Abstract
As compared to peptide/protein-based vaccines, naked DNA vectors and even traditional attenuated or inactived virus vaccines, virus-like particles (VLPs) are an attractive vaccine platform because they offer a combination of safety, ease of production, and both high density B cell epitope display and intracellular presentation of T cell epitopes that induce potent humoral and cellular immune responses respectively. Indeed, human papillomavirus (HPV) vaccines based on VLP production by recombinant expression of major capsid antigen L1 in yeast (Gardasil®, Merck & Co.) or insect cells (Cervarix®, GlaxoSmithKline) have been licensed for the prevention of cervical and anogenital infection and disease associated with the genotypes targeted by each vaccine. These HPV vaccines however have not been demonstrated as effective to treat existing infections, and efforts to develop a therapeutic HPV vaccine continue. Furthermore, current HPV L1-VLP vaccines provide type-restricted protection, requiring highly multivalent formulations to broaden coverage to the dozen or more oncogenic HPV genotypes. This raises the complexity and cost of vaccine production. The lack of access to screening and high disease burden in developing countries has spurred efforts to develop second generation HPV vaccines that are more affordable, induce wider protective coverage and offer therapeutic coverage against HPV-associated malignancies. Given the previous success with L1 VLP-based vaccines against HPV, VLPs have been also adopted as platforms for many second generation HPV and non-HPV vaccine candidates with both prophylactic and therapeutic intent. Here we examine the progress and challenges of these efforts, with a focus on how they inform VLP vaccine design.
Keywords: Human Papillomavirus, Cervical cancer, Virus-like particles, L1, L2, minor capsid protein, HPV Prophylactic vaccines, VLPs
Introduction: The case for Virus-like particle vaccine systems
It is estimated that 16.1% of all cancers worldwide are caused by known infectious agents[1]. This knowledge that certain infectious agents can trigger cancer has driven the development and licensure of prophylactic vaccines. Two small DNA viruses, hepatitis B virus (HBV) and human papillomavirus (HPV) are each responsible for 5% of cancer worldwide. The concept of preventing cancer via vaccination was demonstrated within the last thirty years with the widespread use of the Hepatitis B virus (HBV) vaccines beginning in the late 1980s with Recombivax-HB® (Merck & Co.) and Engerix-B® (GlaxoSmithKline, GSK). A similar approach is being taken with Human Papillomavirus (HPV) vaccines, beginning with Gardasil® (Merck & Co.) and Cervarix® (GSK) in the late 2000s. These HPV vaccines are remarkably efficacious in protecting healthy individuals from acquiring new infections for at least a decade and exhibit an excellent safety profile[2-5]. Both the HBV and HPV vaccines are based on recombinant virus-like particles (VLPs) [6-9]. As a result of their success in terms of reductions in HBV and HPV related diseases and safety, the focus on VLPs has intensified in the vaccine development field.
VLPs offer several advantages over other types of vaccine platforms. Firstly, VLPs are very safe compared to live or attenuated vaccines. The major structural protein(s) of many viruses when expressed alone are able to self-assemble into highly immunogenic VLPs that mimic the structure of their respective native virion. VLPs however lack the infectious viral genome and this distinction makes VLPs safer vaccine candidates compared to their traditional counterparts such as attenuated or inactive viruses which could revert back into infectious form[10]. It also overcomes many issues of manufacture as viruses generally are prepared in mammalian cells lines, whereas VLPs can be produced in bacteria, yeast or insect cells, greatly facilitating scale up and reducing safety concerns. Secondly, compared to linear peptide vaccines, VLP vaccines are typically highly immunogenic; most VLPs are able to induce very high titers of neutralizing antibodies, often in the absence of adjuvants, and these responses are durable. This is primarily due to VLPs having a highly ordered structure which allows for presentation of repetitive epitopes to B-cells for potent activation or to dendritic cell for stimulation of cell-mediated immunity [11-13]. Thirdly, using molecular biology, one can also use VLPs as scaffolds for heterologous antigens against other infectious diseases. While this approach is promising, it is still unproven in the clinic. Nevertheless, the potential of this approach has led numerous vaccine research groups to continue to focus their efforts on VLP display platforms using either bacterial, plant or mammalian viruses. Fourthly, VLPs are able to break B-cell tolerance in many instances, and this may offer a way to induce antibody to self antigens e.g. to block their deleterious effects by active vaccination rather than using passive therapy with monoclonal antibodies [7]. Fifthly, VLP are taken up by dendritic cells and can present epitopes along the MHC class 1 pathway [8]. This offers an avenue to attach therapeutic T cell epitopes to VLP and develop vaccines to treat pre-existing, established infectious disease or cancer. As our review focuses on VLPs in the context of HPV, our discussion will first give a brief overview on the HPV vaccine field before touching on the successes and failures of recent efforts to create a second generation widely protective HPV vaccine using VLP platforms in both the prophylactic and therapeutic setting. HPV VLPs containing heterologous epitopes are being tested as a platform against other diseases, and technical considerations and key challenges with this approach will be discussed.
An overview of HPV biology
The Human Papillomaviruses are a family of non-enveloped viruses with a T=7d icosahedral capsid of approximately 60nm in diameter. Within the HPV capsid is a double-stranded, covalently-closed, circular DNA genome that is approximately 7800 base pairs. The genome encodes six early genes which regulate viral transcription and genome replication (E1, E2, E4, E5, E6, and E7) and two late genes (L1 and L2) which are involved in the viral capsid structure. L1 is the major capsid protein and it forms the capsid by self-assembly of72 pentamers of L1 (termed capsomers). The other capsid protein, L2, is present in the capsid at a ratio as high as 1 L2 per 5 L1, i.e. 72 L2 proteins within each HPV virion, although a ratio as low as 1:30 has been described [14,15]. Understanding HPV virology and its role in tumorgenesis is important for facilitating second generation HPV vaccine development, however due to space limitations, this will not be discussed in full detail in this article [reviewed in [14,16]]. Briefly, during intercourse, the cervicovaginal epithelium may experience micro-abrasions which can give rise to the exposure of HPV to their host cells-basal undifferentiated keratinocyte cells of the cervix. Here in the basal cells the viral early genes are expressed and the genome is replicated as an episome at approximately 102 copies/cell [14]. After establishing the virus infection in the basal cells, the HPV late gene expression program is triggered by differentiation of the infected basal cell as it divides and leaves the basement membrane. Late gene expression is associated with vegetative over-replication of the viral genome, E4 and capsid gene expression and virion formation. During neoplastic transformation, HPV DNA typically integrates into the host cell genome and loses its episomal form. Integration normally leads to disruption of E2 and loss of late gene expression, i.e. L1 and L2. The inactivation of E2 de-represses the expression of E6 and E7 whose expression drives, and is necessary to maintain, the transformed state. E6 compromises TP53 while E7 inactivates retinoblastoma (most prominent among many other growth regulatory pathways impacted by these two viral oncoproteins) and together, their synergistic effects lead to genomic instability and subsequently, cellular transformation and loss of differentiation, and advancing from high-grade squamous intraepithelial lesions (HSIL) into metastatic disease [10-14].
