Abstract
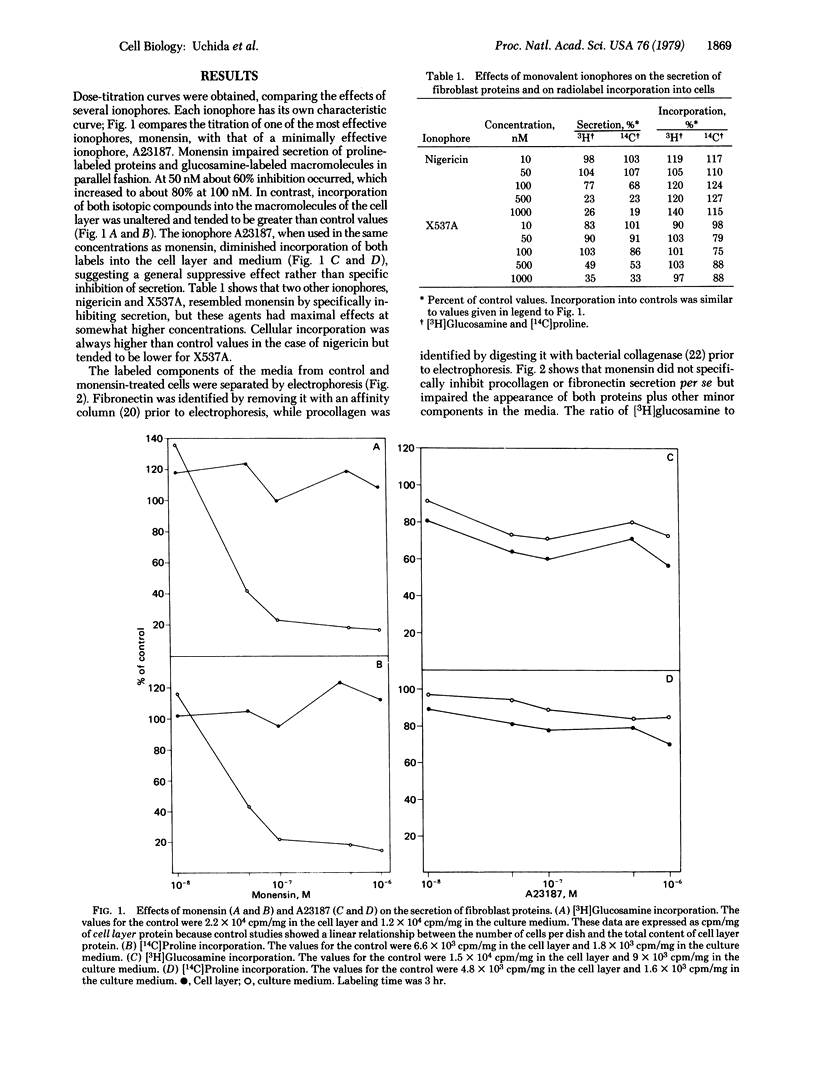
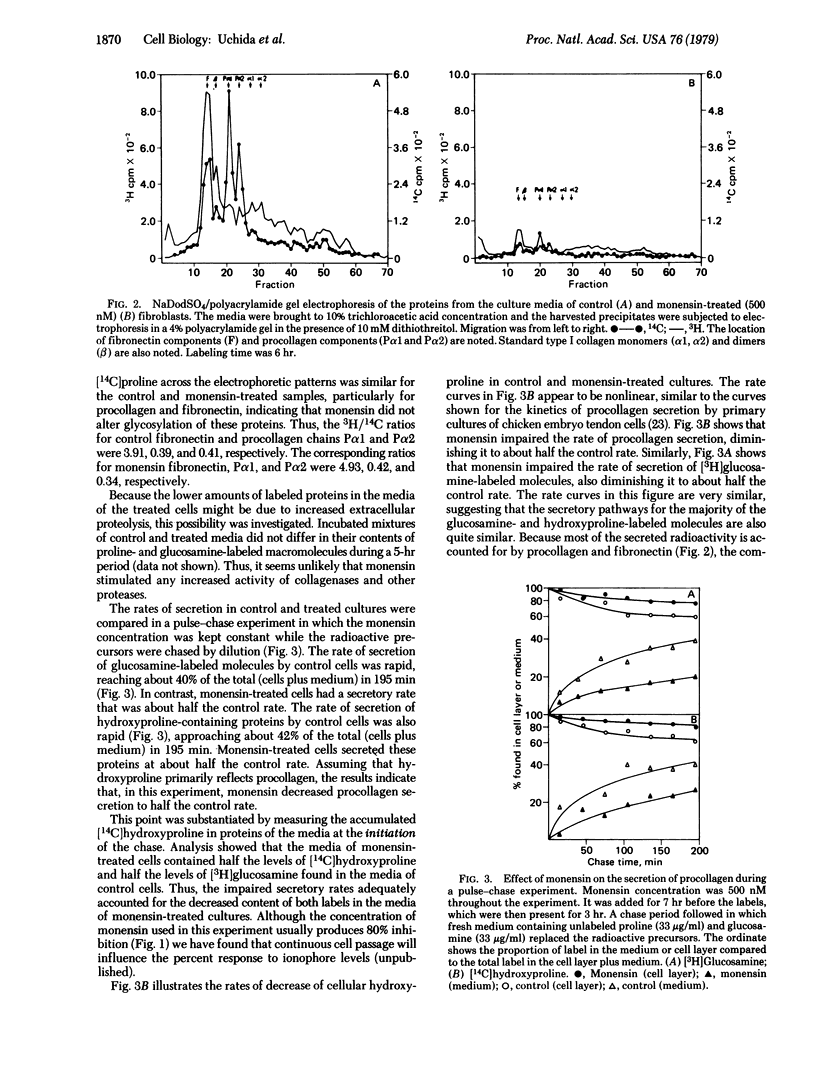
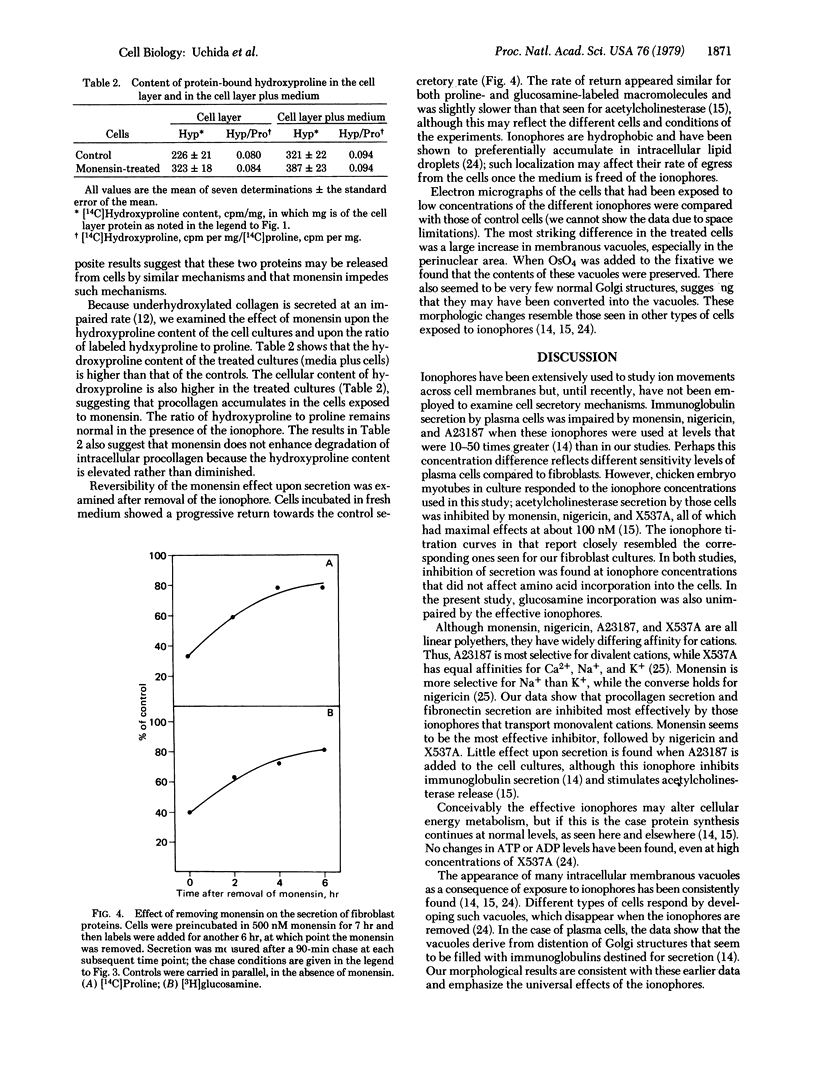
Procollagen and fibronectin are major products of confluent fibroblasts in culture and both are released from the cells. Procollagen is secreted by known pathways, while the mechanism of fibronectin release is controversial. We find that the secretion of both these proteins can be reduced to 20% by low concentrations (0.1-1 μM) of ionophores that have affinity for monovalent cations. In contrast, little effect upon secretion was found for similar concentrations of an ionophore that binds divalent cations. Electron microscopy showed that the inhibition of secretion is accompanied by accumulation of membranous vacuoles. We believe that the ionophores impede secretion by acting on the secretory structures rather than on the proteins themselves. Biochemical studies supported this interpretation because no changes were detected in hydroxylation or glycosylation of procollagen or glycosylation of fibronectin, nor were significant changes in cellular amino acid incorporation observed. Pulse-chase studies indicated that the rates of secretion were impaired by the ionophore without enhancing intracellular degradation. The decreased secretory rates accounted for the lower levels of procollagen and fibronectin in the culture medium; no evidence for increased catabolism of the secreted proteins was found. Secretion could be readily restored by removing the ionophore from the culture medium. The results indicate that procollagen and fibronectin may be simultaneously secreted, possibly utilizing a common pathway for secretion; the ionophores effectively interfere with cellular secretory pathways without impairing protein synthesis or protein glycosylation or altering protein catabolism.
