Abstract
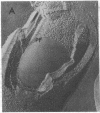
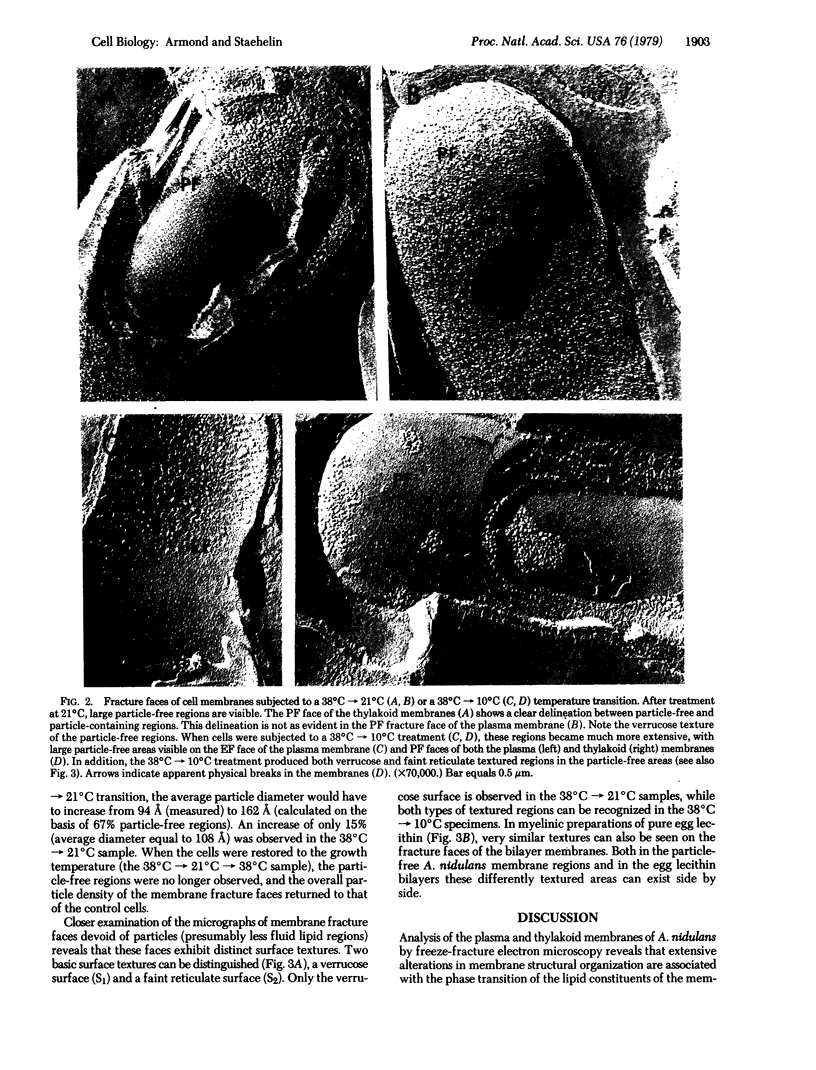
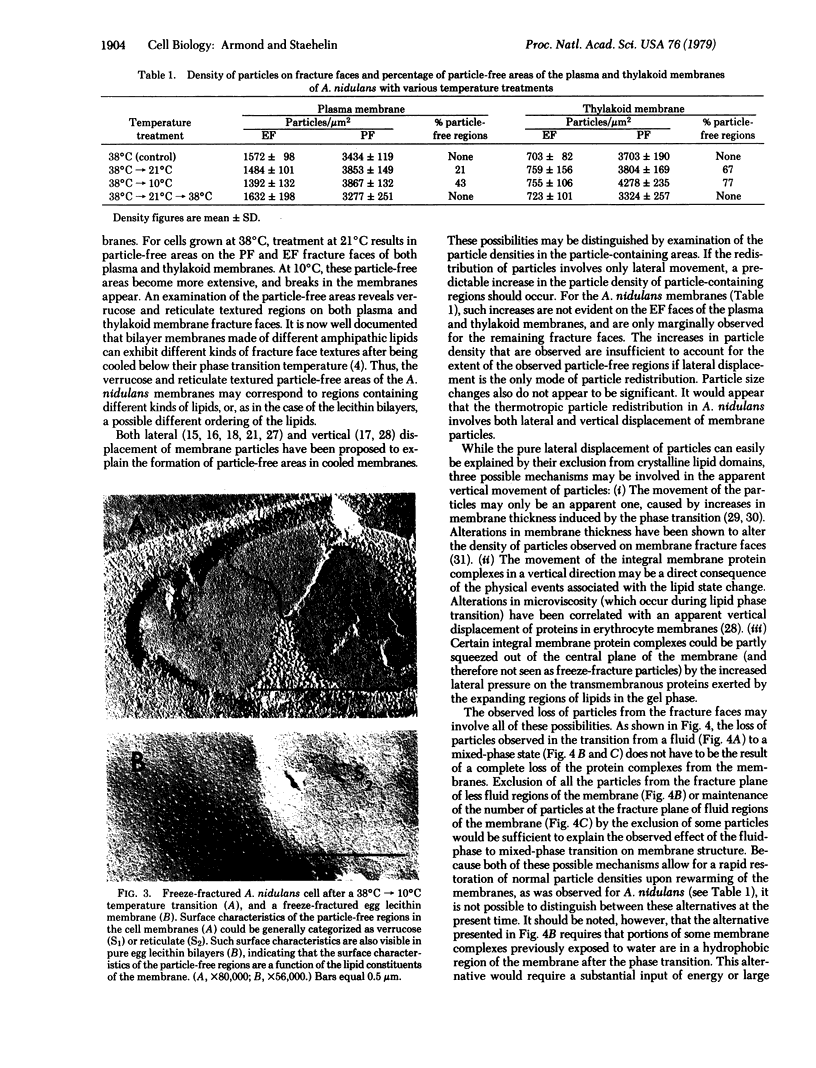
Alterations in membrane structure as a result of lipid phase transitions have been studied in Anacystis nidulans, a blue-green alga. Cells grown at 38 degrees C were subjected to temperature transitions of 38 degrees C leads to 21 degrees C and 38 degrees C leads to 10 degrees C, previously shown to produce substantial changes in photosynthetic activities, and examined by freeze-fracture electron microscopy. As a result of these treatments, large particle-free regions appeared on the fracture faces of both the plasma and thylakoid membranes. Particle density measurements suggest that the displacement of the integral membrane protein complexes occurs in both lateral and vertical directions. Returning the cells to 38 degrees C resulted in the restoration of normal membrane morphology, indicating that the proteins were not lost from the membrane. Such displacement of the integral membrane protein complexes could contribute significantly to the temperature-dependent alterations in the functional activity of membrane-bound enzymatic complexes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borochov H., Shinitzky M. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4526–4530. doi: 10.1073/pnas.73.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Duppel W., Dahl G. Effect of phase transition on the distribution of membrane-associated particles in microsomes. Biochim Biophys Acta. 1976 Mar 19;426(3):408–417. doi: 10.1016/0005-2736(76)90386-2. [DOI] [PubMed] [Google Scholar]
- Gulik-Krzywicki T. Structural studies of the associations between biological membrane components. Biochim Biophys Acta. 1975 Mar 25;415(1):1–28. doi: 10.1016/0304-4157(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Parsons D. F., Cowden M. Electron diffraction of wet phospholipid bilayers. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5068–5072. doi: 10.1073/pnas.71.12.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Parsons D. F. Direct observation of domains in wet lipid bilayers. Science. 1975 Oct 24;190(4212):383–384. doi: 10.1126/science.1179216. [DOI] [PubMed] [Google Scholar]
