Abstract
The adsorption method of Tenax-TA absorbent with GC-MS was used to analyze diurnal rhythms of volatiles from undamaged holly plants, Viburnum awabuki Kock (Dipsacales: Adoxaceae) holly infested by the white-striped longhorned beetle, Batocera lineolata Chevrolat (Coleoptera: Cerambycidae). Electroantennography and a Y-tube olfactometer were used to compare and analyze electroantennogram and behavioral responses of unmated male and female adults to the volatiles from V. awabuki (both undamaged and infested plants). The results of the GC-MS analysis showed that phytosterol and alkane are major volatiles for V. awabuki. The relative content of V. awabuki volatiles changed during the day. Electroantennogram and behavioral responses of unmated male and female adults to the volatiles from both undamaged and infested plants of V. awabuki were stronger between 08:00 and 10:00 and 16:00 and 18:00, which is consistent with early morning and evening feeding behaviors of adults in the field.
Keywords: electroantennogram response, physiological condition
Introduction
The white-striped longhorned beetle, Batocera lineolata Chevrolat (Coleoptera: Cerambycidae), is a polyphagous wood-boring insect that feeds on more than 20 plant species belonging to taxonomically distant plant families (Salicaceae, Juglandaceae, Fagaceae, Rosaceae, Caprifoliaceae, Betuleae, Oleaceae, Moraceae, Euphorbiaceae) (Sun and Zhao 1991; Gao et al. 1995; Li et al. 2008a, 2009a; Liang et al. 2008;). B. lineolata is among the most important wood-boring pests on poplar and walnut in Southern and Central China (Mei et al. 1998; Luo et al. 2000; Chen and Luo 2001) and can also damage white wax, birch, olive, rose, and holly (Xiao et al. 2003; Yang et al. 2010). B. lineolata is mainly distributed in China, Vietnam, Japan, India, and Myanmar (Chen et al. 1959). This species has one generation every 2–3 years and overwinters as larvae and adults in trunks (Yu et al. 2007). Overwintered adults exit their holes and feed on fresh bark at the end of April (Xia et al. 2005). Holly, Viburnum awabuki Kock (Dipsacales: Adoxaceae), is considered to be the preferred food for adults (Liang et al. 2008; Yang et al. 2011).
The larvae of B. lineolata damage the host plant, their borings criss-crossing within the trunk, and cause the eventual death of whole plants, which leads to a loss of commercial timber value (Li et al. 2009b). Currently, biological (nematode, parasitoid, and white mucardine fungi) and chemical (spraying with pyrethroids, organophosphates, and phenylpyrazoles) measures are applied to control B. lineolata larvae, but both methods are difficult and relatively ineffective (Zhu et al. 1995; Lu et al. 1996; Xiao et al. 2003; Li et al. 2008b). When B. lineolata adults emerge from their holes, they demonstrate a unique and obvious behavioral orientation (Liang et al. 2008), i.e., a tendency to move toward the preferred plant. Thus, it may be possible to develop new and effective control measures based on the method of detection of plants by B. lineolata adults.
Many phytophagous insects use odors as cues for orientation to food resources, either for their nutrition, mate location, or oviposition (Stanjek et al. 1997; Ruther et al. 2000; Inui et al. 2003; van Der Goes et al. 2006; Hu et al. 2009). Plants synthesize and release blends of volatile organic compounds (VOCs). Many plant VOCs have been identified, and these kairomones include fatty acid derivatives, phenyl-propanoids, and isoprenoids, among others (Visser 1986; Bernays and Graham 1988; Bruce et al. 2005). The plant VOCs released vary with factors such as age, physiological condition, diurnal rhythm, season, microhabitat, and environment (Lu et al. 2003). V. awabuki has been used as a trapping crop to lure B. lineolata adults in China (Liang et al. 2008; Yang et al. 2010, 2011), but the differences between undamaged and infested V. awabuki were unknown. Furthermore, when VOCs gave the strongest lure to B. lineolata during 1 day was also unknown.
In this study volatile chemical composition and amounts were compared between undamaged and infested V. awabuki branches, and electrophysiological and behavioral responses of B. lineolata to volatiles under different physiological conditions were assessed. This work may lead to the identification of plantbased attractants to be used in control and monitoring strategies for B. lineolata.
Materials and Methods
Insects
B. lineolata adults were collected in fields near Luojiang, China, during April and May 2010. The adult beetles had just finished their emergence and had not mated. Female and male beetles were held in separate cages (60 cm × 60 cm × 60 cm, stainless steel mesh) at room temperature (25 ± 2° C) until the experiment. They were fed with fresh bark of V. awabuki. The characteristics used to identify mated B. lineolata were the villi on the abdomen of mated males and the obvious mating plaques on the backside of the mated females (Ji et al. 1996).