There are over 100 HPV genotypes with distinct tissue tropism for either cutaneous and mucosal tissue regions[17]. Most HPV infections induce benign and self-limited tumors in the skin (e.g. plantar and planar warts), or the genitals (genital warts). However, certain ‘high risk’ or ‘oncogenic’ types of HPV are a necessary cause of cancers of the cervix, and well as a subset of cancers of the anus, vaginal, vulva, penis and oropharyngeal (head and neck) regions. The vast majority (85-99%) of cervical cancers contain HPV[1,18]. However, while HPV infection is a necessary cause, fortunately most HPV infections do not lead to cancer because they are controlled or eliminated by the host's immune system [19,20]. There are over a dozen high risk types HPV types with the propensity to initiate cancers; however HPV16 is the most potent by far, causing50% of cervical cancer, whereas HPV18 casues another 20%, with the remainder each contributing relatively small fractions of cases. Notably, in the cancers of sites other than the cervix, HPV16 is even more predominant, causing over 90% of other HPV-related anogenital cancers and head and neck cancers [14]. As mentioned earlier, two prophylactic vaccines have been licensed on the basis of preventing persistent HPV infection and the precursors of HPV-related anogenital malignancies, high grade CIN, VIN, VaIN and AIN. The clinical trials also demontrate that Gardasil is effective in preventing genital warts[21]. Both HPV vaccines prevent for at least a decade the acquisition of the precursor lesions of cervical cancer associated with the types targeted by the vaccines, HPV16 and HPV18 [2-5,18]. Data also suggest that HPV vaccination prevents other HPV-associated neoplasia in the vagina, vulvar and anus, as well as HPV16 detection in oral rinses[22]
Current Licensed HPV Vaccines and their recognized limitations
Gardasil® and Cervarix® are based on VLP technology derived by expressing L1, the major protein capsid of the HPV virus, in yeast and insect cells respectively. L1 is able to self-assemble and form immunogenic but non-infectious VLPs that are able to induce similarly high titers of neutralizing antibodies as vaccination with native virions [23]. Administration of L1-based VLPs allows the immune system to generate antibody titers that are 100 fold greater than occur upon natural infection [2-4,9,24-27]. In addition, passive transfer studies using L1-VLP vaccinated animal serum into naïve animals confers protection [24,28,29]. These studies strongly suggest that the HPV VLP induced protection is mediated by humoral immunity but roles for innate immunity and cell-mediated immunity cannot be ruled out.
Despite the recent availability of two commercially licensed HPV vaccines, cervical cancer remains the third most common global cancer killer among women for several reasons (Table 1) [30]. Firstly, the availability of the vaccine is too recent to impact cervical cancer rates, because the cancers evolve over more than a decade and thus the impact on cancer incidence and associated deaths will most likely be felt in 2-3 decades and beyond from its introduction. Secondly, vaccination programs have not reached many low income regions of the world, partly reflecting the relatively high cost of the vaccine. Indeed, 85% of the global burden of cervical cancer occurs in developing countries where most are unable to afford this vaccine (∼$360 for full regimen in the US, although tiered pricing has been introduced) [31,32]. However, there are ongoing efforts at regional production of generic vaccines that will likely drive down costs and greatly improve availability.
Table 1. Recognized limitations of current licensed HPV Vaccines- Cervarix® (GSK) and Gardasil® (Merck & Co.).
| Limitation | Impact | Solution and Alternatives |
|---|---|---|
| Cost of manufacturing, trials and IP leads to high vaccine price | Developing countries that do not have the economic means to afford the vaccine continue to bear the burden of cervical cancer | Investigate manufacturing generic L1 VLP vaccines in cheaper production systems like plants or bacteria, and reduce number of doses or combining with childhood vaccines |
| Type restriction | Vaccine does not protect against all HPV types causing cervical cancer, thus screening must continue for HPV-vaccinated women. | More HPV VLP types to create a highly multivalent HPV vaccine Alternative vaccine targeting broadly protective antigen L2 |
| No therapeutic value | No effect on individuals with pre-existing HPV infection | Alternative vaccine constructs displaying E6 and E7 |
Current licensed HPV vaccines elicit type-restricted protection, i.e. the vaccines confer protection primarily against the genotypes from which the VLPs in the vaccine were derived [8,33,34]. While there is some clinical evidence for cross-protection against the most closely related types, the levels of cross-protective antibodies detected are often very low or absent and it is unclear whether such cross-reactive immunity can be sustained in the long term [32,35,36]. Current formulations of both licensed HPV L1 VLP vaccines are made based on two oncogenic HPV genotypes, HPV 16 and 18 which account for ∼70% of all cervical cancers cases. Gardasil® (Merck) also targets HPV 6 and HPV11 that cause benign genital warts. Vaccinated individuals however are either weakly or ineffectively protected from most of the remaining dozen oncogenic HPV genotypes that collectively cause ∼30% of all cervical cancer cases [14,19,37,38]. Furthermore, the vaccines do not target the cutaneous genotypes such HPV1 and HPV2 that cause benign skin warts such as plantar and common warts. A solution towards the problem of type-restricted immunity would be to create more highly valent L1 VLP vaccines for comprehensive coverage [39]. Indeed, Merck currently has a nonavalent vaccine in advanced clinical trials which will likely broaden the efficacy to the other oncogenic HPV types. However, this is a complex and costly approach, and may therefore be difficult to produce at low cost [33]. Therefore, in addition to a therapeutic HPV vaccine, there remains a need to develop a second generation HPV vaccine that is both affordable and provides broad coverage protection against diverse oncogenic and benign HPV types.
Extending HPV vaccine coverage
HPV L2 as an alternative candidate
L2 is the other protein that makes up the HPV capsid and is necessary for PV infection [40,41]. Given its presence in the capsid, it was hypothesized that L2 could also be used as an antigen for HPV prophylactic vaccination. Initial studies using rabbit and bovine papillomavirus models showed that vaccination with the cognate L2 protein prepared by recombinant expression in bacteria confers protection against experimental PV challenge at both mucosal and cutaneous sites and induces low titers of neutralizing antibody[28,42-45]. Further studies showed that there are several cross-neutralizing epitopes on the N-terminus of L2 around amino acid regions 11-88 that offer the potential for broad protection from a single antigen [46-51]. One limitation of these seminal studies was that these animal PV models were unable to dissect the serologic relationship between HPV types [52]. However, this issue has been largely resolved with the development of HPV pseudovirion (PsV) technologies [53-55]. Briefly, HPV PsVs contain the outer capsid structures that mimic native HPV virions but instead of the viral genome they contain a reporter plasmid such as GFP or luciferase that is expressed upon successful “infection”. Importantly, these PsV can be used to challenge mice intra-vaginally or cutaneously [55] and mouse vaccination studies using PsV are consistent with earlier animal PV studies. This can also be done with ‘quasivirions’ that contain bovine or cottontail rabbit papillomavirus genomes inside HPV capsid proteins to provide a measurable phenotype (e.g. focal transformation of mouse fibroblasts or warts in rabbits respectively)[56]. Studies using these HPV pseudovirions show that antisera to L2 can cross-neutralize a diverse array of medically significant HPV genotypes [57-60]. Cross-neutralization of a broad range of HPV genotypes by sera and protection against a diversity of HPV genotypes upon vaccination with a single L2 epitope is an important distinction from current L1 VLP vaccines because it suggests the possibility of a simple pan-HPV prophylactic vaccine derived from L2. L2 vaccines can be produced in bacteria, a simple, inexpensive and well characterized system [19] and provide broad immunity despite producing levels of neutralizing antibodies two orders of magnitude lower than L1 VLP. Thus bacterial production of a single broadly protective antigen in a low cost expression system represents an interesting alternative approach to highly multivalent L1 VLP vaccines. However, because of its weak immunogenicity compared to L1 VLP, a key challenge in L2-based vaccine development is effective display of these broadly cross-neutralizing L2 epitopes to the immune system to trigger high titer, high avidity and long lived neutralizing antibody responses.
Challenges in utilizing L2 in HPV vaccines
L2 is not required in HPV VLP formation but does co-assemble with L1 into VLPs. Thus, initial studies attempted to extend protection by purifying L1/L2-VLPs produced in insect cells for vaccination [61]. Disappointingly, this approach was unsuccessful as L2 antibody responses were either absent or very much sub-dominant to L1 in animals vaccinated with L1/L2-VLPs [61]. L1/L2 VLPs produced in insect cells contain approximately 12 copies of L2 per virion in contrast to the expected number of 72 copies [62]. This might explain in part L2's sub-dominance in the context of an L1/L2VLP produced in insect cells as the higher number and ordered arrangement of L1 epitopes would skew the immune response towards L1. However, natural infections trigger L1-specific but not significant L2 responses, suggesting that low L2 occupancy in L1/L2 VLPs is not the main reason for lack of response to L2. Rather in vitro data suggests that the virus capsid hides L2 until initiation of the infectious process [14,40] which occurs with the virion binding the extracellular matrix in vitro, or the basement membrane in vivo.
