with the Baerveldt glaucoma drainage implant. Ophthalmology. 1995;102:1298–1307. doi: 10.1016/s0161-6420(95)30871-8. [DOI] [PubMed] [Google Scholar]
- 91.Singh K., Byrd S., Egbert P.R., Budenz D. Risk of hypotony after primary trabeculectomy with antifibrotic agents in a black west African population. J. Glaucoma. 1998;7:82–85. [PubMed] [Google Scholar]
- 92.Morinelli E.N., Sidoti P.A., Heuer D.K., Minckler D.S., Baerveldt G., LaBree L., Lee P.P. Laser suture lysis after mitomycin C trabeculectomy. Ophthalmology. 1996;103:306–314. doi: 10.1016/s0161-6420(96)30699-4. [DOI] [PubMed] [Google Scholar]
- 93.Leydheeker W., Akiyama K., Neumann H.G. Intraocular pressure in normal human eyes. Augen Klin Monatsbl Augenheilkd. 1958;133:662–670. [PubMed] [Google Scholar]
- 94.Raamakrishnan R., Michon J., Robin A., Krishnada S.R. Safety and efficacy of mitomycin C trabeculectomy in Southern India. Ophthalmology. 1993;100:1619–1623. doi: 10.1016/s0161-6420(93)31427-2. [DOI] [PubMed] [Google Scholar]
- 95.Schubert H.D. Postsurgical hypotony: Relationship to fistulization, inflammation, chorioretinal lesions, and the vitreous. Surv. Ophthalmol. 1996;41:97–125. doi: 10.1016/S0039-6257(96)80001-4. [DOI] [PubMed] [Google Scholar]
- 96.Weikert M.P. Update on bimanual microincisional cataract surgery. Curr. Opin. Ophthalmol. 2006;17:62–67. doi: 10.1097/01.icu.0000193069.32369.e1. [DOI] [PubMed] [Google Scholar]
- 97.Trivedi D., Denniston A.K., Murray P.I. Safety profile of anterior chamber paracentesis performed at the slit lamp. Clin. Exp. Ophthalmol. 2011;39:725–728. doi: 10.1111/j.1442-9071.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 98.Lam D.S., Chua J.K., Tham C.C., Lai J.S. Efficacy and safety of immediate anterior chamber paracentesis in the treatment of acute primary angle-closure glaucoma: A pilot study. Ophthalmology. 2002;109:64–70. doi: 10.1016/s0161-6420(01)00857-0. [DOI] [PubMed] [Google Scholar]
- 99.Carnahan M.C., Platt L.W. Serial paracenteses in the management of acute elevations of intraocular pressure. Ophthalmology. 2002;109:1604–1606. doi: 10.1016/s0161-6420(02)01126-0. [DOI] [PubMed] [Google Scholar]
- 100.Arnavielle S., Creuzot-Garcher C., Bron A.M. Anterior chamber paracentesis in patients with acute elevation of intraocular pressure. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:345–350. doi: 10.1007/s00417-006-0465-5. [DOI] [PubMed] [Google Scholar]
- 101.Kim H.C., Hayashi A., Shalash A, de Juan E., Jr. A model of chronic hypotony in the rabbit. Graefes Arch. Clin. Exp. Ophthalmol. 1998;236:69–74. doi: 10.1007/s004170050045. [DOI] [PubMed] [Google Scholar]
- 102.Fine H.F., Biscette O., Chang S., Schiff W.M. Ocular hypotony: A review. Compr. Ophthalmol. Update. 2007;8:29–37. [PubMed] [Google Scholar]
- 103.Nussenblatt R.B., Gery I., Ballintine E.J., Wacker W.B. Cellular immune responsiveness of uveitis patients to retinal S-antigen. Am. J. Ophthalmol. 1980;89:173–179. doi: 10.1016/0002-9394(80)90108-7. [DOI] [PubMed] [Google Scholar]
- 104.Ham D.I., Gentleman S., Chan C.C., McDowell J.H., Redmond T.M., Gery I. RPE65 is highly uveitogenic in rats. Invest. Ophthalmol. Vis. Sci. 2002;43:2258–2263. [PubMed] [Google Scholar]