Plants
V. awabuki (1.5 m high and 2 cm DBH) taken from poplar woods in Luojiang County, Deyang City, Sichuan Province, were placed in pots (one plant per pot) in March 2010. The plants were watered once every 2 days and grew at room temperature. Potted V. awabuki were placed into the breeding cages (one pot per cage). After which placing the plants in the cages, 4 pairs of B. lineolata were allowed to feed on the plant for 24 hours. Beetles and their waste were removed before volatile extraction. Intact, undamaged plants were used for comparison.
Extraction and identification of volatiles
A polyester cooking bag (355 mm × 508 mm; Reynolds, www.reynoldskitchens.com)equipped with 2 glass tubes in a row, a charcoal filter tube, and a Tenax-TA tube (50 mg absorbent, 60–80 mesh; glass outer diameter: 6 mm; length: 85 mm; Supelco, www.sigmaaldrich.com) were used to cover V. awabuki. Air was pumped in by a pocket pump QC-2B (Beijing Labor Protection Research Institute, China) from the charcoal filter tube to the polyester cooking bag, and then pumped into the Tenax-TA tube at 0.5 L/min. VOCs were collected for 1 hr at room temperature. Then, 5 mL of steamed n-hexane(chemically pure by Chengdu Kelong Chemical Reagent Factory, www.cdkelong.com) was used repeatedly to clean the Tenax-TA tube, and the solution was placed into the sample bottle of 8 mL. Next, N2 was used to concentrate the solution by 2 mL, and the solution was then stored in an ultra cold freezer (Sanyo, www.panasonic.net/sanyo) and used for volatile identification, electroantennogram (EAG), and behavioral measurement. One sample of the volatile of undamaged and infested V. awabuki was taken every 2 hours from 08:00 to 18:00.
Extracts were analyzed by GC-MS using a Shimadzu gas chromatograph (model 17A, www.shimadzu.com) coupled to a Shimadzu QP5050A electron ionization mass detector. The GC was operated in the splitless mode and was equipped with a DB-5 capillary column (30 m × 0.25 mm × 0.25 µm) (Agilent Technologies, www.agilent.com). The column's oven temperature was programmed to rise from an initial temperature of 40° C (3 min) to 220° C at 5° C per minute and then to 250° C at 8° C per minute. The temperature was then maintained for 5 min. In the mass spectrometer, the electron impact ion source voltage was 70 eV. The temperature for the GC/MS connector was 250° C, and the temperature of the ion source was 200° C. The scanning speed was 0.4 sec, and the scanning range was 40–450 m/z. The electric current in the filament was 150 µA. The mass spectrogram was checked by the standard mass spectrogram from the NIST database in this device, and the relative materials were consulted (Hao and Ha 2000; Zhao et al. 2001) to determine the chemical component.
Electroantennogram experiments
Recordings of the responses of female and male B. lineolata antennae to plant VOCs were made using Syntech (www.syntech.nl)equipment comprising micromanipulators, a CS-05 stimulus air controller, and an IDAC signal connection box for data acquisition. EAG signals and data were analyzed using a customized software package (EAG for Windows XP; Syntech. The antennae of B. lineolata were excised and mounted between Ag and AgCl glass electrodes filled with Ringer solution (Roelofs 1984; Teodora et al. 2010).
A 2 µL odor source sample was taken with a micro-sampler. It was uniformly dripped onto folded filter paper (1.5 × 1.5 cm) that was put into a 10 cm sample tube. The end of the sample tube was connected to the odor stimulating control device. When the baseline was stable, the antenna was stimulated. The stimulation time was 0.5 sec, and the interval between stimulations was 30 sec, which permitted recovery of the antennal receptors. For each compound, 6 antennae (from 6 different adults) were tested, and each antenna was stimulated 5 times. Distilled hexane was used as the standard, and the mean of the observed value for each sample was divided by the mean of the 2 standard values to give the relative value of antennal responses.
