Abstract
Objective
The objective of this study was to investigate the role of Kruppel-like factor (KLF) 10, a zinc-finger transcription factor, in bone marrow-derived cell responses to arterial endothelial injury. Accumulating evidence indicates that bone marrow-derived progenitors are recruited to sites of vascular injury and contribute to endothelial repair.
Approach and Results
In response to carotid artery endothelial denudation, KLF10 mRNA expression was markedlyincreased in both bone marrow and circulating lin− progenitor cells. To examine the specific role for KLF10 in arterial re-endothelialization, we used two models of endothelial denudation (wire- and thermal-induced injury) of the carotid artery in WT and KLF10−/− mice. WT mice displayed higher areas of re-endothelialization compared to KLF10−/− mice following endothelial injury using either method. Bone marrow (BM) transplant studies revealed that re-constitution of KLF10−/− mice with WT BM fully rescued the defect in re-endothelialization and increased lin−CD34+KDR+ progenitors in the blood and injured carotid arteries. Conversely, reconstitution of WT mice with KLF10−/−BM re-capitulated the defects in re-endothelialization and peripheral cell progenitors. The media from cultured KLF10−/− BM progenitors was markedly inefficient at promoting endothelial cell growth and migration compared to the media from WT progenitors, indicative of defective paracrine trophic effects from KLF10−/− BM progenitors. Finally, BM-derived KLF10−/− lin− progenitors from reconstituted mice had reduced CXCR4 expression and impaired migratory responses.
Conclusions
Collectively, these observations demonstrate a protective role for BM-derived KLF10 in paracrine and homing responses important to arterial endothelial injury and highlight KLF10 as a possible therapeutic target to promote endothelial repair in vascular disease states.
Keywords: Re-endothelialization, vascular injury, Kruppel-like factor 10, bone marrow
Introduction
Injury to the vascular endothelium represents a critical upstream event that contributes to a broad spectrum of vascular disease states such as stent thrombosis, stent restenosis, prosthetic vascular graft disease, or transplantation arteriopathy, among others.1-3 Accumulating evidence suggests that circulating endothelial progenitor cells (EPCs), broadly defined as pro-angiogenic cells, constitute a population of bone marrow (BM)-derived stem and progenitor cells responsible for repairing injured tissue and initiating vascular endothelial repair primarily by incorporating into the vessel wall and elaborating paracrine factors.4-6 Inhibition of EPC mobilization, homing, or adhesion, has been shown to delay the reestablishment of the neoendothelium.4 However, the signaling pathways and transcriptional events orchestrating this complex interplay between the bone marrow niche and the vascular endothelium remain incompletely defined.
Krüppel-like factors (KLFs), a subclass of the zinc-finger family of transcription factors, participate in various aspects of cellular growth and differentiation and gene expression.7 Our laboratory and others have identified KLF10 as a critical regulator of diverse biological functions such as in osteoblasts, hepatocytes, cardiomyocytes, T cells, and bone marrow-derived progenitors.8-10 With respect to the latter, KLF10−/− bone marrow-derived EPCs were found to have reduced EPC function and TGF-β1 responsiveness, effects associated with impaired blood flow recovery after hindlimb ischemia in systemic KLF10−/− mice.9 These studies suggest the hypothesis that bone marrow-derived KLF10 expression may promote vascular reparative responses. To investigate the role of bone marrow KLF10 in endothelial arterial injury in vivo, we performed bone marrow transplant (BMT) studies in WT and KLF10−/− mice.
Our studies, herein, reveal a protective role for BM-derived KLF10 expression in orchestrating vascular endothelial repair mechanisms after carotid injury. These studies identify that KLF10-deficient BM progenitors exhibit both intrinsic defects in their ability to migrate and incorporate into the vessel wall and extrinsic paracrine defects in their ability to facilitate endothelial cell (EC) growth and migration.
Materials and Methods
A detailed Methods section is available in the Online Supplement.
