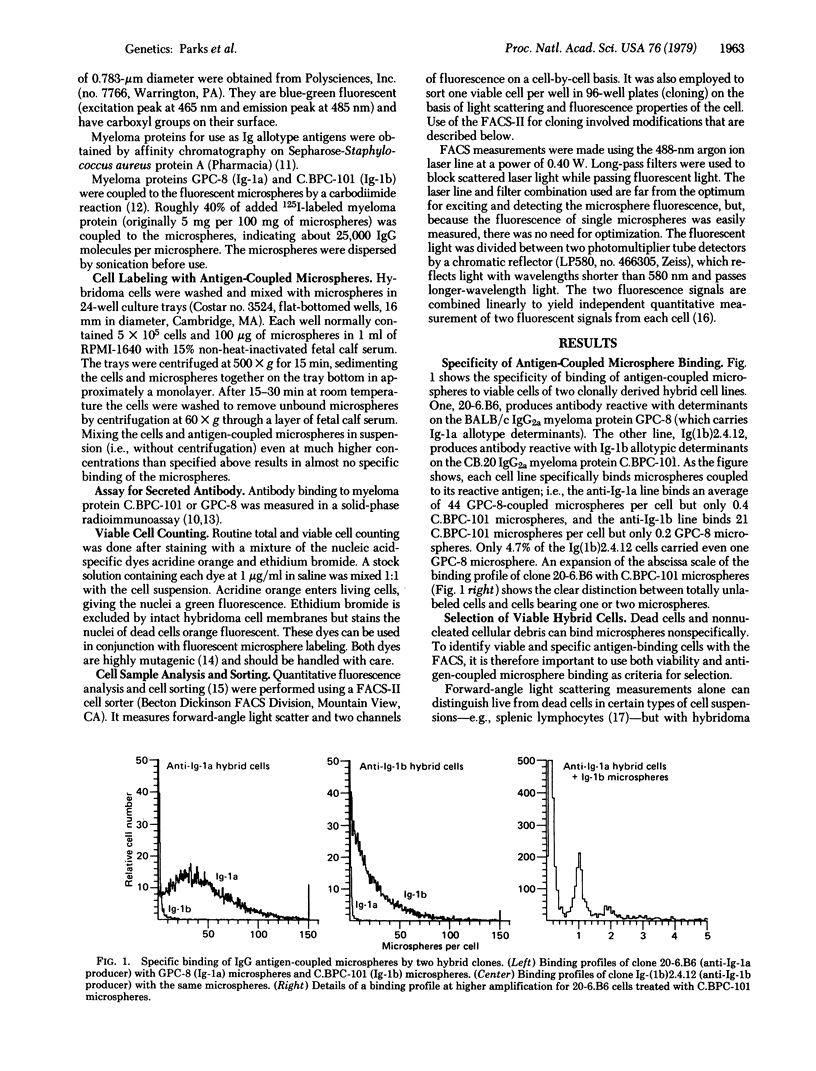
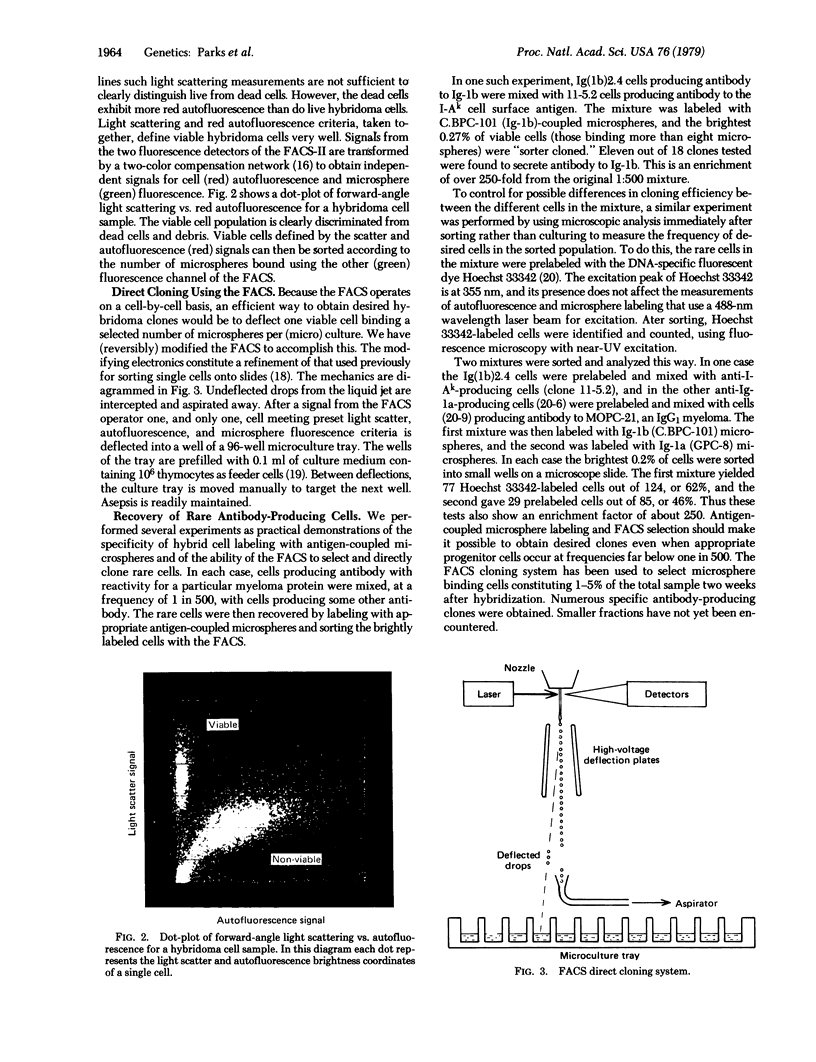
Abstract
Myeloma-spleen cell hybrids (hybridomas) producing antibody to mouse immunoglobulin allotypes have been labeled with fluorescent microspheres coupled with myeloma protein antigens. The ratio of specific to nonspecific microsphere binding by viable hybridoma cells was about 100:1. By using a modified fluorescence-activated cell sorter (FACS), selected hybridoma cells in a mixture have been sorted individually into media in microculture wells, where, with thymocyte feeder cells, they developed into clones producing a desired monoclonal antibody. Viable cells were selected by measurement of their light scattering and autofluorescence properties. Rare antibody-producing clones were obtained without laborious screening and repeated subculturing. This technique should expand the range of monoclonal antibodies readily obtained from hybridomas and greatly facilitate the process of obtaining desired hybridomas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Jovin T. M. Analysis and sorting of living cells according to deoxyribonucleic acid content. J Histochem Cytochem. 1977 Jul;25(7):585–589. doi: 10.1177/25.7.70450. [DOI] [PubMed] [Google Scholar]
- Bonner W. A., Hulett H. R., Sweet R. G., Herzenberg L. A. Fluorescence activated cell sorting. Rev Sci Instrum. 1972 Mar;43(3):404–409. doi: 10.1063/1.1685647. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Jones P. P., Cebra J. J., Herzenberg L. A. Immunoglobulin (Ig) allotype markers on rabbit lymphocytes: separation of cells bearing different allotypes and demonstration of the binding of Ig to lymphoid cell membranes. J Immunol. 