- 105.Broekhuyse R.M., Winkens H.J., Kuhlmann E.D. Induction of experimental autoimmune uveoretinitis and pinealitis by IRBP. Comparison to uveoretinitis induced by S-antigen and opsin. Curr. Eye Res. 1986;5:231–240. doi: 10.3109/02713688609020048. [DOI] [PubMed] [Google Scholar]
- 106.Chan C.C., Nussenblatt R.B., Wiggert B., Redmond T.M., Fujikawa L.S., Chader G.J., Gery I. Immunohistochemical analysis of experimental autoimmune uveoretinitis (EAU) induced by interphotoreceptor retinoid-binding protein (IRBP) in the rat. Immunol. Invest. 1987;16:63–74. doi: 10.3109/08820138709055713. [DOI] [PubMed] [Google Scholar]
- 107.Nussenblatt R.B., Rodrigues M.M., Wacker W.B., Nussenblatt R.B., Rodrigues M.M., Wacker W.B., Cevario S.J., Salinas-Carmona M.C., Gery I. Cyclosporin a. Inhibition of experimental autoimmune uveitis in Lewis rats. J. Clin. Invest. 1981;67:1228–1231. doi: 10.1172/JCI110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boyd S.R., Young S., Lightman S. Immunopathology of the noninfectious posterior and intermediate uveitis. Surv. Ophthalmol. 2001;46:209–233. doi: 10.1016/s0039-6257(01)00275-2. [DOI] [PubMed] [Google Scholar]
- 109.Whitcup S.M., Nussenblatt R.B. Immunologic mechanisms of uveitis. New targets for immunomodulation. Arch. Ophthalmol. 1997;115:520–525. doi: 10.1001/archopht.1997.01100150522013. [DOI] [PubMed] [Google Scholar]
- 110.Foxman E.F., Zhang M., Hurst S.D., Muchamuel T., Shen D., Wawrousek E.F., Chan C.C., Gery I. Inflammatory mediators in uveitis: Differentieal induction of cytokines and chemokines in Th1- versus Th2-mediated ocular inflammation. J. Immunol. 2002;168:2483–2492. doi: 10.4049/jimmunol.168.5.2483. [DOI] [PubMed] [Google Scholar]
- 111.Limb G.A., Soomro H., Janikoun S., Hollifield R.D., Shilling J. Evidence for control of tumor necrosis factor-alpha (TNF-α) activity by TNF receptors in patients with proliferative diabetic retinopathy. Clin. Exp. Immunol. 1999;115:409–414. doi: 10.1046/j.1365-2249.1999.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang H., Gandhi J.K., Zhong X., Wei Y., Gong J., Duh E.J., Vinores S.A. TNFα is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest. Ophthalmol. Vis. Sci. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rowland F.N., Donovan M.J., Lindsay M., Weiss W.I., O’Rourke J., Kreutzer D.L. Demonstration of inflammatory mediator-induced inflammation and endothelial cell damage in the anterior segment of the eye. Am. J. Ophthalmol. 1982;110:1–12. [PMC free article] [PubMed] [Google Scholar]
- 114.Ben-Zvi A., Rodrigues M.M., Gory I., Schiffmann E. Induction of ocular inflammation by synthetic mediators. Arch. Ophthalmol. 1981;99:1436–1444. doi: 10.1001/archopht.1981.03930020310024. [DOI] [PubMed] [Google Scholar]
- 115.Miyake K., Kayazawa F., Manabe R., Miyake Y. Indomethacin and the epinephrine-induced breakdown of the blood-ocular barrier in rabbits. Invest. Ophthalmol. Vis. Sci. 1987;28:482–486. [PubMed] [Google Scholar]
- 116.Elliot P.J., Mackic J.B., Graney W.F., Bartus R.T., Zlokovic B.V. RMP-7, a bradykinin agonist, increases permeability of blood-ocular barriers in the guinea pig. Invest. Ophthalmol. Vis. Sci. 1995;36:2542–2547. [PubMed] [Google Scholar]
- 117.Deng X., Zhang Q., Hu S., Gao Y., Yan L. Pharmacokinetics of puerarin in the aqueous humor and vitreous of rabbit eye following systemic administration. Yan Ke Xue Bao. 2006;22:275–279. [PubMed] [Google Scholar]