Results
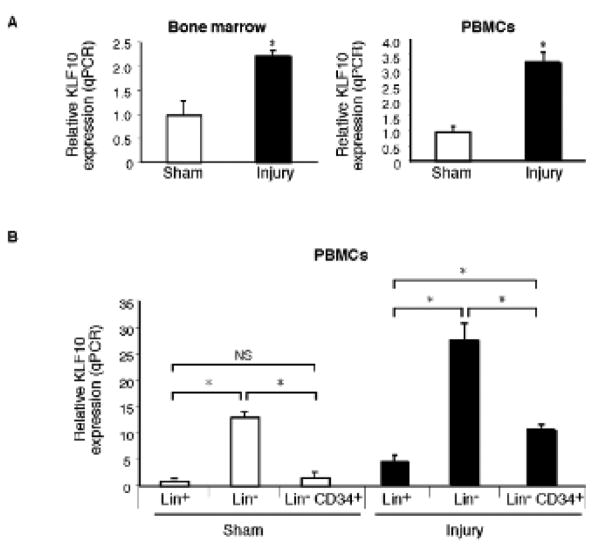
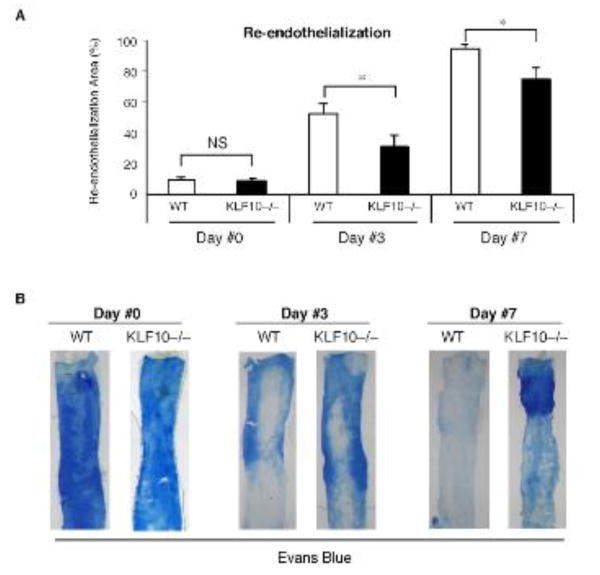
To examine the specific role for KLF10 in arterial re-endothelialization, we first used wire-induced endothelial injury and validated that carotid artery endothelial denudation was achieved using independent strategies including: 1) Evans blue staining; 2) silver nitrate perfusion staining11; 3) staining for β-gal in Tie2-LacZ mice; and 4) scanning electron microscopy (SEM).As shown in Supplemental Figure 1A,en face carotid artery preparations demonstrated highly efficient endothelialdenudation of the carotid artery using a 0.014″-PTCA guidewire as previouslydescribed.12 In response to wire-induced carotidartery endothelial denudation injury, KLF10 expression was induced ∼2-fold in the bonemarrow and ∼3-fold in circulating peripheral blood mononuclear cells (PBMCs) (Fig. 1A). Remarkably, examination of KLF10 mRNA expression in lin− and lin+ PBMCs demonstrated that KLF10 mRNA expression was strongly expressed in both lin− and lin−CD34+ cells by ∼13-fold and ∼2-fold, respectively, compared to lin+ circulating cells. Furthermore, in response to carotid artery endothelial denudation, KLF10 mRNA expression increased in lin− and lin− CD34+ cells by ∼6-fold and ∼2-fold, respectively, compared to lin+ circulating cells. To gain initial insights into whether KLF10-deficiency may impact re-endothelialization, WT and systemic KLF10−/− mice underwent wire-induced carotid artery endothelial denudation injury. The amount of re-endothelialization in the carotids was determined after Evans blue systemic perfusion on days 0, 3, and 7 after injury. WT mice displayed higher areas of re-endothelialization compared to KLF10−/− mice (day 3: WT 52.4% vs. KLF10−/− 30.6%, P=0.045; Day 7: WT 95.1% vs. KLF10−/− 75.1%, P=0.033; Figure 2A-B). In contrast, no differences were observed at day 0 after wire-injury. Using thermal-induced carotid injury as a complementary strategy, a 3-mm segment proximal of the carotid artery was denuded and re-endothelialization was verified after staining with Evans blue as described previously13, 14 (Supplemental Figure 2). Similar to the wire-induced model, re-endothelialization was significantly reduced in KLF10−/− mice compared to WT mice in response to thermal injury (Supplemental Figure 3).
Figure 1. KLF10 expression is increased in bone marrow and circulating lin− progenitor cells in response to carotid artery endothelial injury.
A, Expression of KLF10 in the bone marrow and peripheral blood mononuclear cells (PBMCs) after carotid endothelial artery denudation as quantified by qPCR. B, Expression of KLF10 in PBMCs FACS sorted for lin− and lin+ cells in the presence or absence of carotid artery endothelial injury (n=5-6/group). * P <0.05.
Figure 2. KLF10 deficiency impairs re-endothelialization in response to endothelial injury.
Wild-type (WT) or KLF10−/− mice were subjected to carotid artery endothelial wire-induced injury (A-B). The percent area of re-endothelialization was determined after Evans blue systemic perfusion for Days # 0, 3, and 7 (n=5-6/group). * P <0.05, one-way ANOVA, WT vs KLF10−/− mice.
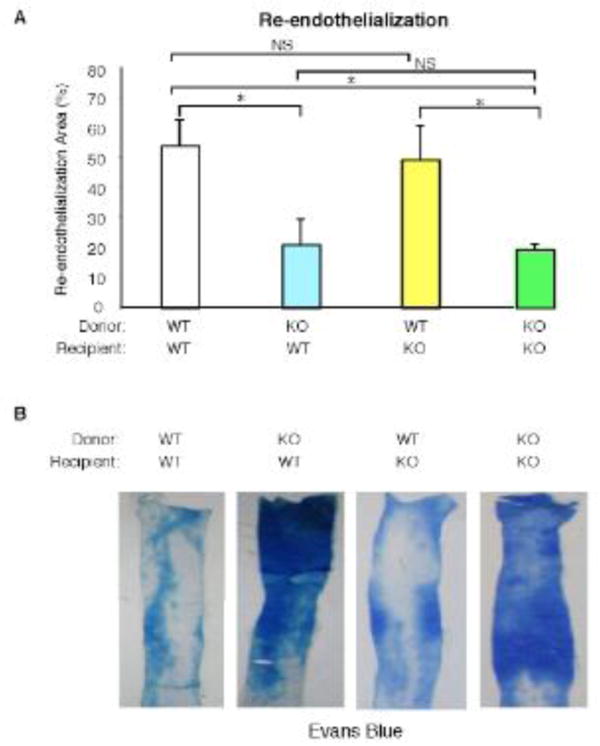
To examine the contribution of KLF10 from the bone marrow, we performed bone marrow transplantation (BMT) studies and examined re-endothelialization after 3 days in 4 groups (n=4-8 mice/group): WT BM into WT recipient mice (WT->WT), KLF10−/− BM into WT recipient mice (KLF10−/−->WT), WT BM into KLF10–/– recipient mice (WT-> KLF10−/−), and KLF10−/− BM into KLF10−/− recipient mice (KLF10−/−–> KLF10−/−). One month after BM reconstitution, wire-induced endothelial injury of the carotid arteries was performed and re-endothelialization areas were examined at 3 days. As shown in Figure 3, WT mice reconstituted with KLF10−/− BM displayed a 61% reduction in re-endothelialization compared to WT mice reconstituted with WT BM; conversely, KLF10−/− mice reconstituted with WT BM exhibited full recovery in re-endothelialization. The re-endothelialization areas were: WT->WT 54.4%; KLF10−/− ->WT 21.1%; WT-> KLF10−/− 49.4%; and KLF10−/−-> KLF10−/− 19.5%, n=5-8 mice/group). Taken together, these data indicate that bone marrow-derived KLF10 expression confers a protective effect in establishing the neoendothelium after carotid artery endothelial denudation.
