Abstract
Recurrent and/or metastatic head and neck squamous cell carcinoma (R/M HNSCC) is a devastating malignancy with a poor prognosis. Treatment is limited to chemotherapeutic approaches. Cisplatin is an established and effective treatment for R/M HNSCC, and many studies have investigated cisplatin treatment in combination with other agents. Even when being treated with first line therapy (cisplatin + 5-fluorouracil + cetuximab), overall survival is only 10 months, indicating the need for novel chemotherapeutics and treatment regimens. Current research is focused on molecular targeting therapies inhibiting epidermal growth factor receptor, phosphoinositide-3-kinase/Akt/mammalian target of rapamycin, and vascular endothelial growth factor pathways. A variety of clinical trials have been completed and are currently underway with encouraging results. Finally, future directions of cisplatin-based R/M HNSCC treatment may include targeting specific pathways known to induce cisplatin resistance, such as nucleotide excision repair and inhibition of apoptosis, in hopes to enhance response to cisplatin therapy.
Keywords: head and neck cancer, chemotherapy, cisplatin
Introduction
In 2012, an estimated 40,250 new cases of head and neck squamous cell carcinoma (HNSCC) were diagnosed in the United States,1 of which 50% are predicted to recur or metastasize (R/M).2 R/M HNSCC carries a poor prognosis, with an average overall survival of about 10 months even with first-line therapy.3 While treatment for locoregionally recurrent HNSCC may include surgery or radiation therapy (RT), systemic chemotherapy remains a therapeutic cornerstone for R/M HNSCC, especially for patients with distant metastases. One of the few advances in R/M HNSCC chemotherapy has been the introduction of cisplatin.
Cisplatin acts via crosslinking DNA and making its repair impossible, thus activating apoptosis in quickly dividing cells.4 The utility of cisplatin in HNSCC treatment was first described in 1977.5 Morton et al performed a randomized phase III 2 × 2 factorial trial of cisplatin and bleomycin, two commonly used R/M HNSCC therapies at the time, in 116 patients with advanced or recurrent HNSCC. This trial found that treatment with cisplatin, but not bleomycin or even combination therapy, prolonged median overall survival (OS) by 10 weeks (P < 0.05), even though there was no statistically significant improvement in response rate (RR).6 Since these studies, cisplatin has become the most extensively studied and widely used chemotherapeutic agent in R/M HNSCC.
Because of its different spectrum of toxicity compared to cisplatin, carboplatin has also been examined as a R/M HNSCC therapeutic agent. When studying its efficacy in the treatment of 31 patients with R/M HNSCC, OS was found to be 4.5 months with a RR of 25.8%.87 This response was comparable to trials of cisplatin monotherapy at the time, and thus, carboplatin was determined to be a similarly efficacious treatment for R/M HNSCC.
While the introduction of cisplatin therapy was a significant landmark in the treatment of R/M HNSCC, response to treatment and patient outcomes were still poor. This instigated the investigation of other chemotherapeutic agents in addition to cisplatin to potentially improve response and survival. Past studies mainly focused on combining cisplatin therapy with additional cytotoxic agents, which led to the current first-line therapy for R/M HNSCC (cisplatin + 5-fluorouracil [5-FU] + cetuximab [a monoclonal epidermal growth factor receptor (EGFR) antibody].) After the discovery of cetuximab and its utility in combination with cisplatin, much of the current research and trials are studying the utility of other novel molecular targeted therapies with cisplatin therapy, with the hopes of demonstrating increased efficacy when using combination treatments that affect multiple tumorgenic pathways. Finally, the future directions of cisplatin combination therapy examine the targeting of signaling pathways that are specifically indicated in cisplatin resistance.
Historical Trials
Several trials have examined the efficacy of combination cytotoxic chemotherapy using cisplatin as the cornerstone of treatment in R/M HNSCC, leading to the current first-line therapy recommendations (Table 1). These combinations and trials are explored further below. Although patients may have received previous induction chemotherapy that could theoretically affect response to treatment regimens, insufficient clinical data are published to allow for a comprehensive review here.
Table 1.