Keywords: cations, intracellular vesicles, protein glycosylation, collagen synthesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Church R. L., Tanzer M. L. Isolation and amino acid composition of human procollagen [Pro alpha 1(I)]2 Pro alpha 2 from skin fibroblasts in culture. FEBS Lett. 1975 Apr 15;53(1):105–109. doi: 10.1016/0014-5793(75)80694-6. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972 Apr;69(4):892–896. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Bornstein P. Microtubules in transcellular movement of procollagen. Nat New Biol. 1972 Aug 30;238(87):257–260. doi: 10.1038/newbio238257a0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Fessler J. H. Protein assembly of procollagen and effects of hydroxylation. J Biol Chem. 1974 Dec 10;249(23):7637–7646. [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The route of secretion of procollagen. The influence of alphaalpha'-bipyridyl, colchicine and antimycin A on the secretory process in embryonic-chick tendon and cartilage cells. Biochem J. 1976 Apr 15;156(1):81–90. doi: 10.1042/bj1560081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRREVERRE F., PIEZ K. A., WOLFF H. L. The separation and determination of cyclic imino acids. J Biol Chem. 1956 Dec;223(2):687–697. [PubMed] [Google Scholar]
- Jimenez S. A., Dehm P., Olsen B. R., Prokop D. J. Intracellular collagen and protocollagen from embryonic tendon cells. J Biol Chem. 1973 Jan 25;248(2):720–729. [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Kinetics for the secretion of procollagen by freshly isolated tendon cells. J Biol Chem. 1977 Dec 10;252(23):8391–8397. [PubMed] [Google Scholar]
- Kruse N. J., Bornstein P. The metabolic requirements for transcellular movement and secretion of collagen. J Biol Chem. 1975 Jul 10;250(13):4841–4847. [PubMed] [Google Scholar]
- Nist C., Von Der Mark K., Hay E. D., Olsen B. R., Bornstein P., Ross R., Dehm P. Location of procollagen in chick corneal and tendon fibroblasts with ferritin-conjugated antibodies. J Cell Biol. 1975 Apr;65(1):75–87. doi: 10.1083/jcb.65.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Garfield R. E., Chacko S., Somlyo A. V. Golgi organelle response to the antibiotic X537A. J Cell Biol. 1975 Aug;66(2):425–443. doi: 10.1083/jcb.66.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Rowland F. N., Murray L. W., Kaplan J. Inhibitory effects of tunicamycin on procollagen biosynthesis and secretion. Biochim Biophys Acta. 1977 Nov 7;500(1):187–196. doi: 10.1016/0304-4165(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Détraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978 Dec;79(3):694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L., Coulombre A. J. Morphogenesis of the collagenous stroma in the chick cornea. J Cell Biol. 1971 Sep;50(3):840–858. doi: 10.1083/jcb.50.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger J. A., Church R. L., Tanzer M. L. Structure of cultured fibroblasts from dermatosparaxic calves. Vet Pathol. 1975;12(1):16–31. doi: 10.1177/030098587501200104. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]