Figure 3. Bone marrow-derived KLF10 regulates arterial endothelial repair.
WT or KLF10−/− mice reconstituted with either WT or KLF10−/− bone marrow (BM) were subjected to carotid artery endothelial wire injury (n=4-8/group). The percent area of re-endothelialization at three days was determined (A) by Evans blue systemic perfusion (B). * P <0.05; NS, non-significant.
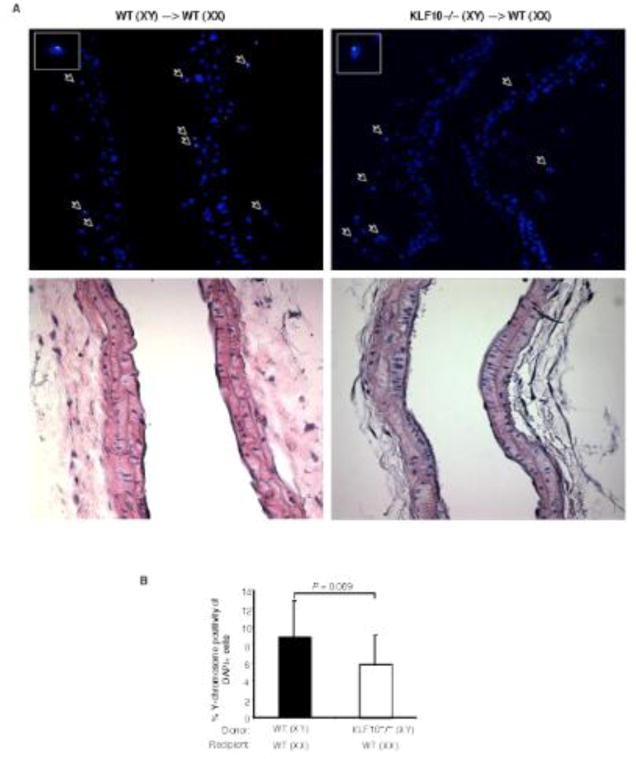
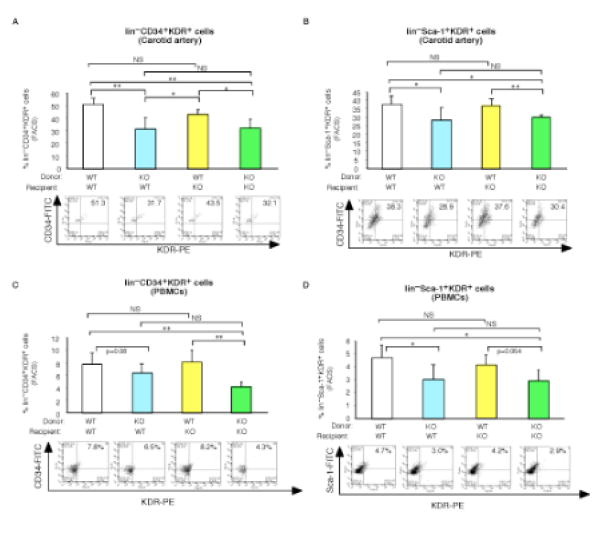
Accumulating studies demonstrate that circulating progenitors may contribute to vascular repair though both direct incorporation in the vessel wall or via paracrine effects.4, 15, 16 To examine the contribution of direct incorporation in the vessel wall, we performed gender mismatched, Y-chromosome tracking studies after BMT using female recipient mice reconstituted with male WT or KLF10−/− donor bone marrow cells. As shown in Figure 4, the percentage of DAPI cells that were Y-chromosome positive in the injured carotid artery of mice reconstituted with KLF10-deficient cells showed a non-significant lower trend compared to mice reconstituted with WT cells (WT–>WT, 9.83% of 3265 DAPI+cells vs. KLF10−/− –>WT, 5.37% of 2050 DAPI+ cells, P=0.069) as detected by fluorescence in situ hybridization (FISH). However, a closer examination of the carotid arteries specifically for lin−progenitor cells by flow cytometry revealed significant reductions in lin−CD34+KDR+ progenitors and lin−Sca-1+KDR+ progenitors by 38.2% and 24.4%, respectively, (Fig. 5A-B) in WT mice reconstituted with KLF10−/− BM, an effect that was completely rescued in KLF10−/− mice reconstituted with WT BM. Similar findings were observed for these progenitors from peripheral blood mononuclear cells (Fig. 5C-D). These findings suggest that KLF10-deficient lin− BM progenitors may have intrinsic functional defects in their ability to be recruited into the vessel wall after injury.