Randomized phase III trials of cisplatin combination therapies in recurrent and/or metastatic head and neck squamous cell carcinoma.
| Combination agent | Treatment groups | RR (%) | Os (mo.) | Reference |
|---|---|---|---|---|
| 5-FU | Cisplatin + 5-FU | 32* | 5.5 | 13 |
| Cisplatin | 17 | 5.0 | ||
| 5-FU | 13 | 6.1 | ||
| Cisplatin + 5-FU | 32* | 6.6 | 14 | |
| Cisplatin | 10 | 5.6 | ||
| CABO | 34* | 6.7 | 15 | |
| Cisplatin + 5-FU | 31* | 6.7 | ||
| Cisplatin | 15 | 6.7 | ||
| Paclitaxel | Cisplatin + 5-FU | 27 | 8.7 | 23 |
| Cisplatin + paclitaxel | 26 | 8.1 | ||
| Pemetrexed | Cisplatin + pemetrexed | 48 | 7.3 | 27 |
| Cisplatin | 32 | 6.2 | ||
| Cetuximab | Cisplatin + cetuximab | 26* | 9.2 | 31 |
| Cisplatin + placebo | 10 | 8.0 | ||
| 5-FU + cetuximab | Platinum + 5-FU + cetuximab | 36* | 10.1* | 3 |
| Platinum + 5-FU | 20 | 7.4 |
Note: Indicates statistically significant difference (P < 0.05).
Abbreviations: RR, response rate; OS, overall survival; 5-FU, 5-fluorouracil; CABO, cisplatin, methotrexate, bleomycin, vincristine.
Cisplatin and 5-fluorouracil
In 1979, treatment of advanced HNSCC with 5-fluorouracil exhibited a RR of 31%, comparable to results observed with several chemotherapeutic agents being used at the time.7 Cisplatin and 5-FU were also found to not have overlapping toxicities, thus making it an attractive combination therapy. This was studied by several groups in the 1980s and appeared promising in multiple phase II trials, demonstrating significant response rates (60%–94%) in patients with advanced HNSCC.8–12 These trials compared patients who did and did not respond to treatment, showing significantly increased survival in responders. Ethical concerns would not permit inclusion of a placebo group for comparison of survival. No single-agent treatment arms were included in any of these studies.
Unfortunately, when cisplatin + 5-FU combination was studied in randomized, phase III trials including cisplatin-only treated groups, no significant improvement in overall survival (OS) was observed when comparing combination therapy to cisplatin-treatment alone.13–15 Jacobs et al found that while RR was significantly increased with doublet therapy over cisplatin monotherapy (32% vs. 17%, P < 0.05), this did not correlate with increased OS (5.5 months vs. 5.0 months, P = 0.49). Paradoxically, treatment with 5-FU alone had the longest OS (6.1 months), but this was not statistically significant.13
Another trial compared the combination of cisplatin + 5-FU to carboplatin + 5-FU to cisplatin treatment alone. Again, cisplatin + 5-FU demonstrated significant improvement of RR when compared to cisplatin-only (32% vs. 10%, P < 0.05) without increased OS (6.6 months vs. 5.6 months). Carboplatin + 5-FU did not significantly improve RR or OS (21%, 5.0 months).14
CABO
CABO (cisplatin, methotrexate, bleomycin and vincristine) is an aggressive combination treatment first described in 1984, combining several frequently used monotherapies into one treatment regimen that included cisplatin, methotrexate, bleomycin, and vincristine. In 72 patients with R/M HNSCC, 50% of patients responded to treatment; effects on survival were not reported.16 The effect of cisplatin on this treatment regimen was demonstrated in a trial comparing CABO and ABO (methotrexate, bleomycin and vincristine) treatment, finding a significant effect on RR (50% vs. 28%, P = 0.0003) but no increase in OS.17
Clavel et al completed a randomized, phase III trial in 382 patients comparing CABO, cisplatin + 5-FU, and cisplatin-only treatment. This study found that RR was significantly improved with both CABO (34%) and cisplatin + 5-FU (31%) over cisplatin monotherapy (15%) (P < 0.001, P = 0.003, respectively), but OS was 6.7 months for all three groups with no significant difference. This study confirmed previous results comparing cisplatin + 5-FU doublet therapy with cisplatin monotherapy, while also demonstrating no improvement in outcome with CABO combination treatment.15
Cisplatin and taxanes
With the development of taxanes in the 1990s, groups began to study the efficacy of combining taxane treatment with cisplatin in HNSCC. The initial phase II trial conducted by the Eastern Cooperative Oncology Group (ECOG) studying cisplatin + paclitaxel treatment in 34 patients found encouraging results with a RR of 40% but with the significant toxicity of granulocytopenia.18 Docetaxel + cisplatin treatment was found to have a similar RR (42%), further indicating the combination of cisplatin with taxane treatment as an attractive therapeutic option.19 ECOG then studied this treatment combination in a phase III trial comparing high dose paclitaxel + granulocyte colony stimulating factor + cisplatin vs. low dose paclitaxel + cisplatin, finding no significant difference in RR or OS. This trial had to be terminated early due to severe hematologic toxicity.20
Additional phase II trials were completed that delineated safer but still active dosing regimens,21,22 leading to a randomized, phase III trial comparing cisplatin + paclitaxel vs. cisplatin + 5-FU in 218 patients with advanced or R/M HNSCC. This trial found no significant difference in RR (26% vs. 27%, respectively) or OS (8.1 months vs. 8.7 months, respectively).23 While a cisplatin monotherapy arm was not included in this trial, it can be presumed that because cisplatin + paclitaxel showed no significant difference from cisplatin + 5-FU treatment (and actually decreased RR and OS), combination treatment would not show significant improvement over treatment with cisplatin alone.