After binding, the virus undergoes a series of conformational changes which exposes the N-terminus of L2 on the capsid surface (Figure 1) [6,29,63-65]. L2 contains a furin cleavage site at its N-terminus and is thus susceptible to a single cut by the cellular protease furin to remove the conserved, positively charged amino terminus, and this cleavage of L2 by furin is essential to infection. These events display upon the capsid surface several broadly cross-neutralizing epitopes at the N-terminus of L2, including residues 17-36 recognized by the cross-neutralizing monoclonal antibody RG-1 [40,49,65,66]. At present, it is unclear how the altered conformation of L1 and/or cleaved L2 binds to secondary receptors on the basal keratinocyte to facilitate cell entry[61,63,67]. Nonetheless, this transient exposure of L2 epitopes prior to cell entry suggests a need to hide the broadly cross-protective epitopes from the immune system. The cloaking of L2 and subdominance to L1 in the context of the capsid may help explain the lack of evolutionary pressure for these epitopes to change, thereby resulting in the maintenance of this broadly cross-reactive neutralizing epitope. The conservation of the L2 neutralizing epitopes also suggests an evolutionary constraint that may reflect a role in binding to a cellular entry factor during infection. The challenge ahead for L2 vaccines thus, is to find methods to induce and maintain protective levels of antibodies to these L2 epitopes for many decades.
Figure 1.
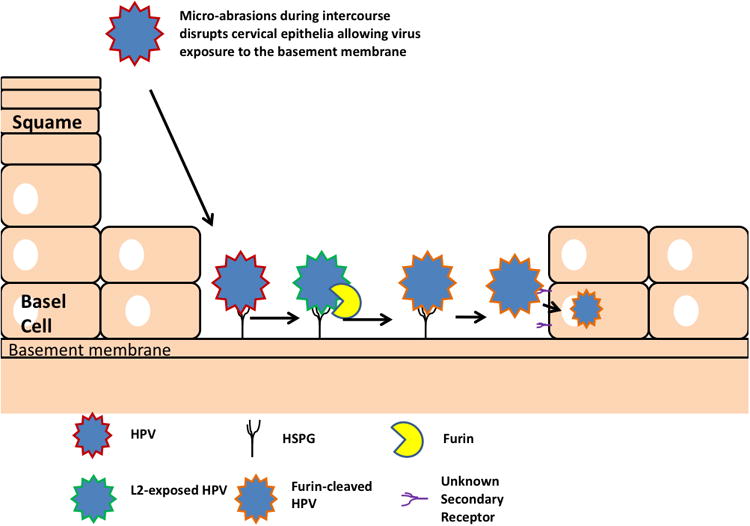
Model of infection of the cervical epithelium by HPV (Developed by Day et al.). Upon access to the basement membrane, HPV binds to the basement membrane before undergoing a series of conformational changes in capsid structure that ultimately leads to the transient exposure of its L2 minor capsid protein (See text for full details). Upon its exposure, L2 is cleaved by furin, and the furin-cleaved virion is then taken up by the basal epithelial cells to initiate infection.
Methods to improve L2-based vaccines
HPV L1-VLPs vaccines displaying L2
To potentially overcome HPV L1 immune dominance by increasing the density of surface display of L2, several groups have produced chimeric HPV VLPs by inserting L2 neutralizing epitopes into the immunodominant L1 neutralizing epitopes of HPV L1-VLPs or BPV1 (Bovine Papillomavirus Type 1) L1-VLPs[68-70]. This concept is promising in that by displaying L2 epitopes on L1 the theoretical amount of L2 epitope exposure would increase approximately 5 fold from the estimated number of 72 to 360. Further, the L2 epitope would no longer be buried and the protective effect of L1 might also be retained. Vaccination studies utilizing this approach reported the presence of L2-specific antibodies via ELISA assays. However, in the context of HPV serology, ELISA assays have been criticized for overestimating the protective antibodies HPV L2 can produce because HPV L2 contains regions that can produce non-neutralizing antibodies. As a result, the HPV PsV in vitro neutralization assay is used for measuring HPV specific neutralizing antibodies. Most studies using this approach [68,69,71] showed a low but detectable amount of L2-specific cross-neutralizing antibodies in addition to the high titer L1-specific neutralizing antibody response. While these studies showed that placing L2 epitopes in an immunodominant loop of L1 epitopes did induce a cross-neutralizing L2-specific response, its impact on the antibody avidity and longevity remains to be assessed.
Display in L1 capsomers (made of 5 L1 proteins and the building block for VLPs) could also be another strategy. Garcea and colleagues[72] showed that by deleting the 9 and 26 amino acids from the N and C terminus of L1 respectively, L1 readily forms capsomers (made of 5 L1 proteins) instead of full VLPs. Interestingly, like L1-VLP, L1 capsomers (abbreviated as L1Δ onwards) retain the ability to induce a type-restricted neutralizing antibody, although at somewhat lower antibody titers [59,72]. It is possible that utilizing L1Δ as a platform for cross-presentation of L2 epitopes, one could overcome the problem of L2 epitopes being hidden in normal L1/L2-VLPs. In addition, high amounts of L1Δ can be made in bacteria readily. This vaccine display design and its subsequent production method would potentially solve both the cost of HPV L1-VLP manufacturing as well as the presentation of L2 epitopes.
Our group has also previously developed a broadly protective L2 peptide vaccine candidate (L211-88X5) by fusing 5 different HPV L2 regions 11-88 (HPV 1, 5, 6, 16, 18) and expressing this multimer in bacteria. While broadly protective against numerous HPV genotypes in vivo, our in vitro neutralization titer data of L211-88X5 are still lower than L1-VLP vaccines [58]. HPV VLPs can directly activate dendritic cells and therefore it is possible that they could act as an adjuvant. Thus a possible approach to augment the L2-antibody titers and broaden vaccine coverage at the same time would be to combine our L211-88X5 with current licensed HPV vaccines to extend protective coverage. To test this hypothesis, we examined mixing of L211-88X5 protein with either an L1-VLP (Cervarix®) or L1Δ. Neutralization assay data using HPV 16 revealed that L211-88X5-Cervarix® mixture was comparable to Cervarix® alone and no improvement in antibody titers was seen in the presence of L1Δ. In addition, there was no improvement in the L2-specific antibody titers against other genotypes. These two observations show that when mixing L1-VLPs or L1Δ with L2 peptide vaccines, the L1-specific and L2-specifc responses act essentially independently[59], and that the L2 epitopes likely need to be displayed directly on VLP rather than mixed with them to boost the response.
In another study [73], the L2 residues 13-47 from 3 different high risk HPV types (HPV 18, 31 and 45) were fused in tandem to the C-terminus of HPV16L1Δ, forming the vaccine candidate HPV16L1Δ-L2X3, this candidate was expressed in bacteria with a glutathione-S-transferase (GST) tag for subsequent downstream protein purification. Vaccination with either HPV16L1Δ or HPV16L1Δ-L2X3 with alum and monophosphoryl lipid A (MPL) induced similar L1-specific protective responses, but the response to the L213-47x3 fusion peptide was weak suggesting that the epitopes need to be displayed in the immunodominant epitopes of the L1 capsomer structure.
Non-HPV VLPs displaying L2
Since display of L2 in the immunodominant epitopes of L1 VLP induced L2-specific antibody responses that were robust but still much lower than the L1-specific titers, others have opted to display L2-neutralizing epitopes using non-HPV VLPs (Table 2). The potential of non-HPV VLPs displaying L2 epitopes was first demonstrated by Palmer et al.[74] who created recombinant tobacco mosaic virus (rTMV) particles that either contained the L2 peptide region 94-122 of cottontail rabbit papillomavirus (CRPV) or rabbit oral papillomaviruses (ROPV). Vaccination studies show efficient protection against homologous challenges. More importantly, because CRPV and ROPV are distantly related, the authors highlighted that vaccination of rTMV-ROPV with an RIBI adjuvant conferred a weak but measurable cross-protective response against cutaneous CRPV challenge which further substantiates the cross-neutralizing ability of L2 in PVs. In a more recent study using HPV L2 instead of animal PVs, Cerovskaet al. [75] utilized recombinant DNA technology to fuse the DNA of HPV L2 regions 108-120 onto the N or C terminus of a plant viral vector expressing the coat protein of potato virus X (PVX). Theoretically, expression of this viral vector in plants would yield Potato virus X coat-protein (PVX CP) virus-like particles with HPV L2 at the N or C-terminus of the coat protein. The study revealed that only the HPV L2108-120 fused at the N-terminus of the PVX CP (L2108-120-PVX CP) could be expressed. Subsequent vaccination in mice with L2108-120-PVX CP via subcutaneous injection or via tattoo administration followed by ELISA of the vaccinated sera showed that this L2-VLP method was able to induce L2-specific antibodies. However the study did not test neutralization titers for comparison with L1 VLP. Nonetheless, both of these studies show the potential of using a non-HPV VLP for L2 display, although challenges remain in optimizing display. Another highlight of both studies was the use of the well-established plant protein expression system, Nicotiana benthamiana to generate the VLPs displaying L2. This has several advantages over current manufacturing methods of licensed VLP vaccines since plants are cheap and easy to grow for large biomass and most importantly, the proteins expressed are usually free from bacterial or animal pathogen toxins that might contaminate the vaccine[76].
