Abstract
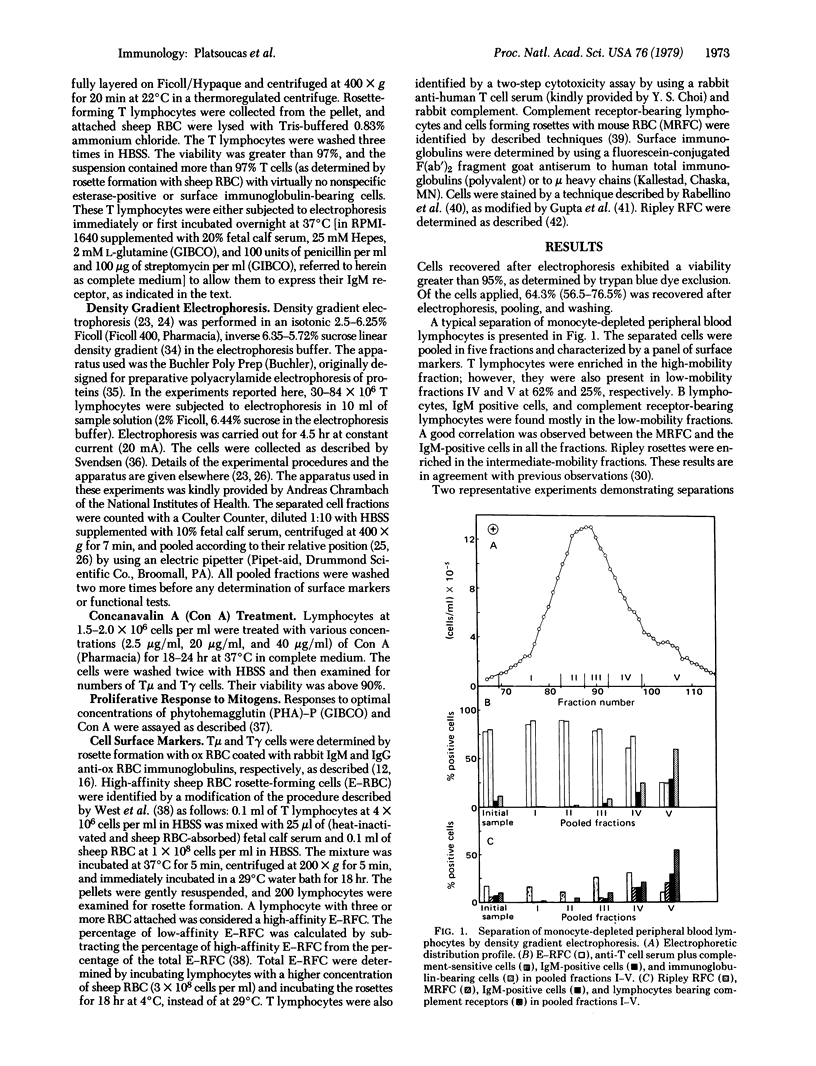
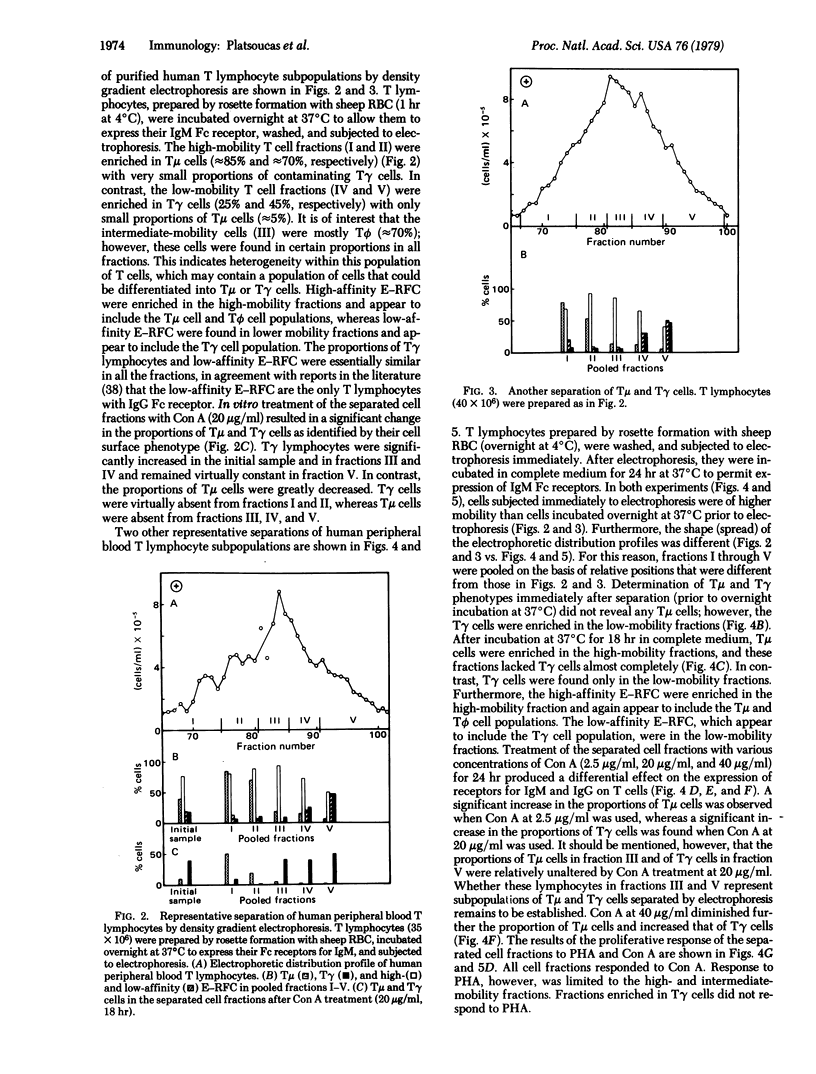
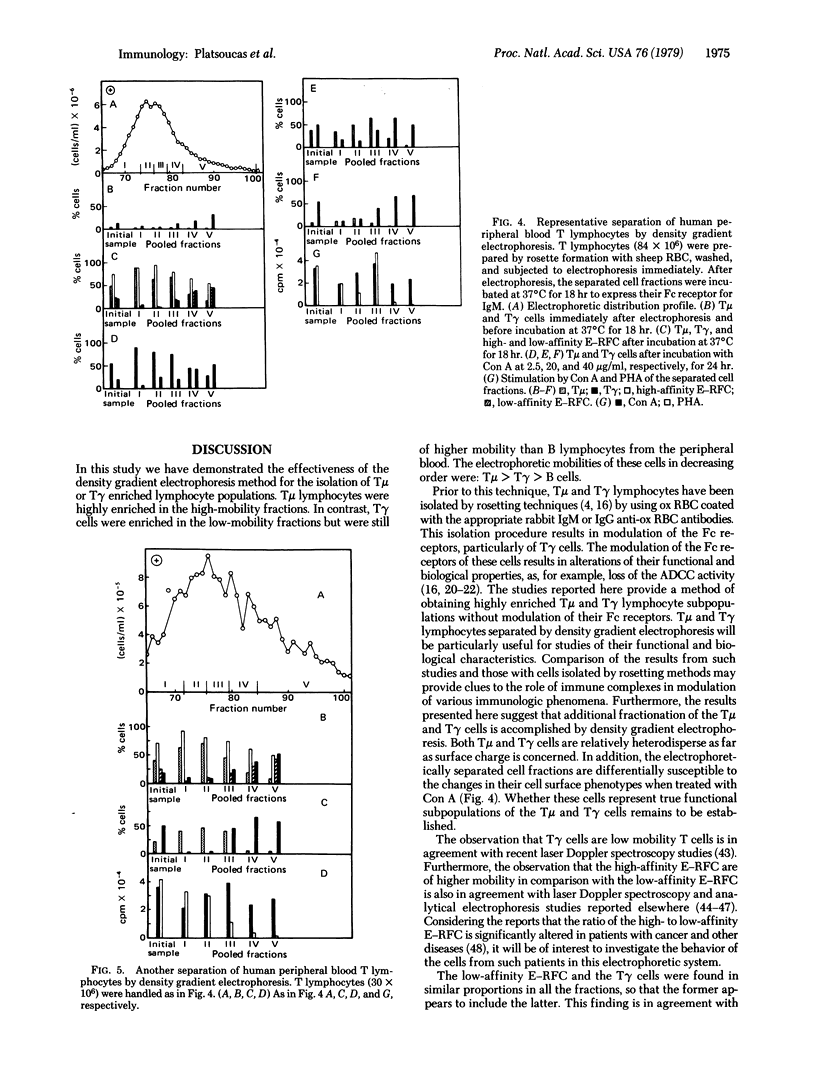
Purified human peripheral blood T lymphocytes were fractionated by density gradient electrophoresis on the basis of their surface charge. The high mobility cell fractions were found to be enriched in T cells having receptors for IgM (Tμ cells) with only minor proportions of T cells having receptors for IgG (Tγ cells). In contrast, the low mobility cell fractions were enriched in Tγ cells with very low numbers of contaminating Tμ cells. Both populations were of higher mobility than human peripheral blood B lymphocytes. High-affinity sheep erythrocyte resette-forming cells (E-RFC) were relatively enriched in the high and intermediate mobility fractions and appear to include the Tμ cells and the T cells without receptors for IgG or IgM (Tϕ). The low affinity E-RFC were found only in the lower mobility fractions that included the Tγ cell population. A direct correlation was observed between the number of Tγ lymphocytes and the low affinity E-RFC in all the fractions. The separated cell fractions were treated in vitro with different concentrations of concanavalin A (Con A) and examined for the numbers of Tμ and Tγ cells. Low concentrations of Con A (2.5 μg/ml) significantly increased the number of Tμ cells, whereas high concentrations of Con A (20 μg/ml and 40 μg/ml) markedly reduced the number of Tμ cells and increased the number of Tγ cells. Furthermore, all fractions (both Tμ and Tγ cell enriched) responded by proliferation to Con A, whereas only the high and intermediate mobility fractions (enriched in Tμ cells) responded to phytohemagglutinin. Fractions enriched in Tγ cells responded very poorly to phytohemagglutinin. This method provides another technique for separating human Tμ- and Tγ-enriched lymphocyte subpopulations and does not modulate the Fc receptors of the cells, in contrast to the rosetting techniques currently in use for the separation of these lymphocytes.
Keywords: T cell subsets, cell surface charge, Fc receptors, mitogen response