Figure 4. Gender mismatched, Y-chromosome tracking studies after bone-marrow transplant and carotid artery endothelial injury.
Female recipient mice were reconstituted with male WT or KLF10−/− donor bone marrow cells and underwent carotid artery endothelial injury. (A) Two days after injury, fluorescence in situ hybridization (FISH) (top) and H&E staining (bottom) was performed and the percentage of DAPI cells that were Y-chromosome positive in the injured carotid artery of WT female mice reconstituted with KLF10−/− or WT cells was determined (B). (n=3 mice/group) * P =0.069; one-tailed Student's t-test.
Figure 5. Bone marrow-derived KLF10 regulates accumulation of lin− progenitor cells in the injured carotid artery and PBMCs.
A-D, WT or KLF10−/− mice reconstituted with either WT or KLF10−/− bone marrow (BM) were subjected to carotid artery endothelial wire injury (n=4-8/group). Representative FACS panels are shown of the number of lin−CD34+KDR+ (A, C) and lin−Sca-1+KDR+ (B, D) progenitor cells in the carotid artery and PBMCs, respectively. * P <0.05; ** P <0.01; NS, non-significant.
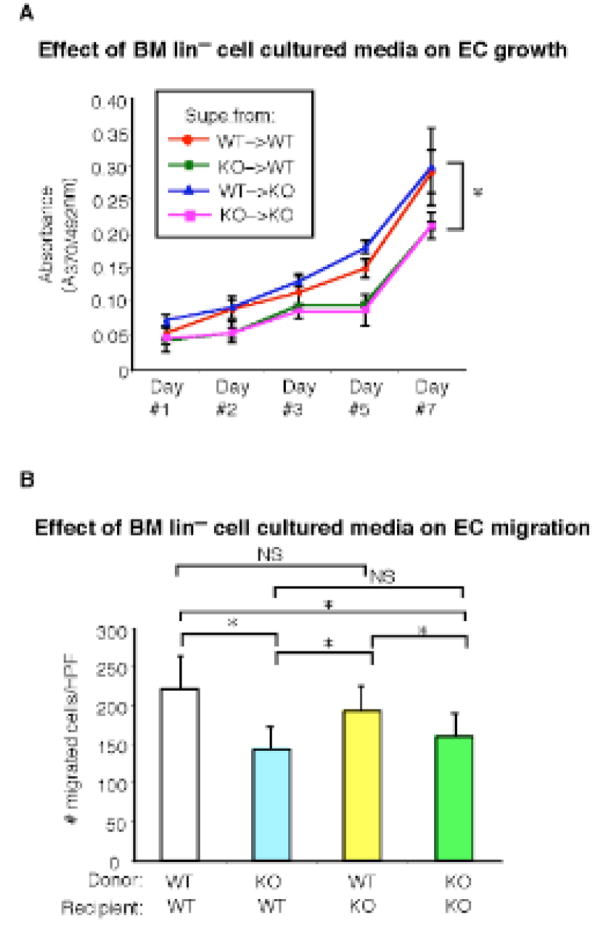
To explore whether the progenitors derived from the BM of reconstituted mice may possessaltered expression for key chemokine receptor markers implicated in bone marrow mobilization and homing, flow cytometry was performed across the four groups. As shown in Fig. 6A, WT mice reconstituted with KLF10−/− BM (Supplemental Fig. 4A) had significantly reduced expression on lin−c-kit+CD34+ progenitors for CXCR4 by 36.5%, a chemokine receptor implicated in EPC mobilization into tissues,17 whereas there was only a modest decrease in a different chemokine receptor CCR7(6.6%; Fig. 6B) or the VEGF receptor KDR (19%;Supplemental Fig. 4B). Moreover, BM from KLF10−/− mice reconstituted with WT BM completely rescued the reduction in CXCR4 and CCR7 expression (Fig. 6A-B). To investigate whether reduced expression for either CXCR4 or CCR7 may be associated with altered migratory responses, transwell Boyden chamber assays were performed using SDF-1α (ligand for CXCR4) and CCL21 (ligand for CCR7). As shown in Fig. 6C-D, WT mice reconstituted with KLF10−/− BM had lin−CD34+ progenitors with decreased migratory responses by 24.3% and 29.2% for SDF-1α and CCL21, respectively. In contrast, BM from KLF10−/− mice reconstituted with WT BM completely rescued the migratory defects in response to both ligands. In addition, BM lin−CD34+ progenitors from WT mice reconstituted with KLF10−/− BM exhibited reduced adhesion to fibronectin-coated plates, whereas KLF10−/− mice reconstituted with WT BM had no adhesion defects (Fig. 6E). Finally, to explore the paracrine and trophic effects of KLF10−/− BM lin− progenitors, media taken from each group of reconstituted BM progenitors grown in culture was used in BrdU endothelial growth assays and migration studies. As shown in Fig. 7A-B, media derived from KLF10−/− BM progenitors exhibited reduced ability to promote EC growth and migration by ∼60% and ∼36%, respectively, compared to WT reconstituted BM progenitors. Moreover, KLF10−/− reconstituted BM progenitor cells also displayed reduced cell surface CXCR4 expression by flow cytometry and impaired migratory responses (Supplemental Fig. 5). Collectively, these studies strongly implicate that KLF10-deficient BM progenitors exhibit both intrinsic defects in their ability to migrate and incorporate into the vessel wall and extrinsic paracrine defects in their ability to facilitate EC growth and migration.