Table 2. Summary of VLP-based prophylactic HPV vaccines displaying L2.
| 2nd generation prophylactic HPV VLP candidate vaccines based on HPV L2 | Potential or current advantages over licensed HPV L1-VLP vaccine | Potential or current disadvantages | References |
|---|---|---|---|
| L1-VLPs expressing L2 epitopes | Broad spectrum protection | L2-specific neutralizing antibody titers detected were low. Uses same expression system as L1-VLPs (Cervarix® or Gardasil®) which does not bring down cost of potential vaccine | [68-70] |
| L1-Capsomers | Bacterial expression system lowers the cost of manufacturing | Lower antibody avidity compared to L1-VLPs. Type restricted protection but can be overcome by creating a variety of capsomeres or combinatorial vaccination methods with L2 | [59,72,88] |
| Plant-VLPs e.g Tobacco Mosaic Virus |
Broad spectrum protection. Plant expression system lowers the cost of manufacturing. Reduced issues of bacterial endotoxin contamination | Studies using this method showed L2 antibody neutralizing titers that were low | [75,87] |
| Bacteriophage VLPs | Broad spectrum protection. Bacterial expression system lowers the cost of manufacturing. No adjuvant needed? | Endotoxin contamination | [77,78] |
| Non-HPV Mammalian virus VLPs e.g AAVLP |
Broad spectrum protection | Requires adjuvant. Pre-existing neutralizing antibodies against the AAV or other carrier | [79] |
Bacteriophage VLPs also have been utilized in generating a pan-HPV VLP vaccine by the Chackerian group whereby they utilized an RNA bacteriophage, PP7, to display the broadly neutralizing L2 epitope (regions 17-31) on the surface loop of the PP7 coat protein[77,78]. Initial studies were focused on creating two L2-PP7 VLPs derived from HPV16 and HPV45 sequences. The vaccination studies with incomplete Freund's adjuvant showed substantial cross-protection, for example, HPV 16 L2-PP7 vaccinated mice were significantly protected from HPV45 PsV vaginal challenge compared to PP7 VLP alone although not completely [78]. Subsequently in another study by the same group, a total of 8 different L2-PP7 VLPs were produced, each displaying the L217-31 of a different HPV type respectively (HPV 1,5,6,11,16,18,45 and 58)[77]. These high and low-risk HPV types were chosen presumably because they represent common members of medically relevant HPV types. Vaginal- and cutaneous- challenges were performed subsequently using these different PsV types and HPV31 whose L2 was not included in L2-PP7. Overall, the results showed the greatest protection was exhibited in mice that were vaccinated with a mixture containing all 8 types of L2-PP7 VLPs. These L2-PP7 mixture vaccinated mice also were protected from HPV31 PsV challenge highlighting substantial cross-protection. There are several useful features of this system. Firstly, PP7 can be simply expressed at very high levels in bacteria. Secondly, two rounds of immunizations were sufficient in their studies to elicit protection. Thirdly, no adjuvant was requied for the L2-PP7 mixture vaccine as PP7 particles carry ssRNA which can potentially act as an endogenous adjuvant to activate TLR 7 or 8 signaling [77]. Taken together, the results suggest the potential of this bacteriophage method as a pan-HPV vaccine. It would be interesting to see future studies looking at the longevity and avidity of the L2 epitope-specific neutralizing responses to L2-PP7 vaccination compared to other approaches.
Non-HPV mammalian VLPs, such as Adeno-Associated virus-like particles (AAVLP), have also been explored as platforms to display L2. AAVLPs are a promising platform for heterologous HPV L2 display because the viral capsids are able to self-assemble efficiently despite insertion of foreign peptide epitopes into the sequence. In a recent pilot study to explore the potential of AAVLPs, Nieto et al. [79] generated prophylactic HPV vaccines made of AAVLPs displaying HPV16 and 31 regions 17-36 of L2. This method showed cross-neutralizing serum in both rabbits and mice. However, an adjuvant was required to elicit the high titers and the duration of the response is not known.
Therapeutic VLP-based HPV vaccines
Rationale of therapeutic HPV vaccination
Pap screening and intervention is a proven strategy in the control of cervical cancer[80] and can reduce the incidence by 60-90% within 3 years of its introduction to populations naïve to screening. It is possible that prophylactic HPV vaccines, when widely administered and proven as effective in reducing cervical cancer rates, will impact the need/interval for screening services or developing therapies such as therapeutic vaccination. However, at present, HPV infection and associated diseases are widespread, and the need for a therapeutic vaccine remains to help those currently afflicted with HPV disease and to accelerate the reduction of cancer rates. Furthermore, both vaccination and cytologic screening implementation is far from complete in many countries. This need is also compounded by the potential advent of first line HPV DNA testing for cervical screening, which will identify millions of infected individuals. The HPV infected patients must be continually screened until they clear the infection or develop high grade neoplasia, whereupon they undergo an ablative therapy. These ablative treatments of high grade neoplasia are associated with significant side effects and costs, and do not impact non-cervical disease. Therefore a therapeutic HPV vaccine could be helpful for these patients. Current licensed HPV L1-VLP vaccines (as well as the various L2 strategies discussed so far) are utilized for prophylaxis and offer little or no therapeutic value [25]. New, more effective treatments for existing non-cervical high grade neoplasia and HPV-associated cancer, which remain prevalent, are urgently needed [33].
During the viral life cycle, detectably expression of L1 and L2 capsid proteins occurs only upon terminal differentiation of the infected keratinocytes. Thus, neither cervical cancer cells, nor the infected basal cells attached to the basement membrane, express the capsid proteins. As a result, the early genes of HPV are more appropriate targets for therapeutic immunization. E1 and E2 are possible candidates but they are not typically expressed in cancer. E5 is very small and poorly immunogenic, and E4 is more of a late protein, being undetectable in basal cells. In contrast, E6 and E7 are expressed in all HPV infected cells, and are up-regulated in cancer cells, and thus are generally considered the logical targets for therapeutic vaccination. E6 and E7 have already been targeted in numerous HPV therapeutic vaccination strategies including peptide, protein, live vector, DNA and whole-cell based approaches. Due to space limitations, our discussion will be focused on the efforts using VLP-based therapeutic vaccine approaches. This is an attractive approach that can potentially combine both prophylactic with therapeutic effects. For other therapeutic strategies, the reader is directed to [81,82].
Chimeric VLPs for therapeutic vaccination
Conventional therapies for cervical cancer are often invasive and toxic because they do not directly target the virus. The use of therapeutic vaccination against cervical cancer is thus attractive since it introduces the possibility of a non-invasive approach to directly target HPV infected cells via the induction of CD8 cytotoxic T-lymphocyte responses (CTLs), thereby reducing the potential for side effects. Indeed, most HPV infections are spontaneously cleared by the immune system, but this becomes a much less frequent outcome in immunosuppressed patients, or those with more advanced disease. HPV E6 and E7 peptide sequences contain several T cell epitopes which can elicit an anti-tumor CTL response [82-84]. However, typically the T-cell responses induced by natural infection are very low or undetectable, possibly reflecting the localized nature of the infection and viral immune evasion mechanisms, and indicating the potential for vaccination to improve E6/E7-specific immune responses. As mentioned, HPV VLPs are able to directly activate dendritic cells, and despite being an exogenous antigen, they can provide MHC Class I presentation by pseudo-infecting cells [12,85]. Thus, in addition to inducing high antibody titers, VLPs contain features that readily induce cell-mediated immunity.