Bioassay of the behavioral response
A glass Y-tube olfactometer with an inside diameter of 15 cm was used to conduct the bioassays. The main arm of the device was 30 cm long, and the 2 side arms were 25 cm long. The angle between the 2 side arms was 75°, and the ends of the arms had ground-glass edges. The 2 side arms were connected to 2 250 mL volumetric flasks by Teflon tubes. A micro-sampler was used to extract 10 µL VOCs and solution (distilled hexane) for the comparison respectively, and then each was dropped on filter papers (1 × 1 cm). Filter papers were put into the 2 volumetric flasks separately. The volumetric flasks were connected by Teflon tubes to a distilled water humidification bottle and a charcoal filter. The air flow speed was controlled at 0.5–…0.6 L/min. Tests were run 08:00–12:00 when the temperature of the laboratory was 25 ± 2° C. B. lineolata adults were introduced into the inlet of the main arm of the Y-tube, and timing began after they had moved forward 10 cm from the inlet. Adults had to make a choice at the junction of the Y-tube to 1 of the side arms. Tests were conducted for 5 minutes to observe each B. lineolata adult. If an insect went forward 10 cm into a side arm and stayed for at least 1 minute, it was recorded as having made a choice of this odor; otherwise, it was recorded to have made no choice. After every 5 adults, the 2 arms of the Y-tube were interchanged to eliminate the possible influence of the different arms on the behavior of B. lineolata adults. When each treatment was finished, the Y-tube olfactometer, Teflon tubes, and volumetric flasks were washed with alcohol and allowed to air dry. Each treatment was performed for 30 B. lineolata adults in the same eclosion time, and each B. lineolata adult was used only once.
Statistical analyses
Statistical analyses was performed using the SPSS10.0 statistical package (SPSS Inc, IBM, www.ibm.com). A one-factor randomized complete block ANOVA was conducted on the EAG data and the relative content of VOCs. Fisher's protected least significant difference LSD multiple comparison procedure was used in EAG responses of unmated male and female adults to VOCs from V. awabuki and relative content change of V. awabuki VOCs at different times (Dong et al. 2000; Liu et al. 2005). A Chi-square test was used to compare the rate of attraction (Sokal and Rohlf 1995). The rate of attraction was calculated according to the following formulas (Ding et al. 1996; Yan et al. 2006):
Results
Comparison of compositions of VOCs from undamaged and infested V. awabuki
The results of GC-MS analysis showed that phytosterols such as benzyl alcohol, octanol, and isohexyl alcohol, and alkanes such as decane, tridecane, hexadecane, and 2-methyl octane were major VOCs for V. awabuki. There were 14 compounds in undamaged plants (Figure 1) and 18 compounds in plants infested by B. lineolata (Figure 2).
Daily changes in relative contents of V. awabuki VOCs
For relative contents of V. awabuki VOCs from undamaged hosts, there was an obvious daily change in nonanal, decanal, butyl acrylate, and diisobutyl phthalate (Figure 3). Relative peak content of nonanal and butyl acrylate appeared at 18:00, while peak content of decanal appeared at 10:00 and peak content of diisobutyl phthalate appeared at 16:00.
For relative contents of V. awabuki VOCs from infested hosts, there was an obvious daily change in limonene, 3-butyric acid methyl, octanol, and (Z)-3-hexene-1-alcohol (Figure 4). The relative peak content of limonene and 3-butyric acid methyl appeared at 18:00, while the peak content of octanol and (Z)-3-hexene1-alcohol appeared at 08:00.
EAG response of B. lineolata adults to V. awabuki VOCs at different times
In order to evaluate the electrophysiological activity, VOCs in different periods were tested for the EAG responses they elicited in B. lineolata antennae. The highest EAG responses of unmated females to VOCs from undamagedand infested plants occurred at 18:00 (p >0.05; Figure 5). For the 2 different physiology states of V. awabuki, there was a significant difference among EAG responses of unmated males at 08:00, 12:00, 16:00, and 18:00 (p < 0.05).
Figure 1.
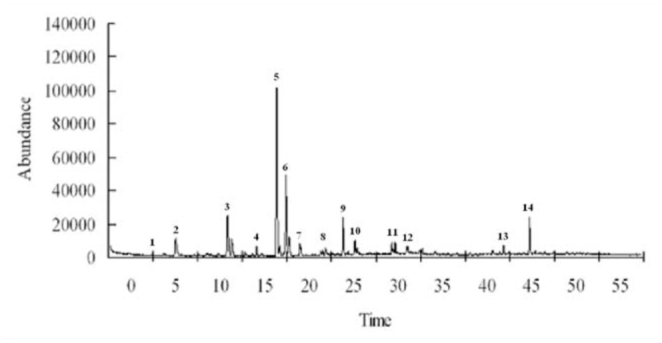
GC-MS analyses of volatiles from undamaged Viburnum awabuki. The 14 compounds are: benzyl alcohol (1), decane (2), nonanal (3), tridecane (4), limonene (5), 3-methyl-butanoic acid (6), decanal (7), isohexyl alcohol (8), hexadecane (9), isobutyl phthalate (10), butanamide (11), 2-methyloctane (12), butyl acrylate (13), and (Z)-3-hexen-l-ol (14). High quality figures are available online.