Figure 6. Bone marrow-derived KLF10 regulates chemotaxis and adhesion.
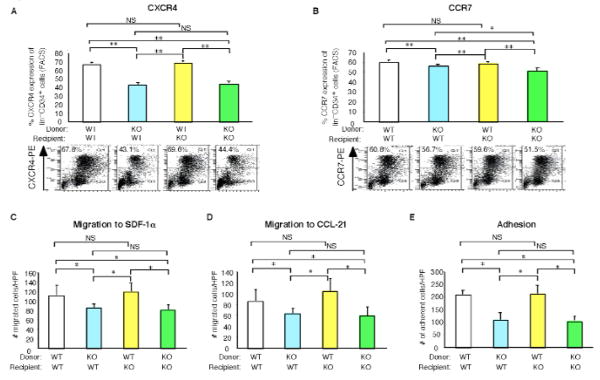
A-E, WT or KLF10−/− mice reconstituted with either WT or KLF10−/− bone marrow (BM) were subjected to carotid artery endothelial wire injury (n=4-8/group). Expression of CXCR4 (A) and CCR7 (B) by flow cytometry in BM lin−progenitors is shown. Migration of BM lin−CD34+ progenitors in response to SDF-1 (C) or CCL21 (D) by transwell Boyden chamber assays. (E) Adhesion of BM lin−CD34+ progenitors to fibronectin-coated plates was determined. (n=6/group) * P <0.05; ** P <0.01; NS, non-significant.
Figure 7. Media from cultured BM-derived KLF10−/− progenitors exhibits impaired ability to promote endothelial cell growth or migration.
A-B, WT or KLF10−/− mice reconstituted with either WT or KLF10−/− bone marrow (BM) were subjected to carotid artery endothelial wire injury (n=5 mice/group). The media of cultured BM lin−CD34+ progenitors from each group was used in BrdU endothelial cell growth assays (A) or transwell Boyden chamber endothelial migration assays (B) (n=10/group). In (A), for groups WT–>WT vs KO–>WT, WT–>WT vs KO–>KO, WT–>KO vs KO–>KO, and KO–>WT vs WT–>KO, * P <0.01, one-way ANOVA. For groups WT–>WT vs WT–>KO and KO–>WT vs KO–>KO, P =NS, one-way ANOVA. In (B), * P <0.05; NS, non-significant.
Discussion
The transcriptional events in the bone marrow niche that help orchestrate vascular arterial re-endothelialization after injury have not been well-defined. To our knowledge, this is the first experimental study addressing the importance of an endogenous transcription factor expressed in the bone marrow on arterial re-endothelialization. Our data show that KLF10 deficiency inhibits re-endothelialization following endothelial denudation, an effect associated with reduced circulating progenitors in the blood and within the carotid artery. BM reconstitution studies demonstrated that KLF10 expression in the bone marrow mediated both the reduced arterial re-endothelialization and the reduced lin− progenitor cells in the periphery and injured carotid artery. In addition, these studies revealed that KLF10−/− lin− progenitors exhibited impaired paracrine and trophic effects on endothelial cells. Finally, KLF10−/− lin− progenitors also displayed intrinsic defects in their ability to migrate with markedly reduced expression for the chemokine receptor CXCR4.
Our studies indicate that KLF10 is a novel regulator of bone marrow progenitor mobilization in response to endothelial injury. In support of these observations, progenitors from reconstituted mice with KLF10−/− BM have reduced expression of chemokine receptors CXCR4 and to a modest extent CCR7. Indeed, KLF10−/− BM progenitors had impaired migratory responses to their cognate ligands. Reduced expression of CXCR4, a critical receptor in regulating EPC homing and recruitment, has been associated with impaired progenitor homing to sites of endothelial injury despite an increase of SDF-1 expression locally in injured areas after endothelial denudation.18 Furthermore, disruption of SDF-1/CXCR4 signaling axis has been implicated in various pathophysiological contexts such as vascular injury, left ventricular remodeling after myocardial infarction, myocardial regeneration, neovascularization, and wound healing.18-23 We have previously demonstrated that systemic deficiency of KLF10 markedly reduces blood flow recovery and neovascularization after hindlimb ischemia.9 Whether, specifically, BM-derived KLF10 contributes to reparative functions in response to hindlimb ischemia or in the context of other cardiovascular injury states will require further study. KLF10−/− BM progenitors also exhibited reduced adhesion properties which may also adversely contribute to the endothelial reparative response. Taken together, these studies suggest that KLF10 positively regulates the bone marrow response to arterial endothelial injury.
These observations add to a growing list of factors that may contribute to BM progenitor cell mobilization and homing including colony-stimulating factors (ie. G-CSF, GM-CSF), growth factors (e.g. VEGF, TGF-β1), chemokines (e.g. SDF-1), angiogenic factors (e.g angiopoietin-1, thrombospondin-1), hormones (e.g. estrogen), drugs (e.g. PPARγ agonists, statins, erythropoietins), and other regulators of the bone marrow niche (e.g. eNOS, MMP-9, parathyroid hormone).17 Our previous studies revealed that in response to TGF-β1, KLF10 is induced in lin− BM progenitor subsets.9 Whether KLF10 participates in the signaling of these other diverse stimuli to mediate specific ligand-triggered BM progenitor cell homing will require additional investigation.