Utilizing the advantages of VLPs, numerous groups have attempted to improve E6/E7 T-cell responses by forming chimeric HPV VLPs (cVLPs) in which the T-cell epitopes of HPV E6, E7 or both are fused to the C-terminus of either HPV L1-VLP or L1/L2-VLPs. Chimeric VLPs can thus act like a ‘delivery vehicle’ carrying the extra epitopes of the non-structural HPV proteins into the target cell for entry into the class I presentation pathway. Fusion of E7 protein to the C-terminus of L1 in VLPs provides a strong specific CTL response. However, there are significant constraints to the size of the E7 polypeptide fused to L1 before it results in impaired cVLP production. Conversely, fusing E7 to L2 in L1/L2E7-cVLPs is more tolerant to larger fusions without disrupting assembly, and we note that L2 is required for viral infection but not class I antigen presentation of the E7 epitopes included in the cVLPs [86]. Indeed, both approaches provide in vivo tumor protection in mouse models. The cVLPs have also been modified for inexpensive production in bacterial or plant expression systems [87-92].
The data discussed in this section on therapeutic cVLPs is largely pre-clinical and there is limited information about the safety and therapeutic efficacy of using cVLP in humans affected with cervical cancer. The first phase 1 clinical trial of cVLP was published in 2007. HPV16 L1E7 cVLPs were administered 4 times to patients with HPV16-positive high-grade cervical intraepithelial neoplasia (CIN2/3) [93]. The HPV16 L1E7 cVLPs were well tolerated and able to induce cellular immune responses in vaccinated patients. Unfortunately, in terms of clinical efficacy which was measured via 1) 50% lesion size reduction and 2) disappearance of HPV 16 DNA, there was no significant difference in either parameter between placebo and vaccinated groups. In addition, even though cellular immune responses were detected in patients, they did not clearly correlate with clinical response. This is in contrast to pre-clinical mouse data whereby the clearance of HPV induced tumors is clearly correlated with specific E6 or E7-CTL responses induced by the cVLPs.
One possible reason for the poor clinical efficacy of the HPV16 L1E7 cVLPs could be pre-existing neutralizing antibodies against HPV16 L1 (triggered by natural infection) that would limit the effectiveness of the cVLP vaccination, as shown in cVLP vaccinated mice by Da Silva and colleagues [94]. To circumvent this issue, Da Silva et al[95] successfully showed that a prime/boost strategy using heterologous cVLPs made from BPV and COPV but still fused with HPV16 E7 epitope could potentially bypass the pre-existing neutralizing antibody effects. Since the safety profile of the vaccine is high and a strong T-cell response was observed, it would be interesting to see if this heterologous prime/boost vaccination method could improve correlative responses in future human trials with HPV cVLP vaccines. Another possible reason for the difference between the response to the cVLP vaccination against human high grade cervical disease and the mouse model is the locality of the disease. The mouse TC-1 challenge model is systemic, whereas CIN2/3 is restricted to the patient's cervical epithelium. Therefore, a vaccination strategy to target or ‘pull’ the induced E7-specific CTL to the site of infection in the cervical epithelium may be required.
In the case of cervical cancer, specific CTLs against the cervical lesions are ‘stuck’ at the lymphatic regions and are unable to interact with the tumor lesions. The factors hampering this have not been fully identified although it has been attributed to a combination of T-regulatory cells and myeloid-derived suppressor cells that impact the proper homing and killing function of tumor-specific CTLs [96-98]. Clearly, the local tumor microenvironment plays a huge role in hindering the success of immunotherapeutic approaches. Taken together, it would be pertinent to consider combining therapeutic cVLPs with either immunomodulatory agents or other therapeutic modalities to alter the tumor micro-environment to improve vaccine potency (e.g via CTL access to the lesion and overcoming the local immune suppressive environment). Such combinatorial studies have already been tested using HPV therapeutic DNA vaccines whereby the depletion of regulatory T-cells has helped to improve therapeutic potency [99]. In another interesting study, HPV therapeutic DNA vaccination combined with chemotherapeutic agents resulted in enhanced anti-tumor effects [100]. These approaches however have been tested in mouse models only and thus it remains to be seen if such methods can be used in clinical settings.
HPV VLPs as delivery platforms
HPV L1/L2-Pseudovirions as carriers for DNA vaccines
Naked DNA-based vaccines have great potential for the induction of therapeutic T cell responses. However, one major obstacle in the field of DNA vaccination is the delivery of the DNA vaccines into target cells, especially antigen presenting cells. The utilization of L1/L2 VLPs to encapsidate a DNA vaccine that creates infectious but non-replicative HPV pseudovirion (PsV) is a promising technology to overcome the challenge of plasmid delivery in vivo [53]. Several groups have tried previously using L1-VLPs to facilitate DNA vaccine delivery; however because L1-VLPs lack L2 that is necessary for HPV infection, this approach is likely inferior compared to using HPV PsV. HPV PsV have been tested as a vaccine delivery platform against non-HPV diseases such as respiratory syncytical (RSV) virus and in Human Immunodeficiency virus (HIV) vaccine research using an SIV (Simian immunodeficiency virus) model[101,102]. In both studies, the specific CD8+T cell responses towards the respective DNA antigen were far higher than naked DNA vaccination, and the mice were vaccinated intra-vaginally because HPV PsV do not infect intact normal epithelium. Intra-vaginal vaccination warrants particular consideration for mucosal immunization as many viruses such as HIV, Herpes simplex viruses, share this same site of infection; induction of immune responses at the mucosa (e.g vagina) locally could thus be advantageous in several disease systems. Peng et al[103] generated HPV 16 PsV encapsidating a DNA vaccine encoding the ovalbumin (OVA) antigen. The results showed that PsV delivery method was able to generate a stronger OVA-specific CD8+ response compared to other routes of administration such as gene gun delivery, providing further support for HPV PsV as a promising platform for DNA-based therapeutic vaccination.
Expert commentary and 5 year outlook
Although vaccine development has been successful, there are still numerous issues that need to be addressed. It is important to recognize that pre-cancer was the primary efficacy endpoint (high grade cervical intraepithelial neoplasia (CIN) or adenocarcinoma in situ (AIS)) of the vaccine trials. Thus the demonstration of the true impact of the vaccines on cervical cancer rates in the population will take time, reflecting the 10-30 year process of HPV carcinogenesis and the delays in vaccine delivery nationally. However, we feel that there is reason to be optimistic since efficacy has been demonstrated in preventing both genital warts and precursors of cervical cancer in a national vaccine program setting in Australia. In addition, there is also no information on the duration of vaccine efficacy beyond a decade given its recent development and we will have to wait for these phase 4 studies to mature. If immunity wanes, then a booster may be required. The sufficiency of only 2 doses instead of 3 (to increase cost effectiveness), or the need for an additional booster (4th immunization) to achieve lifetime immunity remain open questions.
We also await the outcome of the efficacy studies of Merck's nonavalent HPV L1 VLP vaccine that are currently ongoing. This vaccine targets the two most common HPV types in genital warts, and most importantly, the seven most common oncogenic HPV types found in cervical cancer (HPV16, 18,31,33, 35, 52, 58). Should this vaccine prove safe and effective in providing the type of broad and durable coverage of oncogenic HPV types needed to eventually eradicate HPV-related anogenital malignancies, it would likely dramatically reduce or eliminate the need for cytologic screening in the appropriately vaccinated population.
From a public health perspective, the introduction of Merck's nonavalent HPV vaccine is unlikely to solve the problem of cost. Hence, in parallel with this are numerous efforts to develop generic HPV L1 VLP vaccines and L2-based approaches intended for local production at low cost to broaden access to this important opportunity for cancer prevention across the globe and to better reach under-served populations. Furthermore, efforts to simplify delivery of HPV vaccines must continue, including examining co-delivery of HPV VLP vaccines with vaccines for other agents, and whether HPV vaccination could be initiated with childhood vaccination. A careful consideration of the impact of HPV vaccination on screening also must be made with respect to cost benefit, and whether the consequent reduction in the prevalence of cervical neoplasia renders the Pap smear insufficiently predictive as a first line screening tool. Another critical issue to address is whether HPV VLP vaccines protect men and women from HPV infection in the oral cavity, and thereby from developing HPV-associated head and neck cancers. Currently there is early evidence to support this possibility[22] and rates of HPV-associated head and neck cancers should be followed in phase 4 vaccine studies in men and women.