- Höchli M., Hackenbrock C. R. Thermotropic lateral translational motion of intramembrane particles in the inner mitochondrial membrane and its inhibition by artificial peripheral proteins. J Cell Biol. 1977 Feb;72(2):278–291. doi: 10.1083/jcb.72.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. E., Hudson B., Andersen H. C. A theory of the chain melting phase transition of aqueous phospholipid dispersions. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3993–3997. doi: 10.1073/pnas.72.10.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Branton D. Lipid- and temperature-dependent structural changes in Acholeplasma laidlawii cell membranes. Biochim Biophys Acta. 1973 Oct 25;323(3):378–390. doi: 10.1016/0005-2736(73)90183-1. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. I. Lipid phase transitions. Biochim Biophys Acta. 1977 Aug 9;472(2):237–281. doi: 10.1016/0304-4157(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. II. Mictures involving lipids. Biochim Biophys Acta. 1977 Nov 14;472(3-4):285–344. doi: 10.1016/0304-4157(77)90001-6. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. R., Staehelin L. A. Fine structure of the chloroplast membranes of Euglena gracilis as revealed by freeze-cleaving and deep-etching techniques. Protoplasma. 1973;77(1):55–78. doi: 10.1007/BF01287292. [DOI] [PubMed] [Google Scholar]
- Murata N., Fork D. C. Temperature dependence of the light-induced spectral shift of carotenoids in Cyanidium caldarium and higher plant leaves. Evidence for an effect of the physical phase of chloroplast membrane lipids on the permeability of the membrane to ions. Biochim Biophys Acta. 1977 Sep 14;461(3):365–378. doi: 10.1016/0005-2728(77)90226-2. [DOI] [PubMed] [Google Scholar]
- Murata N. Relationships between the Transition of the Physical Phase of Membrane Lipids and Photosynthetic Parameters in Anacystis nidulans and Lettuce and Spinach Chloroplasts. Plant Physiol. 1975 Oct;56(4):508–517. doi: 10.1104/pp.56.