Cisplatin and pemetrexed
Pemetrexed was discovered in 1992 to be a thymidylate synthase inhibitor and a possibly effective chemotherapeutic agent.24 Single-agent treatment of HNSCC with pemetrexed was studied in a phase II trial with a RR of 26.5%, comparable to previously identified monotherapies.25 Furthermore, a phase III trial comparing pemetrexed + cisplatin vs. cisplatin alone in 456 patients with malignant pleural mesothelioma resulted in significant enhancement of RR (41.3% vs. 16.7%, P < 0.001) and OS (12.1 months vs. 9.3 months, P = 0.02).26 Even though this was not an HNSCC trial, it demonstrated cisplatin + pemetrexed as a promising chemotherapeutic approach.
Therefore, a randomized, phase III trial was performed in patients with R/M HNSCC comparing cisplatin + pemetrexed to cisplatin alone; there was no significant difference in either RR (48% vs. 32%, respectively) or OS (7.3 months vs. 6.3 months, respectively). Further analysis demonstrated that patients with a performance status (PS) (a measure of activity and ability to self-care developed by ECOG,)6 of 0 or 1 exhibited significant improvement of OS vs. patients with a PS of 2.27
Cetuximab
The development of cetuximab, a monoclonal antibody targeting EGFR has been an exciting advancement in treatment of HNSCC. Cetuximab was found to be an effective treatment option for patients who failed cisplatin treatment in multiple phase II trials (RR: 10%–15%).28–30 This led to 2 phase III trials. The first compared cetuximab + cisplatin treatment vs. cisplatin monotherapy in never treated R/M HNSCC, finding similar results to cytotoxic combination studies: RR was significantly improved (26% vs. 10%, P = 0.03) but OS was not prolonged (9.2 months vs. 8.0 months, P = 0.45).31
After a phase I/II trial of 53 patients with R/M HNSCC showed a RR of 30% and OS of 9.9 months,32 Vermorken et al completed the EXTREME trial, a randomized, phase III trial of 442 patients with R/M HNSCC comparing cisplatin + 5-FU + cetuximab treatment to cisplatin + 5-FU doublet therapy. This was the first trial to demonstrate significant improvement of both RR (36% vs. 20%, P < 0.001) and OS (10.1 months vs. 7.4 months, P = 0.04) with combination therapy over treatment with only cisplatin.3 These results led to new National Comprehensive Cancer Network (NCCN) treatment recommendations for R/M HNSCC.33
Even with these advances, the OS of patients with R/M HNSCC is still only 10.1 months. It is imperative that additional therapies be developed and treatment regimens be designed to improve the outcome for those with this aggressive malignancy. Because of the success found when adding a molecular targeted therapy, numerous novel agents of this nature have been created and are currently being tested in preclinical and clinical studies for efficacy in R/M HNSCC.
Current Trials of Molecular Targeted Therapies
Several pathways are currently under investigation for therapeutic targeting in R/M HNSCC, with the 3 mostly intensively studied being the EGFR pathway, the phosphoinositide-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway, and the vascular endothelial growth factor pathway (VEGF; Fig. 1). Table 2 summarizes the completed trials of agents targeting these pathways in HNSCC, while Table 3 summarizes trials that are currently in progress.
Figure 1.
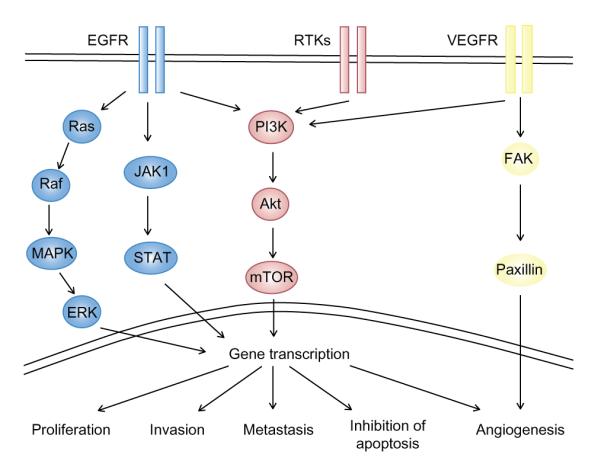
Targeting molecular pathways in conjunction with cisplatin therapy.