For further improvements in the field of VLP vaccinology against HPV or other pathogens and diseases, it is important that the dissection of the B cell epitope display and T cell epitope deliver mechanisms continues. It is clear that VLPs do not all behave the same way; for example HPV VLP directly activates dendritic cells but human polyomavirus VLPs do not. The mechanisms and consequences of this for the immune response are not fully understood, and may impact the induction of cellular immune responses to heterologous T cell epitopes carried by chimeric VLP. The insertion of heterologous epitopes into VLP often reduces particle assembly warranting structural studies of the folding and assembly processes. The immunodominant VLP epitopes are typically conformational, i.e. formed from residues on multiple different loops, rather than linear like the heterologous epitopes being inserted (e.g. L2 17-36). This may explain why the inserted heterologous epitope is immunogenic, but not nearly to the level of the original immunodominant epitope site. More research is needed to understand structurally how best to present these epitopes to B cells such that they are even more immunogenic to the level of HPV VLP. Perhaps a better approach is to use a monoclonal antibody with the appropriate properties to select the VLP structure from a random phage library, for example, especially if the monoclonal antibody recognizes a conformation epitope. We believe that this will be a fruitful area in VLP design in the near term for both xenogenic epitopes of infectious agents and even potentially self-epitopes such as those of tumor antigens. Indeed there is growing interest in prophylactic vaccination against tumor antigens in patients genetically at high risk for particular malignancies to generate protective humoral and cellular immune responses.
Conclusion
Since HPV is currently responsible for genital warts and 5% of all cancer cases worldwide, the licensure in numerous countries and implementation of many national immunization campaigns for the prophylactic L1 VLP vaccines is potentially of profound benefit to human health. Early indications of such benefits are already seen in Australia and we are optimistic that this vaccine or a more broadly protective 2nd generation successor will be a important complement to the demonstrated success of Pap screening in cervical cancer prevention, and the emerging implementation of HPV DNA screening. Nevertheless, much work remains to ensure global access to HPV vaccination and broadening coverage to all oncogenic HPV genotypes. How these HPV VLP vaccines are so effective should be studied in detail, and information about the key mechanisms can be utilized to drive rational development of VLP vaccines to targets other than HPV. Efforts to display broadly protective L2 epitopes in various VLP to develop a pan-HPV vaccine are ongoing, but much remains to be learned about the optimal approach to insert heterologous epitopes into conformation-specific immunodominant VLP epitopes. HPV VLP can also be utilized to display other epitopes and generate robust humoral responses, even to self-antigens, suggesting promise in many other applications such as combating arthritic disease, Alzheimer disease and other infectious agents. HPV VLPs are particularly interesting in their ability to directly active dendritic and other antigen-presenting cells, and to present heterologous epitopes, including viral and tumor antigens, via MHCI to generate therapeutic immune responses. With only a single trial using such chimeric HPV L1-E7 VLP, these studies remain in their infancy. However, it is clear that in addition to triggering robust E6 and/or E7 antigen-specific T cell responses, considerations of targeting cytotoxic T cell responses to disease sites, and overcoming local tolerogenic factors are likely to influence the success of therapeutic HPV vaccines based on chimeric VLP vaccines.
Key Issues.
Cervical cancer and other HPV-associated malignancies are potentially preventable through HPV VLP vaccination, but continued follow up is required to demonstrate their impact on cancer incidence and mortality.
Implementation of cytologic screening and intervention can reduce cervical cancer rates 60-90% in naïve populations and this must be considered with respect to the cost/benefit of vaccination.
Careful consideration of how to globally implement of HPV vaccination is required to realize a dramatic reduction in rates of HPV-associated cancer, but there is an extended lead time to achieve this goal.
Widespread HPV vaccination is likely to impact cytologic screening approaches (i.e. Pap screening versus HPV DNA testing first), timing (when to start and intervals). The costs and benefits of all these approaches requires careful consideration and proper implementation to achieve the best reduction rates in cervical cancer.
Low cost vaccines and overcoming logistical barriers to delivery are critical as current vaccines are not practical for the developing world. Generic, local production of heat-stable formulations of L1 VLP in low cost expression systems that are effective as a single dose would be advantageous.
To inform rationale design of VLP-based vaccines to other agents, further mechanistic study of the human immune response to HPV VLP, especially early innate events and the induction of longterm memory, is required to understand how they are so immunogenic and effective.
Defining an immune correlate of protection is critical so that individual patients can know that they are effectively vaccinated (i.e. immune to HPV at that time)
Robust and long lived protection must be extended to all oncogenic HPV types by developing highly multivalent L1 VLP formulations, or possibly by use of L2.
VLP show promise for display of heterologous linear epitopes such as L2 to induce robust titers of high avidity and long-lived antibodies, but this potential has not been fully realized suggesting suboptimal presentation to the immune system. More study of immunodominant conformational epitopes in VLP is required to understand how best to generate similar responses to heterologous linear epitopes inserted into these structures.
HPV infection and associated diseases are widespread, and the need for a therapeutic vaccine remains.
Chimeric VLP can effectively deliver viral antigens to MHCI and generate a robust cellular immune response. However, to date, cVLP have not generated therapeutic responses in patients, suggesting consideration of targeting cellular immunity to the lesion site (e.g. route and site of immunization) and approaches to modulate the lesion microenvironment are warranted.
Acknowledgments
Funding: Public Health Service grants CA133749, CA118790, P50 CA098252 to RBSR
Footnotes
Financial Disclosure: The study was funded by Public Health Service grantP50 CA098252.
Competing Interests: Richard Roden is an inventor of L2-related patents licensed to Shantha Biotechnics Ltd., GlaxoSmithKline, PaxVax, Inc. and Acambis, Inc. Richard Roden has received research funding from Sanofi Pasteur, Shantha Biotechnic and GlaxoSmithKline and is a founder of Papivax LLC. The terms of these arrangements are managed by Johns Hopkins University in accordance with its conflict of interest policies.