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreeff M., Beck J. D., Darzynkiewicz Z., Traganos F., Gupta S., Melamed M. R., Good R. A. RNA content in human lymphocyte subpopulations. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1938–1942. doi: 10.1073/pnas.75.4.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault K. A., Griffith A. L., Platsoucas C. D., Catsimpoolas N. Partial separation of human blood leukocytes by density gradient electrophoresis:different mobilities of lymphocytes with IgG, those with IgM and IgD, T lymphocytes, and monocytes. J Immunol. 1976 Oct;117(4):1406–1408. [PubMed] [Google Scholar]
- Boltz R. C., Jr, Todd P., Streibel M. J., Louie M. K. Preparative electrophoresis of living mammalian cells in a stationary ficoll gradient. Prep Biochem. 1973;3(4):383–401. doi: 10.1080/00327487308061523. [DOI] [PubMed] [Google Scholar]
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Catsimpoolas N., Griffith A. L., Skrabut E. M., Platscucas C. D., Valeri C. R. Differential Cr uptake of human peripheral lymphocytes separated by density gradient electrophoresis. Cell Immunol. 1976 Aug;25(2):317–321. doi: 10.1016/0008-8749(76)90122-2. [DOI] [PubMed] [Google Scholar]
- Cordier G., Samarut C., Revillard J. P. Changes of Fcgamma receptor-related properties induced by interaction of human lymphocytes with insoluble immune complexes. J Immunol. 1977 Dec;119(6):1943–1948. [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Gmelig-Meyling F., Van der Ham M., Ballieux R. E. Binding of IgM by human T lymphocytes. Scand J Immunol. 1976;5(5):487–495. doi: 10.1111/j.1365-3083.1976.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Griffith A. L., Catsimpoolas N., Wortis H. H. Electrophoretic separation of cells in a density gradient. Life Sci. 1975 Jun 1;16(11):1693–1702. doi: 10.1016/0024-3205(75)90053-3. [DOI] [PubMed] [Google Scholar]
- Grossi C. E., Webb S. R., Zicca A., Lydyard P. M., Moretta L., Mingari M. C., Cooper M. D. Morphological and histochemical analyses of two human T-cell subpopulations bearing receptors for IgM or IgG. J Exp Med. 1978 May 1;147(5):1405–1417. doi: 10.1084/jem.147.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Fernandes G., Nair M., Good R. A. Spontaneous and antibody-dependent cell-mediated cytotoxicity by human T cell subpopulations. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5137–5141. doi: 10.1073/pnas.75.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. Functionally distinct subpopulations of human T lymphocytes--a review. Clin Bull. 1978;8(3):100–106. [PubMed] [Google Scholar]
- Gupta S., Good R. A., Siegal F. P. Rosette-formation with mouse erythrocytes. II. A marker for human B and non-T lymphocytes. Clin Exp Immunol. 1976 Aug;25(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. I. Studies in immunodeficient patients. Clin Exp Immunol. 1977 Nov;30(2):222–228. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. II. Effect of thymopoietin, corticosteroids, and irradiation. Cell Immunol. 1977 Nov;34(1):10–18. doi: 10.1016/0008-8749(77)90224-6. [DOI] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. III. Distribution and quantitation in peripheral blood, cord blood, tonsils, bone marrow, thymus, lymph nodes, and spleen. Cell Immunol. 1978 Mar 15;36(2):263–270. doi: 10.1016/0008-8749(78)90270-8. [DOI] [PubMed] [Google Scholar]
- Gupta S., Pahwa R., Siegal F. P., Good R. A. Rosette formation with mouse erythrocytes. IV. T, B and third population cells in human tonsils. Clin Exp Immunol. 1977 May;28(2):347–351. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Safai B., Good R. A. Rosette-formation with mouse erythrocytes: VI. T, B, and third population lymphoid cells in mycosis fungoides and effect of leukopheresis. Am J Hematol. 1978;4(2):133–140. doi: 10.1002/ajh.2830040205. [DOI] [PubMed] [Google Scholar]
- Gupta S., Safai B., Good R. A. Subpopulations of human T lymphocytes. IV. Quantitation and distribution in patients with mycosis fungoides and Sézary syndrome. Cell Immunol. 1978 Aug;39(1):18–26. doi: 10.1016/0008-8749(78)90078-3. [DOI] [PubMed] [Google Scholar]