Several studies have identified the presence of BM-derived progenitor cells in the vessel wall in response to vascular injury states including carotid endothelial denudation, restenosis, atherosclerosis, and transplant arteriopathy.24-29 Several investigations have demonstrated the presence of bone marrow-derived cells in the media and peri-adventitial regions where they may exert functional paracrine effects on re-endothelialization and vascular repair.20, 24, 27, 29-33 Furthermore, a recent study indicated that the frequency of BM progenitors directly accumulating in the neo-endothelium of injured vessels is negligible.34 We found decreased subsets of key circulating KLF10−/− BM progenitor cells (lin−CD34+KDR+ and lin−Sca-1+KDR+ progenitors) in the vessel wall and revealed that the medium from cultured BM lin− KLF10−/− progenitors exhibited impaired paracrine and trophic functions in their ability to promote EC migration and growth compared to WT lin− progenitors. Our findings are in keeping with the emerging concept that circulating progenitors are not a source for endothelial cells during endothelial regeneration, whereas our studies highlight the importance of BM-derived progenitors for paracrine-mediated effects (e.g. on the flanking non-injured endothelial cells in the vasculature) and homing (e.g. to peri-adventitial regions) in the re-endothelialization response to vascular injury.
Finally, these findings bear several important clinical implications because delayed endothelial repair contributes prominently to the pathophysiology of stent thrombosis, stent restenosis, and prosthetic vascular graft disease.1-3 In particular, re-endothelialization is critically important for arterial healing after therapeutic angioplasty and stenting, and delayed re-endothelialization has been implicated as a major cause of stent thrombosis following drug-eluting stent (DES) implantation.35, 36 Current DES technologies prevent restenosis by releasing cytotoxic drugs which inhibit neointimal formation. Although these drugs prevent restenosis, they also delay re-endothelialization increasing risk of stent thrombosis. Indeed, human autopsy studies have shown that DES can have focal areas of defective re-endothelialization years after initial stent placement.37-39 Furthermore, due to the elevated risk of stent thrombosis with DES, current guidelines recommend extended treatment with antiplatelet therapies to reduce stent thrombosis risk.35, 40
Because of the risk of delayed arterial healing with current technologies, there is intense focus on developing new therapies that both quench inflammation and promote vascular repair. Several strategies that augment the homing or function of circulating cells that promote re-endothelialization are under evaluation in preclinical41, 42 and clinical studies.43-47 In this context, the findings, herein, that BM-derived KLF10 promotes arterial re-endothelialization raises the possibility that increasing KLF10 expression or function may provide a novel strategy to accelerate re-endothelialization after coronary stent placement. Diabetic subjects, in particular, have impaired endothelial repair mechanisms and are at higher risk for both stent thrombosis and stent restenosis.48 Experimentally, diabetes reduces re-endothelialization,12, 49 and we previously demonstrated that KLF10 expression in lin−CD34+ circulating progenitors is reduced by ∼70% in diabetic human subjects compared to healthy subject controls.9 Thus, strategies to increase KLF10 expression may have particular therapeutic potential in this vulnerable patient population.
In summary, in response to arterial endothelial denudation, KLF10 expression is increased in BM and circulating lin- progenitors. KLF10-deficient mice exhibit impaired re-endothelialization and reduced circulating lin- progenitors in the periphery and injured carotid artery, effects mediated by KLF10-deficiency in the BM. KLF10−/− BM lin− progenitors have both cell intrinsic and extrinsic defects that impair re-endothelialization. These defects include reduced expression of CXCR4, which may underlie the reduced homing of these cells to injured sites, and paracrine defects that may underlie the attenuated trophic effects on cultured endothelial cells. Taken together, our work demonstrates a critical role for KLF10 in regulating the bone marrow response to endothelial injury and highlights a potential opportunity to target KLF10 for therapeutic intervention to improve endothelial repair.
Significance
Accumulating evidence indicates that bone marrow (BM)-derived progenitors are recruited to sites of vascular injury and contribute to endothelial repair, primarily by paracrine mechanisms. However, the transcriptional events orchestrating this complex interplay between the BM niche and the vascular endothelium remain incompletely defined. The current study reveals a novel and unexpected role for an endogenous transcription factor, KLF10, expressed in the BM on arterial re-endothelialization. In response to carotid artery endothelial denudation, KLF10 expression is markedly increased in both the BM and circulating lin− progenitor cells. KLF10−/− mice displayed reduced areas of re-endothelialization compared to WT mice in response to injury, an effect verified using BM reconstitution studies. Finally, KLF10−/− mice exhibit defects in paracrine and homing responses important to arterial endothelial injury. Collectively, our findings highlight a protective role for BM-derived KLF10 to promote endothelial repair with therapeutic implications in vascular disease states.
Supplementary Material
Acknowledgments
We thank Richard N Mitchell for insightful suggestions and comments.