References
Papers of special note have been highlighted as:
*of interest
**of considerable interest
- 1.De Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. The lancet oncology. 2012;13(6):607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 2.Dillner J, Kjaer SK, Wheeler CM, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. The lancet oncology. 2012;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann AM, Nitschmann S. Vaccine against human papillomavirus: PATRICIA Study (PApilloma TRIal against Cancer In young Adults) Der Internist. 2010;51410(3):412–413. doi: 10.1007/s00108-009-2575-8. [DOI] [PubMed] [Google Scholar]
- 5**.Kreimer AR, Gonzalez P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. The lancet oncology. 2011;12(9):862–870. doi: 10.1016/S1470-2045(11)70213-3. References 2-5 are recently ended clinical trials that show vaccination with currently licensed VLPs induce high antibody titers and provide protection against vaccine-related HPV types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. Journal of virology. 2008;82(9):4638–4646. doi: 10.1128/JVI.00143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaxosmithkline Vaccine HPVSG, Romanowski B, De Borba PC, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374(9706):1975–1985. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 8.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 9.Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstetrics and gynecology. 2006;107(1):18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 10.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert review of vaccines. 2010;9(10):1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 11**.Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. Journal of immunology. 2002;169(11):6120–6126. doi: 10.4049/jimmunol.169.11.6120. A study showing how HPV VLPs can be utilized to break B-cell tolerance. [DOI] [PubMed] [Google Scholar]
- 12.Lenz P, Day PM, Pang YY, et al. Papillomavirus-like particles induce acute activation of dendritic cells. Journal of immunology. 2001;166(9):5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 13.Lenz P, Thompson CD, Day PM, Bacot SM, Lowy DR, Schiller JT. Interaction of papillomavirus virus-like particles with human myeloid antigen-presenting cells. Clinical immunology. 2003;106(3):231–237. doi: 10.1016/s1521-6616(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 14*.Buck CB, Trus BL. The papillomavirus virion: a machine built to hide molecular Achilles' heels. Advances in experimental medicine and biology. 2012;726:403–422. doi: 10.1007/978-1-4614-0980-9_18. Excellent review on HPV virology and the biology of HPV infection. [DOI] [PubMed] [Google Scholar]
- 15.Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Molecular cell. 2000;5(3):557–567. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- 16.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clinical science. 2006;110(5):525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 17.De Villiers EM, Fauquet C, Broker TR, Bernard HU, Zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 18.De Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. The lancet oncology. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 19.Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nature reviews Cancer. 2006;6(10):753–763. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21*.Donovan B, Franklin N, Guy R, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. The Lancet infectious diseases. 2011;11(1):39–44. doi: 10.1016/S1473-3099(10)70225-5. Study in Australia which has nationalized HPV vaccination showed that mass vaccination can effectively prevent the burden of HPV disease, in this case, genital warts. [DOI] [PubMed] [Google Scholar]
- 22.Rolando H, Wim Quint, Allan Hildesheim, Paula Gonzalez, Linda Struijk, Katki Hormuzd a, Carolina Porras MS, Ana Cecilia Rodriguez, Diane Solomon, Silvia Jimenez, John T, Schiller DRL, Van Doorn Leen-Jan, Sholom Wacholder, Kreimer AR Group FTCV. Efficacy of an HPV16/18 vaccine against oral HPV infections: a randomized clinical trial. Efficacy of an HPV16/18 vaccine against oral HPV infections: a randomized clinical trial. 2012 [Google Scholar]
- 23.Mcneil C. Who invented the VLP cervical cancer vaccines? Journal of the National Cancer Institute. 2006;98(7):433. doi: 10.1093/jnci/djj144. [DOI] [PubMed] [Google Scholar]
- 24.Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Human vaccines. 2009;5(10):705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 25.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. The New England journal of medicine. 2002;347(21):1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 26.Poland GA, Jacobson RM, Koutsky LA, et al. Immunogenicity and reactogenicity of a novel vaccine for human papillomavirus 16: a 2-year randomized controlled clinical trial. Mayo Clinic proceedings Mayo Clinic. 2005;80(5):601–610. doi: 10.4065/80.5.601. [DOI] [PubMed] [Google Scholar]
- 27.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. The lancet oncology. 2005;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 28.Breitburd F, Kirnbauer R, Hubbert NL, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. Journal of virology. 1995;69(6):3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell host & microbe. 2010;8(3):260–270. doi: 10.1016/j.chom.2010.08.003. \Detailed study on how prophylatic HPV L1-VLP or L2-based vaccines exert their antibody mediated protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 31.Clemens JD, Jodar L. Translational research to assist policy decisions about introducing new vaccines in developing countries. Journal of health, population, and nutrition. 2004;22(3):223–231. [PubMed] [Google Scholar]
- 32.Schiffman M, Wacholder S. Success of HPV vaccination is now a matter of coverage. The lancet oncology. 2012;13(1):10–12. doi: 10.1016/S1470-2045(11)70324-2. [DOI] [PubMed] [Google Scholar]
- 33.Lowy DR, Solomon D, Hildesheim A, Schiller JT, Schiffman M. Human papillomavirus infection and the primary and secondary prevention of cervical cancer. Cancer. 2008;113(7 Suppl):1980–1993. doi: 10.1002/cncr.23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 35*.Wheeler CM, Castellsague X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. The lancet oncology. 2012;13(1):100–110. doi: 10.1016/S1470-2045(11)70287-X. A study showing the potential of Cervarix providing cross protection. [DOI] [PubMed] [Google Scholar]
- 36.Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. The lancet oncology. 2012;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 37.Roden R, Monie A, Wu TC. The impact of preventive HPV vaccination. Discovery medicine. 2006;6(35):175–181. [PubMed] [Google Scholar]
- 38.Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecologic oncology. 2010;118(1 Suppl):S12–17. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. The Journal of infectious diseases. 2009;200(2):166–171. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards RM, Lowy DR, Schiller JT, Day PM. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1522–1527. doi: 10.1073/pnas.0508815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fay A, Yutzy WHT, Roden RB, Moroianu J. The positively charged termini of L2 minor capsid protein required for bovine papillomavirus infection function separately in nuclear import and DNA binding. Journal of virology. 2004;78(24):13447–13454. doi: 10.1128/JVI.78.24.13447-13454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campo MS, Grindlay GJ, O'neil BW, Chandrachud LM, Mcgarvie GM, Jarrett WF. Prophylactic and therapeutic vaccination against a mucosal papillomavirus. The Journal of general virology. 1993;74(Pt 6):945–953. doi: 10.1099/0022-1317-74-6-945. [DOI] [PubMed] [Google Scholar]
- 43.Christensen ND, Kreider JW, Kan NC, Diangelo SL. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181(2):572–579. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- 44.Lin YL, Borenstein LA, Selvakumar R, Ahmed R, Wettstein FO. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992;187(2):612–619. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- 45.Embers ME, Budgeon LR, Pickel M, Christensen ND. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. Journal of virology. 2002;76(19):9798–9805. doi: 10.1128/JVI.76.19.9798-9805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campo MS, O'neil BW, Grindlay GJ, Curtis F, Knowles G, Chandrachud L. A peptide encoding a B-cell epitope from the N-terminus of the capsid protein L2 of bovine papillomavirus-4 prevents disease. Virology. 1997;234(2):261–266. doi: 10.1006/viro.1997.8649. [DOI] [PubMed] [Google Scholar]
- 47.Chandrachud LM, Grindlay GJ, Mcgarvie GM, et al. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology. 1995;211(1):204–208. doi: 10.1006/viro.1995.1392. [DOI] [PubMed] [Google Scholar]
- 48.Gambhira R, Jagu S, Karanam B, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. Journal of virology. 2007;81(21):11585–11592. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gambhira R, Karanam B, Jagu S, et al. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. Journal of virology. 2007;81(24):13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawana K, Kawana Y, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Nasal immunization of mice with peptide having a cross-neutralization epitope on minor capsid protein L2 of human papillomavirus type 16 elicit systemic and mucosal antibodies. Vaccine. 2001;19(11-12):1496–1502. doi: 10.1016/s0264-410x(00)00367-4. [DOI] [PubMed] [Google Scholar]
- 51*.Rubio I, Seitz H, Canali E et al. The N-terminal region of the human papillomavirus L2 protein contains overlapping binding sites for neutralizing, cross-neutralizing and non-neutralizing antibodies. Virology. 2011;409(2):348–359. doi: 10.1016/j.virol.2010.10.017. References 43-51 show that vaccination with L2 and its peptides protects animals from experimental papillomavirus infections. [DOI] [PubMed] [Google Scholar]