- Hanjan S. N., Talwar G. P., Kidwai Z., Nath I. Delineation and quantitation of human peripheral blood lymphocyte subpopulations by electrophoretic mobility and role of surface charge in cell to cell interaction. J Immunol. 1977 Jan;118(1):235–241. [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Kay H. D., Bonnard G. D., West W. H., Herberman R. B. A functional comparison of human Fc-receptor-bearing lymphocytes active in natural cytotoxicity and antibody-dependent cellular cytotoxicity. J Immunol. 1977 Jun;118(6):2058–2066. [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M. L., Mingari M. C. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975 Aug;5(8):565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Romanzi C. A. Loss of Fc receptors for IgG from human T lymphocytes exposed to IgG immune complexes. Nature. 1978 Apr 13;272(5654):618–620. doi: 10.1038/272618a0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Webb S. R., Pearl E. R., Lydyard P. M., Grossi C. E., Lawton A. R., Cooper M. D. Imbalances in T cell subpopulations associated with immunodeficiency and autoimmune syndromes. Eur J Immunol. 1977 Oct;7(10):696–700. doi: 10.1002/eji.1830071009. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott D. M., Good R. A., O'Neill G. J., Gupta S. Heterogeneity of locomotion in human T cell subsets. Proc Natl Acad Sci U S A. 1978 May;75(5):2392–2395. doi: 10.1073/pnas.75.5.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platsoucas C. D., Griffith A. L., Catsimpoolas N. Density gradient electrophoresis of mouse spleen lymphocytes: separation of T and B cell fractions. J Immunol Methods. 1976;13(2):145–152. doi: 10.1016/0022-1759(76)90152-6. [DOI] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West W. H., Boozer R. B., Herberman R. B. Low affinity E-rosette formation by the human K cell. J Immunol. 1978 Jan;120(1):90–95. [PubMed] [Google Scholar]
- West W. H., Payne S. M., Weese J. L., Herberman R. B. Human T lymphocyte subpopulations: correlation between E-rosette-forming affinity and expression of the Fc receptor. J Immunol. 1977 Aug;119(2):548–554. [PubMed] [Google Scholar]
- West W. H., Sienknecht C. W., Townes A. S., Herberman R. B. Performance of a rosette assay between lymphocytes and sheep erythrocytes at elevated temperatures to study patients with cancer and other diseases. Clin Immunol Immunopathol. 1976 Jan;5(1):60–66. doi: 10.1016/0090-1229(76)90149-5. [DOI] [PubMed] [Google Scholar]