Sources of Funding: This work was supported by funding from the National Institutes of Health (HL091076 and HL115141 to M.W.F., F32HL088819 to A.K.W.), the American Cancer Society (Research Scholar Grant RSG0719501-LIB to M.W.F.), the Boston Area Diabetes Endocrinology Research Center (Pilot Grant P30DK057521 to M.W.F.), the J.P. Lemann Foundation (Jorge Paulo Lemann Harvard Medical School Cardiovascular Fellowship Grant to J.F.M.), CAPES Foundation, Brazil (to A.M), a Jonathan Levy Research Fund (to M.W.F.), and a Watkins Cardiovascular Medicine Discovery Award (to M.W.F.).
Footnotes
Disclosures: Mark W. Feinberg, Akm Wara, and the Brigham and Women's Hospital have a patent pending related to the work that is described in the present study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular inflammation and repair: implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc Interv. 2011;4:1057–1066. doi: 10.1016/j.jcin.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawamoto A, Asahara T. Role of progenitor endothelial cells in cardiovascular disease and upcoming therapies. Catheter Cardiovasc Interv. 2007;70:477–484. doi: 10.1002/ccd.21292. [DOI] [PubMed] [Google Scholar]
- 3.O' Brien E, Ma X, Simard T, Pourdjabbar A, Hibbert B. Pathogenesis of neointima formation following vascular injury. Cardiovasc Hematol Disord Drug Targets. 2011;11:30–39. doi: 10.2174/187152911795945169. [DOI] [PubMed] [Google Scholar]
- 4.Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends in Cardiovascular Medicine. 2008;18:33–37. doi: 10.1016/j.tcm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78:413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 6.Padfield GJ, Newby DE, Mills NL. Understanding the role of endothelial progenitor cells in percutaneous coronary intervention. J Am Coll Cardiol. 2011;55:1553–1565. doi: 10.1016/j.jacc.2009.10.070. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Sun X, Icli B, Wara AK, Feinberg MW. Role of Kruppel-like factors in leukocyte development, function, and disease. Blood. 2010 doi: 10.1182/blood-2010-05-285353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Z, Wara AK, Icli B, Sun X, Packard RR, Esen F, Stapleton CJ, Subramaniam M, Kretschmer K, Apostolou I, von Boehmer H, Hansson GK, Spelsberg TC, Libby P, Feinberg MW. Kruppel-like factor KLF10 targets transforming growth factor-beta1 to regulate CD4(+)CD25(-) T cells and T regulatory cells. J Biol Chem. 2009;284:24914–24924. doi: 10.1074/jbc.M109.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wara AK, Foo S, Croce K, Sun X, Icli B, Tesmenitsky Y, Esen F, Lee JS, Subramaniam M, Spelsberg TC, Lev EI, Leshem-Lev D, Pande RL, Creager MA, Rosenzweig A, Feinberg MW. TGF-beta1 signaling and Kruppel-like factor 10 regulate bone marrow-derived proangiogenic cell differentiation, function, and neovascularization. Blood. 2011;118:6450–6460. doi: 10.1182/blood-2011-06-363713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramaniam M, Hawse JR, Rajamannan NM, Ingle JN, Spelsberg TC. Functional role of KLF10 in multiple disease processes. Biofactors. 2010;36:8–18. doi: 10.1002/biof.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborne-Pellegrin MJ, Weill D. “Spontaneous” endothelial injury in the rat caudal artery. Exp Mol Pathol. 1983;39:61–79. doi: 10.1016/0014-4800(83)90041-2. [DOI] [PubMed] [Google Scholar]
- 12.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circulation Research. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer S, Steinmetz M, Asdonk T, Motz I, Coch C, Hartmann E, Barchet W, Wassmann S, Hartmann G, Nickenig G. Activation of endothelial toll-like receptor 3 impairs endothelial function. Circ Res. 2011;108:1358–1366. doi: 10.1161/CIRCRESAHA.111.243246. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P, Moons L, Stassen JM, De Mol M, Bouche A, van den Oord JJ, Kockx M, Collen D. Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol. 1997;150:761–776. [PMC free article] [PubMed] [Google Scholar]
- 15.Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925–936. doi: 10.1001/jama.299.8.925. see comment. [DOI] [PubMed] [Google Scholar]
- 16.Napoli C, Hayashi T, Cacciatore F, Casamassimi A, Casini C, Al-Omran M, Ignarro LJ. Endothelial progenitor cells as therapeutic agents in the microcirculation: an update. Atherosclerosis. 2011;215:9–22. doi: 10.1016/j.atherosclerosis.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Dimmeler S. Regulation of bone marrow-derived vascular progenitor cell mobilization and maintenance. Arterioscler Thromb Vasc Biol. 2010;30:1088–1093. doi: 10.1161/ATVBAHA.109.191668. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Zhao X, Fang Y, Yu S, Zhao J, Song M, Huang L. SDF-1alpha involved in mobilization and recruitment of endothelial progenitor cells after arterial injury in mice. Cardiovasc Pathol. 2010;19:218–227. doi: 10.1016/j.carpath.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 20.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 21.Cheng M, Zhou J, Wu M, Boriboun C, Thorne T, Liu T, Xiang Z, Zeng Q, Tanaka T, Tang YL, Kishore R, Tomasson MH, Miller RJ, Losordo DW, Qin G. CXCR4-mediated bone marrow progenitor cell maintenance and mobilization are modulated by c-kit activity. Circ Res. 2010;107:1083–1093. doi: 10.1161/CIRCRESAHA.110.220970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 23.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. see comment. [DOI] [PubMed] [Google Scholar]