Notes: Molecular targeting therapies are currently being developed for the treatment of R/M HNSCC and combined with cisplatin in novel treatment regimens. Frequently targeted pathways include epidermal growth factor receptor (EGFR), phosphoiniositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR), and vascular endothelial growth factor (VEGF) pathways, which each have a variety of downstream effects on tumor proliferation, invasion, metastasis, inhibition of apoptosis, and angiogenesis.
Table 2.
Completed clinical trials of molecular targeted therapies in recurrent and/or metastatic head and neck squamous cell carcinoma.
| Pathway | combination agent |
phase | pts | Treatment groups | RR (%) |
OS (mo.) |
notes | Reference |
|---|---|---|---|---|---|---|---|---|
| EGFR | Panitumumab | III | R/M | CDDP + 5-FU + Panitumumab |
36* | 11.1 | 86 | |
| CDDP + 5-FU | 25.0 | 9.0 | ||||||
| Zalutumumab | III | cis-res | Zalutumumab | 6.0 | 6.7 | 35 | ||
| Supportive care ± MTX | – | 5.2 | ||||||
| Erlotinib | II | R/M | Erlotinib | 4.3 | 6.0 | 37 | ||
| II | R/M | CDDP + erlotinib | 21.0 | 7.9 | 38 | |||
| Gefitinib | II | R/M | Gefitinib | 10.6 | 8.1 | 40 | ||
| II | R/M | Gefitinib | 1.4 | 5.5 | 41 | |||
| II | R/M | Gefitinib | 8.0 | 4.3 | 42 | |||
| III | R/M | High dose gefitinib | 7.6 | 6.0 | 43 | |||
| Low dose gefitinib | 2.1 | 5.6 | ||||||
| MTX | 3.9 | 6.7 | ||||||
| II | LA | CDDP + cetuximab + gefitinib |
– | 66%† | 44 | |||
| PI3K | Everolimus | I | R/M | Everolimus | Safe | 50 | ||
| I/II | CDDP + cetuximab + everolimus |
Terminated | NCT01009346 | |||||
| Temsirolimus | II | CDDP + cetuximab + temsirolimus |
Terminated | NCT01015664 | ||||
| VEGF | Bevacizumab | I | LA | CDDP + IMRT + bevacizumab |
Safe | 87 | ||
| II | LA | CDDP + IMRT + bevacizumab |
– | 88%†† | 58 | |||
| Sorafenib | II | R/M | Sorafenib | 3.7 | 4.2 | 59 | ||
| II | R/M | Sorafenib | 2.0 | 9.0 | 60 |
Notes: Indicates statistically significant difference (P < 0.05)
66% OS at 4 years
88% OS at 2 years.
Abbreviations: RR, response rate; OS, overall survival; R/M, recurrent/metastatic; cis-res, cisplatin-resistant; LA, locally advanced; CDDP, cisplatin; 5-FU, 5-fluorouracil; MTX, methotrexate.
Table 3.
In progress clinical trials of cisplatin + molecular targeted therapies in recurrent and/or metastatic head and neck squamous cell carcinoma.
| pathway | combination agent | phase | pts | Treatment groups | Reference |
|---|---|---|---|---|---|
| EGFR | Panitumumab | II | R/M | CDDP + doc ± panitumumab | NCT00454779 |
| Erlotinib | II | R/M | Carbo + pac + cetuximab + erlotinib | NCT01316757 | |
| II | R/M | CDDP + doc + erlotinib | NCT00076310 | ||
| II | R/M | Platinum + doc + erlotinib | NCT01064479 | ||
| I | LA | CDDP + doc + erlotinib + bevacizumab | NCT00405405* | ||
| PI3K | Everolimus | I/II | R/M | Carbo + pac + everolimus | NCT01333085 |
| I/II | R/M | Carbo + cetuximab + everolimus | NCT01283334 | ||
| I | LA | CDDP + IMRT + everolimus | NCT00935961 | ||
| I | LA | CDDP + doclitaxel + everolimus | NCT00858663 | ||
| Temsirolimus | I/II | R/M | Carbo + paclitaxel + temsirolimus | NCT01016769 | |
| VEGF | Bevacizumab | III | R/M | CDDP + 5-FU or doc ± bevacizumab | NCT00588770 |
| I | LA | CDDP + doc + erlotinib + bevacizumab | NCT00405405* | ||
| Sorafenib | II | R/M | Carbo + paclitaxel + sorafenib | NCT00494182 | |
| Vandetanib | II | LA | CDDP + RT ± vandetanib | NCT00720083 | |
| I | LA | CDDP + RT + vandetanib | NCT00450138 |
Note: Indicates duplicate trial.