Figure 2.
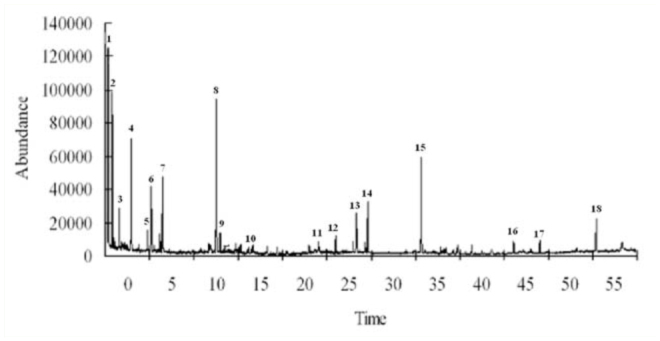
GC-MS analyses of volatiles from infested Viburnum awabuki. The 18 compounds are permethyl 99A (1), octyl alcohol (2), nonanal (3), cyclooctatetraene (4), limonene (5), decanal (6), tridecane (7), 3-methyl-butanoic acid (8), butyl acrylate (9), heptyl chloroacetate (10), hexanal (11), isobutyl phthalate (12), 2-bromo-octane (13), butanamide (14), (Z)-3-hexen-1-ol (15), 3,4,5,6-tetramethyloctane (16), indole (17), and hexadecane (18). High quality figures are available online.
Figure 3.
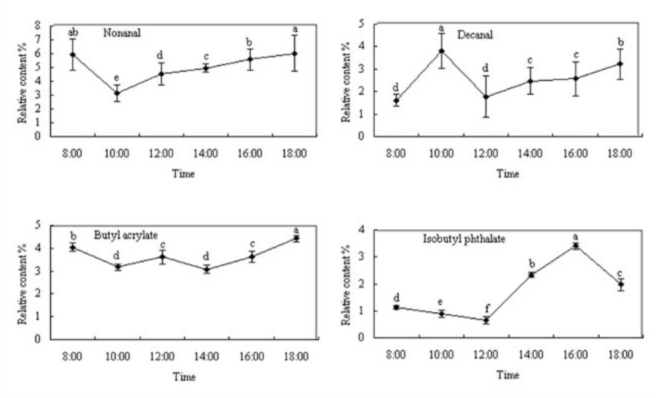
Daily dynamics of 4 volatile chemicals from undamaged Viburnum awabuki. Figures marked with the same lowercase letter were not significantly different (ANOVA followed by LSD, p < 0.05). Error bars ± SE (n = 3). High quality figures are available online.
Figure 4.
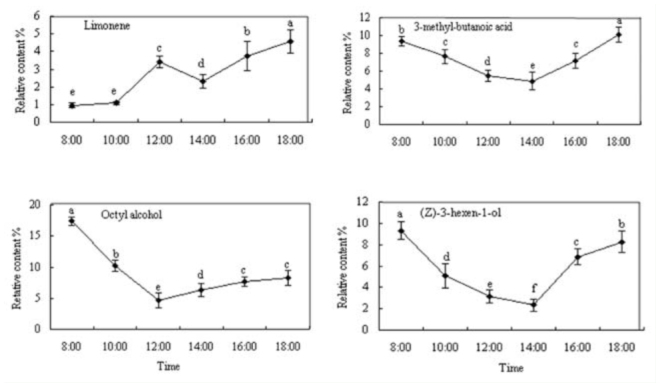
Daily dynamics of 4 volatile chemicals from infested Viburnum awabuki. Figures marked with the same lowercase letter were not significantly (ANOVA followed by LSD, p < 0.05). Error bars ± SE (n = 3). High quality figures are available online.
Figure 5.
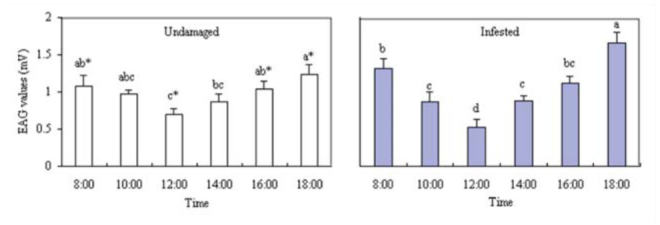
EAG responses of Batocera lineolata unmated females to VOCs from Viburnum awabuki at different times. White bars (undamaged plants) marked with the same lowercase letter were not significantly different. Grey bars (infested plants) marked with the same lowercase letter were not significantly different (ANOVA followed by LSD, p < 0.05). Asterisks signify a significant difference between undamaged and infested plants (t-test, p < 0.05). Error bars ± SE (n = 6). High quality figures are available online.