- 52.Karanam B, Jagu S, Huh WK, Roden RB. Developing vaccines against minor capsid antigen L2 to prevent papillomavirus infection. Immunology and cell biology. 2009;87(4):287–299. doi: 10.1038/icb.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods in molecular medicine. 2005;119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 54.Pastrana DV, Buck CB, Pang YY, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321(2):205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 55.Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nature medicine. 2007;13(7):857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 56.Roden RB, Greenstone HL, Kirnbauer R, et al. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. Journal of virology. 1996;70(9):5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alphs HH, Gambhira R, Karanam B, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(15):5850–5855. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jagu S, Karanam B, Gambhira R, et al. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. Journal of the National Cancer Institute. 2009;101(11):782–792. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jagu S, Kwak K, Garcea RL, Roden RB. Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection. Vaccine. 2010;28(28):4478–4486. doi: 10.1016/j.vaccine.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jagu S, Malandro N, Kwak K, et al. A multimeric L2 vaccine for prevention of animal papillomavirus infections. Virology. 2011;420(1):43–50. doi: 10.1016/j.virol.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roden RB, Yutzy WHT, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270(2):254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 62.Buck CB, Cheng N, Thompson CD, et al. Arrangement of L2 within the papillomavirus capsid. Journal of virology. 2008;82(11):5190–5197. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. An HPV in vitro neutralization assay that recapitulates the in vivo process of infection provides a sensitive measure of L2 infection-inhibiting antibodies. Clinical and vaccine immunology: CVI. 2012 doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. Journal of virology. 2009;83(5):2067–2074. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(48):20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selinka HC, Giroglou T, Nowak T, Christensen ND, Sapp M. Further evidence that papillomavirus capsids exist in two distinct conformations. Journal of virology. 2003;77(24):12961–12967. doi: 10.1128/JVI.77.24.12961-12967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roden RB, Day PM, Bronzo BK, et al. Positively charged termini of the L2 minor capsid protein are necessary for papillomavirus infection. Journal of virology. 2001;75(21):10493–10497. doi: 10.1128/JVI.75.21.10493-10497.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slupetzky K, Gambhira R, Culp TD, et al. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine. 2007;25(11):2001–2010. doi: 10.1016/j.vaccine.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. Journal of virology. 2009;83(19):10085–10095. doi: 10.1128/JVI.01088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varsani A, Williamson AL, De Villiers D, Becker I, Christensen ND, Rybicki EP. Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16. Journal of virology. 2003;77(15):8386–8393. doi: 10.1128/JVI.77.15.8386-8393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kondo K, Ochi H, Matsumoto T, Yoshikawa H, Kanda T. Modification of human papillomavirus-like particle vaccine by insertion of the cross-reactive L2-epitopes. Journal of medical virology. 2008;80(5):841–846. doi: 10.1002/jmv.21124. [DOI] [PubMed] [Google Scholar]
- 72.Rose RC, White WI, Li M, Suzich JA, Lane C, Garcea RL. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. Journal of virology. 1998;72(7):6151–6154. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu WH, Gersch E, Kwak K, et al. Capsomer vaccines protect mice from vaginal challenge with human papillomavirus. PloS one. 2011;6(11):e27141. doi: 10.1371/journal.pone.0027141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmer KE, Benko A, Doucette SA, et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine. 2006;24(26):5516–5525. doi: 10.1016/j.vaccine.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 75.Cerovska N, Hoffmeisterova H, Moravec T, et al. Transient expression of Human papillomavirus type 16 L2 epitope fused to N- and C-terminus of coat protein of Potato virus X in plants. Journal of biosciences. 2012;37(1):125–133. doi: 10.1007/s12038-011-9177-z. [DOI] [PubMed] [Google Scholar]
- 76.Cerovska N, Hoffmeisterova H, Pecenkova T, et al. Transient expression of HPV16 E7 peptide (aa 44-60) and HPV16 L2 peptide (aa 108-120) on chimeric potyvirus-like particles using Potato virus X-based vector. Protein expression and purification. 2008;58(1):154–161. doi: 10.1016/j.pep.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PloS one. 2011;6(8):e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caldeira Jdo C, Medford A, Kines RC, et al. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine. 2010;28(27):4384–4393. doi: 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nieto K, Weghofer M, Sehr P, et al. Development of AAVLP(HPV16/31L2) Particles as Broadly Protective HPV Vaccine Candidate. PloS one. 2012;7(6):e39741. doi: 10.1371/journal.pone.0039741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suba EJ, Raab SS. Lessons learned from successful Papanicolaou cytology cervical cancer prevention in the Socialist Republic of Vietnam. Diagnostic cytopathology. 2012;40(4):355–366. doi: 10.1002/dc.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin K, Doolan K, Hung CF, Wu TC. Perspectives for preventive and therapeutic HPV vaccines. Journal of the Formosan Medical Association = Taiwan yi zhi. 2010;109(1):4–24. doi: 10.1016/s0929-6646(10)60017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hung CF, Ma B, Monie A, Tsen SW, Wu TC. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert opinion on biological therapy. 2008;8(4):421–439. doi: 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu CY, Monie A, Pang X, Hung CF, Wu TC. Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. Journal of biomedical science. 2010;17:88. doi: 10.1186/1423-0127-17-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer immunology, immunotherapy: CII. 2012;61(8):1307–1317. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rudolf MP, Fausch SC, Da Silva DM, Kast WM. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. Journal of immunology. 2001;166(10):5917–5924. doi: 10.4049/jimmunol.166.10.5917. [DOI] [PubMed] [Google Scholar]
- 86.Wakabayashi MT, Da Silva DM, Potkul RK, Kast WM. Comparison of human papillomavirus type 16 L1 chimeric virus-like particles versus L1/L2 chimeric virus-like particles in tumor prevention. Intervirology. 2002;45(4-6):300–307. doi: 10.1159/000067921. [DOI] [PubMed] [Google Scholar]
- 87.Paz De La Rosa G, Monroy-Garcia A, Mora-Garcia Mde L, et al. An HPV 16 L1-based chimeric human papilloma virus-like particles containing a string of epitopes produced in plants is able to elicit humoral and cytotoxic T-cell activity in mice. Virology journal. 2009;6:2. doi: 10.1186/1743-422X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bian T, Wang Y, Lu Z, et al. Human papillomavirus type 16 L1E7 chimeric capsomeres have prophylactic and therapeutic efficacy against papillomavirus in mice. Molecular cancer therapeutics. 2008;7(5):1329–1335. doi: 10.1158/1535-7163.MCT-07-2015. [DOI] [PubMed] [Google Scholar]
- 89.Muller M, Zhou J, Reed TD, et al. Chimeric papillomavirus-like particles. Virology. 1997;234(1):93–111. doi: 10.1006/viro.1997.8591. [DOI] [PubMed] [Google Scholar]
- 90.Schafer K, Muller M, Faath S, et al. Immune response to human papillomavirus 16 L1E7 chimeric virus-like particles: induction of cytotoxic T cells and specific tumor protection. International journal of cancer Journal international du cancer. 1999;81(6):881–888. doi: 10.1002/(sici)1097-0215(19990611)81:6<881::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 91.Sharma C, Dey B, Wahiduzzaman M, Singh N. Human papillomavirus 16 L1-E7 chimeric virus like particles show prophylactic and therapeutic efficacy in murine model of cervical cancer. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Kaufmann AM, Nieland J, Schinz M, et al. HPV16 L1E7 chimeric virus-like particles induce specific HLA-restricted T cells in humans after in vitro vaccination. International journal of cancer Journal international du cancer. 2001;92(2):285–293. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1181>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 93.Kaufmann AM, Nieland JD, Jochmus I, et al. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3) International journal of cancer Journal international du cancer. 2007;121(12):2794–2800. doi: 10.1002/ijc.23022. [DOI] [PubMed] [Google Scholar]
- 94.Da Silva DM, Pastrana DV, Schiller JT, Kast WM. Effect of preexisting neutralizing antibodies on the anti-tumor immune response induced by chimeric human papillomavirus virus-like particle vaccines. Virology. 2001;290(2):350–360. doi: 10.1006/viro.2001.1179. [DOI] [PubMed] [Google Scholar]
- 95**.Da Silva DM, Schiller JT, Kast WM. Heterologous boosting increases immunogenicity of chimeric papillomavirus virus-like particle vaccines. Vaccine. 2003;21(23):3219–3227. doi: 10.1016/s0264-410x(03)00237-8. References 93-95 is about a failed clinical trial using chimeric-HPV VLP as a therapeutic vaccine and two follow up studies (94,95) on why cVLPs might have failed and possible solutions to overcome this. [DOI] [PubMed] [Google Scholar]
- 96.Trimble CL, Clark RA, Thoburn C, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. Journal of immunology. 2010;185(11):7107–7114. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Welters MJ, Kenter GG, De Vos Van Steenwijk PJ, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(26):11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Der Burg SH, Piersma SJ, De Jong A, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(29):12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chuang CM, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+CD25+ T regulatory cells. Vaccine. 2009;27(5):684–689. doi: 10.1016/j.vaccine.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chuang CM, Monie A, Wu A, Hung CF. Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. Journal of biomedical science. 2009;16:49. doi: 10.1186/1423-0127-16-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Graham BS, Kines RC, Corbett KS, et al. Mucosal delivery of human papillomavirus pseudovirus-encapsidated plasmids improves the potency of DNA vaccination. Mucosal immunology. 2010;3(5):475–486. doi: 10.1038/mi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gordon SN, Kines RC, Kutsyna G, et al. Targeting the vaginal mucosa with human papillomavirus pseudovirion vaccines delivering simian immunodeficiency virus DNA. Journal of immunology. 2012;188(2):714–723. doi: 10.4049/jimmunol.1101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peng S, Monie A, Kang TH, Hung CF, Roden R, Wu TC. Efficient delivery of DNA vaccines using human papillomavirus pseudovirions. Gene therapy. 2010;17(12):1453–1464. doi: 10.1038/gt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]