1973 Nov;111(5):1334–1348. [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Lernhardt W., Andersson J., Coutinho A., Melchers F. Cloning of murine transformed cell lines in suspension culture with efficiencies near 100%. Exp Cell Res. 1978 Feb;111(2):309–316. doi: 10.1016/0014-4827(78)90175-1. [DOI] [PubMed] [Google Scholar]
- Liesegang B., Radbruch A., Rajewsky K. Isolation of myeloma variants with predefined variant surface immunoglobulin by cell sorting. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3901–3905. doi: 10.1073/pnas.75.8.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Loken M. R., Parks D. R., Herzenberg L. A. Two-color immunofluorescence using a fluorescence-activated cell sorter. J Histochem Cytochem. 1977 Jul;25(7):899–907. doi: 10.1177/25.7.330738. [DOI] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday R. S., Dreyer W. J., Rembaum A., Yen S. P. New immunolatex spheres: visual markers of antigens on lymphocytes for scanning electron microscopy. J Cell Biol. 1975 Jan;64(1):75–88. doi: 10.1083/jcb.64.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday R. S., Yen S. P., Rembaum A. Application of magnetic microspheres in labelling and separation of cells. Nature. 1977 Aug 4;268(5619):437–438. doi: 10.1038/268437a0. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Stovel R. T., Sweet R. G. Individual cell sorting. J Histochem Cytochem. 1979 Jan;27(1):284–288. doi: 10.1177/27.1.374588. [DOI] [PubMed] [Google Scholar]
- Taylor C. R., Gordon I. L., Rembaum A., Russell R., Parker J., O'Brien R. L., Lukes R. J. Human B lymphocytes in giemsa stained preparations. J Immunol Methods. 1977;17(1-2):81–89. doi: 10.1016/0022-1759(77)90079-5. [DOI] [PubMed] [Google Scholar]