Figure 6.
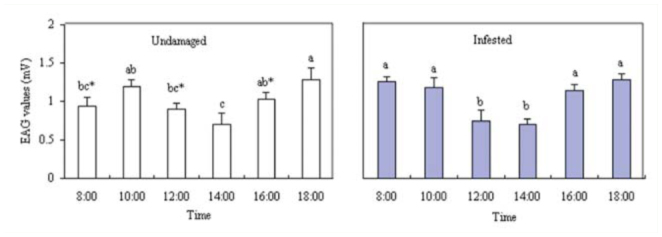
EAG responses of Batocera lineolata unmated males to VOCs from Viburnum awabuki at different times. White bars (undamaged plants) marked with the same lowercase letter were not significantly different. Grey bars (infested plants) marked with the same lowercase letter were not significantly different (ANOVA followed by LSD, p < 0.05). Asterisks signify a significant difference between undamaged and infested plants (t-test, p < 0.05). Error bars ± SE (n = 6). High quality figures are available online.
Figure 7.
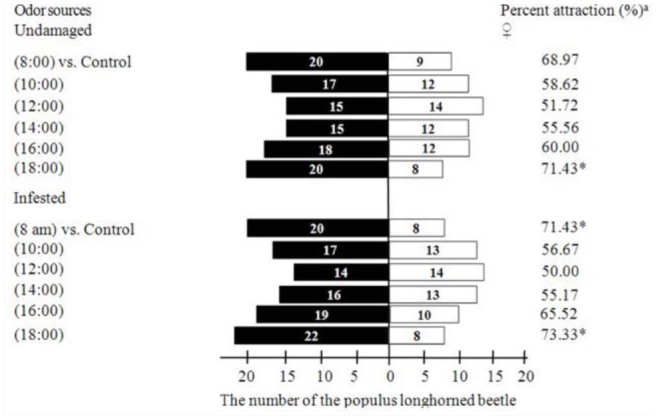
Behavioral response of Batocera lineolata unmated females to VOCs from Viburnum awabuki in different times. (White columns = control arms, black columns = treatment arms, n =30, when time more than 5 min, B. lineolata will not move toward control arms or treatment arms, which is regarded as “no response”). a Asterisk shows a significant rate of attraction (Chi-square test p < 0.05). High quality figures are available online.
There was no difference in EAG responses of unmated male to VOCs from infested plants at 08:00, 10:00, 16:00, and 18:00 (p > 0.05; Figure 6). The highest EAG responses of unmated males were at 18:00 (p > 0.05) from the undamaged plant. For the 2 different physiology states of V. awabuki, there was a significant difference among EAG responses of unmated males at 08:00, 12:00, 16:00, and 18:00 (p < 0.05).
Laboratory bioassay of B. lineolata adults to V. awabuki VOCs at different times
There was a strong attraction (higher rate of attraction) of V. awabuki VOCs to unmated female B. lineolata at different times (Figure 7). The result of the Chi-square test showed that there was a significant attractive effect from undamaged volatiles (18:00) toward unmated female B. lineolata (χ2 = 4.32; df = 1; p < 0.05), and a significant attractive effect from infested hosts’ (08:00) (χ2 = 4.32; df= 1; p < 0.05) volatiles (18:00) (χ2= 5.63; df = 1; p < 0.05) toward unmated female B. lineolata.
The highest rate of attraction of V. awabuki VOCs to unmated male B. lineolata at 18:00 was nearly 77% (Figure 8). The results of the Chi-square test showed that there was a significant attractive effect of undamaged volatiles (10:00) (χ2= 6.04; df = 1; p < 0.05) and infested volatiles (18:00) (χ2= 6.50; df = 1; p < 0.05) toward unmated B. lineolata adults.
Figure 8.