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan W. G., Smillie R. M. Multi-temperature effects on Hill reaction activity of barley chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):461–475. doi: 10.1016/0005-2728(76)90034-7. [DOI] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Moscarello M., Eylar E. H., Isac T. Effects of proteins on thermotropic phase transitions of phospholipid membranes. Biochim Biophys Acta. 1975 Sep 2;401(3):317–335. doi: 10.1016/0005-2736(75)90233-3. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Ladbrooke B. D., Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970 Jan 6;196(1):35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Green J. P., Nichols B. W. The phase behavior of monogalactosyl, digalactosyl, and sulphoquinovosyl diglycerides. Biochim Biophys Acta. 1973 Jul 18;311(4):531–544. doi: 10.1016/0005-2736(73)90128-4. [DOI] [PubMed] [Google Scholar]
- Träuble H., Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973 May 25;307(3):491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- Untrach S. H., Shipley G. G. Molecular interactions between lecithin and sphingomyelin. Temperature- and composition-dependent phase separation. J Biol Chem. 1977 Jul 10;252(13):4449–4457. [PubMed] [Google Scholar]
- Verkleij A. J., Ververgaert P. H., van Deenen L. L., Elbers P. F. Phase transitions of phospholipid bilayers and membranes of Acholeplasma laidlawii B visualized by freeze fracturing electron microscopy. Biochim Biophys Acta. 1972 Nov 2;288(2):326–332. doi: 10.1016/0005-2736(72)90253-2. [DOI] [PubMed] [Google Scholar]
- Verwer W., Ververgaert P. H., Leunissen-Bijvelt J., Verkleij A. J. Particle aggregation in photosynthetic membranes of the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1978 Oct 11;504(1):231–234. doi: 10.1016/0005-2728(78)90021-x. [DOI] [PubMed] [Google Scholar]
- Watts A., Marsh D., Knowles P. F. Characterization of dimyristoylphosphatidylcholine vesicles and their dimensional changes through the phase transition: molecular control of membrane morphology. Biochemistry. 1978 May 2;17(9):1792–1801. doi: 10.1021/bi00602a034. [DOI] [PubMed] [Google Scholar]
- Wunderlich F., Ronai A., Speth V., Seelig J., Blume A. Thermotropic lipid clustering in tetrahymena membranes. Biochemistry. 1975 Aug 26;14(17):3730–3735. doi: 10.1021/bi00688a002. [DOI] [PubMed] [Google Scholar]