- 118.Copland D.A., Liu J., Schewitz-Bowers L.P., Brinkmann V., Anderson K., Nicholson L.B., Dick A.D. Therapeutic dosing of fingolimod (FTY720) prevents cell infiltration, rapidly suppresses ocular inflammation, and maintains the blood-ocular barrier. Am. J. Pathol. 2011;180:672–681. doi: 10.1016/j.ajpath.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oshika T., Araie M., Masuka K. Diurnal variation of aqueous flare in normal human eyes measured with laser flare-cell meter. Jpn. J. Ophthalmol. 1988;32:143–150. [PubMed] [Google Scholar]
- 120.Sawa M. Clinical application of laser flare-cell meter. Jpn. J. Ophthalmol. 1990;34:346–363. [PubMed] [Google Scholar]
- 121.Küchle M., Hannappel E., Nguyen N.X., Ho S.T., Beck W., Naumann G.O. Correlation between tyndallometry with the “laser flare cell meter” in vivo and biochemical protein determination in human aqueous humor. Klin. Monbl. Augenheilkd. 1993;202:14–18. doi: 10.1055/s-2008-1045553. [DOI] [PubMed] [Google Scholar]
- 122.Küchle M. Laser tyndallometry in anterior segment diseases. Curr. Opin. Ophthalmol. 1994;5:110–116. [PubMed] [Google Scholar]
- 123.Tugal-Tutkin I., Herbort C.P. Laser flare photometry: A noninvasive, objective, and quantitative method to measure intraocular inflammation. Int. Ophthalmol. 2010;30:453–464. doi: 10.1007/s10792-009-9310-2. [DOI] [PubMed] [Google Scholar]
- 124.Naumann G.O., Schlötzer-Schrehardt U., Küchle M. Pseudoexfoliation syndrome for the comprehensive ophthalmologist. Intraocular and systemic manifestations. Ophthalmology. 1998;105:951–968. doi: 10.1016/S0161-6420(98)96020-1. [DOI] [PubMed] [Google Scholar]
- 125.Chen M.S., Hou P.K., Tai T.Y., Lin B.J. Blood-ocular barriers. Tzu Chi Med. J. 2008;20:25–34. [Google Scholar]
- 126.Lund-Andersen H., Krogsaa B., la Cour M., Larsen J. Quantitative vitreous fluorophotometruy applying a mathematical model of the eye. Invest. Ophthalmol. Vis. Sci. 1985;26:698–710. [PubMed] [Google Scholar]
- 127.Larsen M. Ocular fluorometry methodological improvements and clinical studies with special reference to the blood-retina barrier permeability to fluorescein and fluorescein glucuronide. Acta Ophthalmol. Suppl. 1993;211:1–52. [PubMed] [Google Scholar]
- 128.Cunha-Vaz J., Bernardes R., Lobo C. Blood-retinal barrier. Eur. J. Ophthalmol. 2010;21:3–9. doi: 10.5301/EJO.2010.6049. [DOI] [PubMed] [Google Scholar]
- 129.Lu V.H., Ho I.V., Lee V., Hunyor A.P. Complications from fluorescein angiography: A prospective study. Clin. Exp. Ophthalmol. 2009;37:826–827. [PubMed] [Google Scholar]
- 130.Metrikin D.C., Wilson C.A., Berkowitz B.A., Lam M.K., Wood G.K., Peshock R.M. Measurement of blood-retinal barrier breakdown in endotoxin-induced endophthalmitis. Invest. Ophthalmol. Vis. Sci. 1995;36:1361–1370. [PubMed] [Google Scholar]
- 131.Li S.K., Lisak M.J., Jeong E.K. MRI in ocular drug delivery. NMR Biomed. 2008;21:941–956. doi: 10.1002/nbm.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cunha-Vaz J.G. The blood-retinal barriers system. Basic concepts and clinical evaluation. Exp. Eye Res. 2004;78:715–721. doi: 10.1016/S0014-4835(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 133.del Amo E.M., Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov. Today. 2008;13:135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]