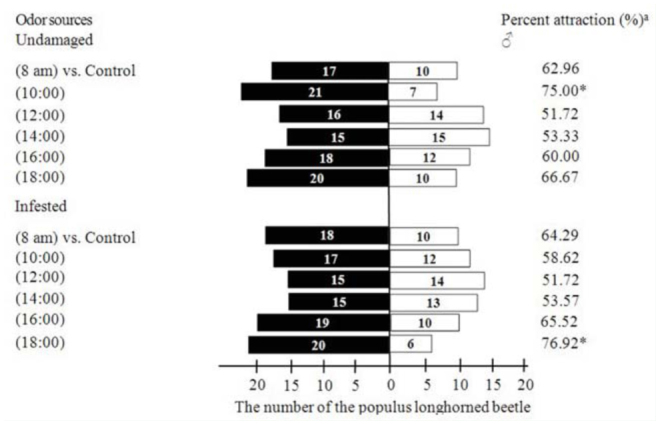
Behavioral response of Batocera lineolata unmated males to VOCs from Viburnum awabuki at different times. White columns = control arms, black columns = treatment arms, n = 30. When the time reached 5 min and the B. lineolata did not move toward the control or treatment arm it was regarded as “no response.” Asterisks signify a significant difference in rate of attraction (Chi-square test p < 0.05). High quality figures are available online.
Discussion
According to the results of GC-MS analysis, the two different V. awabuki states had different volatile compositions. After V. awabuki was used to feed B. lineolata, volatile composition changed and new compositions were found, such as permethyl 99A, octyl alcohol, cyclooctatetraene, heptyl chloroacetate, hexanal, 2-bromo-octane, 3,4,5,6tetramethyloctane, and indole. Research has shown that the type and quantity of volatiles released from plants bitten by phytophagous insects are different from normal volatiles (Mamiya and Enda 1972), and these new compositions are generated by the lure after the plant has been bitten by phytophagous insects (Degenhardt and Lincoln 2006). Paré and Tumlinson (1997) adopted CO2-impulse labeling to prove that those new compositions and VOCs that cannot be released by undamaged plants can be made after the plant has been bitten by phytophagous insects.
The relative content of nonanal, decanal, butyl acrylate, and diisobutyl phthalate released from undamaged V. awabuki, and limonene, 3-butyric acid methyl, octanol, and (Z)-3– hexene-1-alcohol from infested plants was different in 6 periods of time during the same day. According to the analysis of Loughrin et al. (1994), terpenoid content in the volatiles released from Spodoptera exigua has an obvious daily change. After Martin et al. (2003) took methyl jasmonate to spray on Picea abies, the induced volatile also had a rhythm of daily and nightly change. The research of Zhang et al. (1999) showed that monoterpene, sesquiterpene, and green volatiles from the branches of Betula pendula And Sambucus williamsii were influenced by temperature change during day time and increased with temperature rise within the range of 16–24° C, but there was no increase after the temperature rose above 24° C.
The EAG response of undamaged and infested plants of V. awabuki to unmated male and female B. lineolata was stronger between 08:00–10:00 and between16:00–18:00 than between 12:00–14:00. The behavior response results matched the EAG response results. It was found that the feeding behavior of plantfeeding insects matched the changes of certain components in host plants (Li et al. 2009). Yan et al. (1997) found that B. lineolata ate at dawn and dusk in the field, which matches the results of the EAG response test and the laboratory bioassay. Further study is required to determine how the circadian rhythm of the host plant affects the behavior of B. lineolata.
Plant volatiles induced by insects can effect the behavior selection of phytophagous insects and plays a role in chemical communication between individual plants (Visser 1986). Plant volatiles induced by insects are easily detectable to insects, and phytophagous insects can learn the status of host plants by the information in induced volatiles and perform corresponding behavior responses (Loughrin et al. 1995; Bolter et al. 1997; Dicke 1997). In our study, the EAG responses of unmated females to VOCs of infested plants were stronger at 08:00 and 18:00 than they were to undamaged plants at the same times, and the EAG responses for unmated males to VOCs of infested plants were stronger at 8:00 than they were to undamaged plants at the same time. Although there was no analysis of the difference between behavioral responses to VOCs from undamaged and infested plants, the lure rate matched the EAGs responses (Figures 7, 8), which means that VOCs released from infested V. awabuki had stronger attractiveness to B. lineolata.
VOCs could be utilized in developing traps for detecting and monitoring populations of B. lineolata. The behavioral role of other VOCs inducing relatively high electrophysiological activity on B. lineolata antennae needs to be studied in more detail.
Acknowledgements
We thank the anonymous reviewers for valuable comments on the manuscript. This work was funded by the National Natural Science Foundation of China (No. 31270694) and the Financing Project of the Construction of Ecological Forestry Engineering on the upstream of the Yangtze River.