- 24.Lin HH, Chen YH, Yet SF, Chau LY. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost. 2009;7:1401–1408. doi: 10.1111/j.1538-7836.2009.03478.x. [DOI] [PubMed] [Google Scholar]
- 25.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 26.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. Journal of Clinical Investigation. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arteriosclerosis, Thrombosis & Vascular Biology. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 28.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 29.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 30.O'Neill TJt, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97:1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 31.Thyberg J. Re-endothelialization via bone marrow-derived progenitor cells: still another target of statins in vascular disease. Arterioscler Thromb Vasc Biol. 2002;22:1509–1511. doi: 10.1161/01.atv.0000036415.04486.01. [DOI] [PubMed] [Google Scholar]
- 32.Religa P, Bojakowski K, Maksymowicz M, Bojakowska M, Sirsjo A, Gaciong Z, Olszewski W, Hedin U, Thyberg J. Smooth-muscle progenitor cells of bone marrow origin contribute to the development of neointimal thickenings in rat aortic allografts and injured rat carotid arteries. Transplantation. 2002;74:1310–1315. doi: 10.1097/00007890-200211150-00019. [DOI] [PubMed] [Google Scholar]
- 33.Sahara M, Sata M, Morita T, Nakamura K, Hirata Y, Nagai R. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation. 2007;115:509–517. doi: 10.1161/CIRCULATIONAHA.106.655837. [DOI] [PubMed] [Google Scholar]
- 34.Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2012;93:223–231. doi: 10.1093/cvr/cvr278. [DOI] [PubMed] [Google Scholar]
- 35.Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Juni P, Vaina S, Hellige G, Tsuchida K, Morger C, Boersma E, Kukreja N, Meier B, Serruys PW, Windecker S. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol. 2008;52:1134–1140. doi: 10.1016/j.jacc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–1510. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 37.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 38.Virmani R, Farb A, Guagliumi G, Kolodgie FD. Drug-eluting stents: caution and concerns for long-term outcome. Coron Artery Dis. 2004;15:313–318. doi: 10.1097/00019501-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 40.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2011;79:453–495. doi: 10.1002/ccd.23438. [DOI] [PubMed] [Google Scholar]
- 41.Nakazawa G, Granada JF, Alviar CL, Tellez A, Kaluza GL, Guilhermier MY, Parker S, Rowland SM, Kolodgie FD, Leon MB, Virmani R. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv. 2010;3:68–75. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Yang XS, Zhu HG, Yu ZJ, Ren F, Chen YQ, Wu X, Zhang ZC, Zhang LJ, Lu SZ. The union of anti-CD34 antibody can improve the performance of drug-eluting stents. Catheter Cardiovasc Interv. 2012;79:972–978. doi: 10.1002/ccd.23186. [DOI] [PubMed] [Google Scholar]
- 43.Sangiorgi GM, Morice MC, Bramucci E, Ferlini M, Grinfeld L, Petronio AS, Pierli C, Iadanza A, Biondi-Zoccai G, Colombo A. Evaluating the safety of very short-term (10 days) dual antiplatelet therapy after Genous bio-engineered R stent implantation: the multicentre pilot GENOUS trial. EuroIntervention. 2011;7:813–819. doi: 10.4244/EIJV7I7A128. [DOI] [PubMed] [Google Scholar]
- 44.Sethi R, Lee CH. Endothelial Progenitor Cell Capture Stent: Safety and Effectiveness. J Interv Cardiol. 2012 doi: 10.1111/j.1540-8183.2012.00740.x. [DOI] [PubMed] [Google Scholar]
- 45.Rognoni A, Secco GG, Lupi A, Santagostino M, Sansa M, Bongo AS, Rognoni G. Use of endothelial progenitor capture cell stent during percutaneous treatment of coronary bifurcations: a prospective angiographic registry. Crit Pathw Cardiol. 2011;10:189–192. doi: 10.1097/HPC.0b013e318233d57f. [DOI] [PubMed] [Google Scholar]
- 46.Klomp M, Beijk MA, Tijssen JG, de Winter RJ. Significant intimal hyperplasia regression between 6 and 18 months following Genous endothelial progenitor cell capturing stent placement. Int J Cardiol. 2011;147:289–291. doi: 10.1016/j.ijcard.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 47.den Dekker WK, Houtgraaf JH, Onuma Y, Benit E, de Winter RJ, Wijns W, Grisold M, Verheye S, Silber S, Teiger E, Rowland SM, Ligtenberg E, Hill J, Wiemer M, den Heijer P, Rensing BJ, Channon KM, Serruys PW, Duckers HJ. Final results of the HEALING IIB trial to evaluate a bio-engineered CD34 antibody coated stent (GenousStent) designed to promote vascular healing by capture of circulating endothelial progenitor cells in CAD patients. Atherosclerosis. 2011;219:245–252. doi: 10.1016/j.atherosclerosis.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 48.van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, Koolen JJ, Brueren BR, Dambrink JH, Hautvast RW, Verheugt FW, ten Berg JM. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53:1399–1409. doi: 10.1016/j.jacc.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 49.Chang J, Li Y, Huang Y, Lam KS, Hoo RL, Wong WT, Cheng KK, Wang Y, Vanhoutte PM, Xu A. Adiponectin prevents diabetic premature senescence of endothelial progenitor cells and promotes endothelial repair by suppressing the p38 MAP kinase/p16INK4A signaling pathway. Diabetes. 2010;59:2949–2959. doi: 10.2337/db10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.