Glossary
Abbreviations:
- EAG,
electroantennogram;
- VOC,
volatile organic compound
References
- Bernays EA, Graham M. On the evolution of host specificity in phytophagous arthropods. Ecology. 1988;69:886–892. [Google Scholar]
- Bolter CJ, Dicke M, van Loon JJA, Visser JH, Posthumus MA. Attraction of colorado potato beetle to herbivore damaged plants during herbivory and after its termination. Journal of Chemical Ecology. 1997;23:1003–1023. [Google Scholar]
- Bruce TJA, Wadhams LJ, Woodcock CM. Insect host location: a volatile situation. Trends in Plant Science. 2005;10:269–274. doi: 10.1016/j.tplants.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Chen JY, Luo YJ. The harmful characters and control countermeasure of poplar longhorned beetle in Jianghan Plain. China Forestry Science and Technology. 2001;16:46–48. [Google Scholar]
- Chen SX, Xie YZ, Zheng GF. Economic insect fauna of China-Cerambycidae. Science Press. 1959;3:84–85. [Google Scholar]
- Degenhardt DC, Lincoln DE. Volatile emissions from an odorous plant in response to herbivory and methyl jasmonate exposure. Journal of Chemical Ecology. 2006;32(4):725–743. doi: 10.1007/s10886-006-9030-2. [DOI] [PubMed] [Google Scholar]
- Dicke M. Induced indirect plant defense: communication and exploitation in multitrophic context. Mitteilungen Der Deutschen Gesellschaft Fuer Allgemeine Und Angewandte Entomologie. 1997;11:453–464. [Google Scholar]
- Ding HJ, Guo YY, Wu CH. Design and application of four arms olfactometry with the study of olfaction behavior of insect. Entomological Knowledge. 1996;33:241–243. [Google Scholar]
- Dong WX, Wang R, Zhang ZN. Electroantennal responses of a parasitoid (Microplitis mediator) to cotton plant volatiles. Acta Entomologica Sinica. 2000;43:119–125. (Supplement) (in Chinese) [Google Scholar]
- Gao RT, Wang HQ, Xu BX, Zheng SK, Wang XQ, Gong YH. Study on the habit of absorbing replenishing nutrition of Batocera horsfieldi and its relation with the host trees. Forest Research. 1995;8(6):619–623. [Google Scholar]
- Hu J, Angeli S, Schuetz S, Luo Y, Hajek AE. Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis. Agricultural and Forest Entomology. 2009;11:359–375. [Google Scholar]
- Inui Y, Miyamoto Y, Ohgushi T. Comparison of volatile leaf compounds and herbivorous insect communities on three willow species. Population Ecology. 2003;45:41–46. [Google Scholar]
- Ji BZ, Qian FJ, Yan AJ. Improvement of method of Batocera horsfieldi (Hope). Forest Pest and Disease. 1996;1:45–46. [Google Scholar]
- Li J, Wang MQ, Zhang ZC, Chen JY, Zhang GA. Behavioral response of Batocera horsfieldi adults to plant volatiles. Scientia Silvae Sinicae. 2008a;44(6):168–170. [Google Scholar]
- Li JQ, Mei ZX, Yang ZQ, Dong BT. Comparison on the bioassay methods of the toxicity of Beauveria bassiana metabolites to Batocera horsfieldi on poplars. Journal of Anhui Agricultural Sciences. 2008b;36(2):630–631. [Google Scholar]
- Li JQ, Yang ZQ, Mei ZX, Zhang YL. Pest risk analysis and control countermeasure of Batocera horsfieldi. Forestry Research. 2009a;22:148–153. [Google Scholar]
- Li XG, Yang LJ, Liu LP, Liu HX. Host selection of adult Dioryctriapryeri. Scientia Silvae Sinicae. 2009b;45(2):75–81. [Google Scholar]
- Liang XY, Yang W, Yang YL, Yang CP, Yang Y. Preference of Batocera horsfieldi for adults to feeding plants. Chinese Bulletin of Entomology. 2008;45:78–82. [Google Scholar]
- Liu Y, Guo GX, Chen JL, Ni HX. Behavioral and electrophysiological responses of four predatory insect species to semiochemicals of wheat. Acta Entomologica Sinica. 2005;48:161–165. [Google Scholar]
- Loughrin JH, Manukian A, Heach RR, Turlings TCJ, Tumlinson JH. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proceedings National Academy of Sciences USA. 1994;91:11836–11840. doi: 10.1073/pnas.91.25.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrin JH, Potter DA, Hamilton-Kemp TR. Volatile compounds induced by herbivory act as aggregation kairomones for the Japanese beetle (Popillia japonica Newman). Journal of Chemical Ecology. 1995;21(10):1457–1467. doi: 10.1007/BF02035145. [DOI] [PubMed] [Google Scholar]
- Lu XH, Du L, Qu AJ. Orientation of Harmonia axyridis ab. spectabilis to plant and prey combinations. Chinese Journal of Biological Control. 2003;22(4):279–282. [Google Scholar]
- Lu XP, Zhu CX, Liu Y, Wang H. Application on Steinernema feltiae A24 control Batocera horsfieldi larvae. Plant Protection. 1996;22(4):43–44. [Google Scholar]
- Luo YQ, Huang JF, Li JG. Study on the achievement, problem and prospect of the populus longhorned beetle in China. Chinese Bulletin of Entomology. 2000;37(2):116–122. [Google Scholar]
- Mamiya Y, Enda N. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Coleoptera:Cerambycidae). Nematologica. 1972;18:159–162. [Google Scholar]
- Martin D, Gershenzon J, Bohlmann J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of norway spruce. Plant Physiology. 2003;132(3):1586–1598. doi: 10.1104/pp.103.021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei AH, Chen JY, Wu GY, Du XS, Luo FS. The investigation of pests, causes of outbreak and control strategy of Poplar in Jianghan Plain. Forest Pest and Disease. 1998;2:35–39. [Google Scholar]
- Paré PW, Tumlinson JH. Induced synthesis of plant volatiles. Nature. 1997;385:30–31. [Google Scholar]
- Ruther J, Reinecke A, Thiemann K, Tolasch T, Francke W, Hilker M. Mate finding in the forest cockchafer, Melolontha hippocastani, mediated by volatiles from plants and females. Physiological Entomology. 2000;25:172–179. [Google Scholar]
- Stanjek V, Herhaus C, Ritgen U, Boland W, Städler E. Changes in the leaf surface chemistry of Apium graveolens (Apiaceae) stimulated by jasmonic acid and perceived by a specialist insect. Helvetica Chimica Acta. 1997;80:1408–1420. [Google Scholar]
- Sun QY, Zhao ZC. A preliminary study on Batocera horsfieldi. Journal of Jiangsu Forestry Science and Technology. 1991;2:22–25. [Google Scholar]
- van Der Goes, van Naters W, Carlson JR. Insects as chemosensors of humans and crops. Nature. 2006;444:302–307. doi: 10.1038/nature05403. [DOI] [PubMed] [Google Scholar]
- Visser JH. Host odor perception in phytophagous insects. Annual Review of Entomology. 1986;31:121–144. [Google Scholar]
- Xia JP, Dai JH, Liu LD, Hu XY. Progress in research on Batocera horsfieldi. J Hubei Forestry Science and Technology. 2005;132:42–44. [Google Scholar]
- Xiao YB, Zhou JH, Xiao YG, Zhao R, Xiao SF. A primary report on using Scleroderma sichuanensis to control Batoceral horsfieldi Hope. Journal of Sichuan Forestry Science and Technology. 2003;24(4):37–41. [Google Scholar]
- Yan HJ, Ji BZ, Qian FJ. A study on Batocera horsfieldi (Hope). Journal of Nanjing Forestry University. 1997;21(1):1–6. [Google Scholar]
- Yan SC, Cheng H, Yang H, Yuan HE, Zhang J, Chi DF. Effects of plant volatiles on the EAG response and behavior of the grey tiger longicorn, Xylotrechus rusticus (L.) (Coleoptera: Cerambycidae). Acta Entomologica Sinica. 2006;49:759–767. [Google Scholar]
- Yang H, Yang W, Liang XY, Yang MF, Yang CP, Zhu TH, Wu XL. The EAG and behavioral responses of Batocera horsfieldi (Coleoptera: Cerambycidae) to the composition of volatiles. Journal of the Kansas Entomological Society. 2011;84(3):217–231. [Google Scholar]
- Yang H, Yang W, Yang MF, Yang CP, Zhu TH, Huang Q. Effects of plant volatiles on the EAG and behavioral responses of Batocera horsfieldi Hope (Coleoptera: Cerambycidae). Journal of Agricultural and Urban Entomology. 2010;27(1):20–32. [Google Scholar]
- Yu JX, Zhang YR, Zhong WH. Study on the occurrence and non-pollution control of Batocera horsfieldi on poplar. Journal of Huinan Forestry Science and Technology. 2007;34(5):30–31. [Google Scholar]
- Zhang QH, Birgersson G, Zhu JW, Löfstedt C, Löfqvist J, Schlyter F. Leaf volatiles from nonhost deciduous trees: variation by tree species, season and temperature and electrophysiological activity in Ips typographus. Journal of Chemical Ecology. 1999;25(8):1925–1943. [Google Scholar]
- Zhu ZC, Tang JG, Xia MZ, Chen XF, Tian CJ. Tests on the effectiveness of the compound microcapsule and its productive technological design. Journal of Southwest Forestry College. 1995;15(1):44–52. [Google Scholar]