Abbreviations: R/M, recurrent/metastatic; LA, locally advanced; CDDP, cisplatin; 5-FU, 5-fluorouracil; carbo, carboplatin; doc, docetaxel; pac, paclitaxel; IMRT, idensity modulation radiation therapy.
Other EGFR inhibitors
HNSCC tumors generally express increased EGFR levels that correlate with a poor prognosis.34 2 therapies targeting EGFR that are currently in use are monoclonal antibodies directed at the N-terminal, extracellular ligand-binding domain of the receptor and small molecule tyrosine kinase inhibitors (TKIs) targeting the C-terminal, intracellular tyrosine kinase domain.
Monoclonal antibodies
As described above, cetuximab has been found to have significant impact on clinical outcomes when used in combination with cisplatin and 5-FU. Currently, it is the only routinely used targeted therapy in R/M HNSCC. However, studies examining other targeted therapies, and specifically EGFR monoclonal antibodies, are underway.
Panitumumab
Panitumumab is a fully human IgG2 EGFR-directed monoclonal antibody that is currently approved for use in colorectal cancer. The SPECTRUM (Study of Panitumumab Efficacy in Patient with Recurrent and/or Metastatic Head and Neck Cancer) trial was designed much like the EXTREME trial as a randomized, phase III trial comparing patients treated with platinum and 5-FU with or without panitumumab treatment. While statistically significant improvement of RR (36% vs. 25%, P = 0.007) and progression free survival (PFS) (5.8 months vs. 4.6 months, P = 0.004) were noted, OS was not significantly different (11.1 months vs. 9.0 months, P = 0.14; Table 2). There is a phase II trial underway investigating the efficacy of cisplatin + docetaxel + panitumumab in R/M HNSCC (NCT00454779) (Table 3). Currently, panitumumab is not approved for use in R/M HNSCC.
Zalutumumab
Zalutumumab is a human IgG1κ monoclonal antibody targeting EGFR that also induces Fc-mediated antibody-dependent cellular toxicity. A randomized, phase III trial of 286 patients with platinum resistant R/M HNSCC was completed comparing zalutumumab monotherapy vs. best supportive care in addition to the option of weekly methotrexate. 6 percent of patients responded to zalutumumab treatment, and there was a significant impact on PFS when compared to the supportive care ± methotrexate group (9.9 weeks vs. 8.4 weeks, P = 0.0012). Difference in OS was marginally not significant (6.7 months vs. 5.2 months, P = 0.0648;35 Table 2).
No studies have been completed or are underway to examine the effects of combination zalutumumab and cisplatin therapy. When comparing this phase III trial to cetuximab monotherapy for cisplatin-resistant R/M HNSCC, it seems unlikely zalutumumab will be a more clinically significant treatment option than the current cetuximab triplet therapy regimen.
Tyrosine kinase inhibitors
Erlotinib
Erlotinib is a TKI currently used in the treatment of non-small cell lung cancer (NSCLC) and pancreatic cancer. Preclinical in vivo studies show that erlotinib/cisplatin combination therapy has a greater effect on tumor growth inhibition that cisplatin alone.36 In clinical studies the efficacy of erlotinib as single agent therapy was examined in a multicenter, phase II trial. Although the RR was found to be only 4.3%, substantially lower than with traditional cytotoxic therapies, OS was found to be 6.0 months and comparable to other palliative chemotherapeutic approaches37 (Table 2).
Based on these results, a phase I/II trial was undertaken in 51 patients with R/M HNSCC to analyze the efficacy of cisplatin + erlotinib doublet therapy. With a RR of 21% and median OS of 7.9 months, the results from this study were similar to cisplatin + cetuximab treatment. Thus, while cisplatin + erlotinib was found to be a safe and effective treatment, it is hypothesized that a phase III trial of cisplatin + erlotinib would show a similar lack of significant improvement of clinical outcomes when compared to treatment with cisplatin alone38 (Table 2). Currently, several phase II trials are in progress examining R/M HNSCC response to carboplatin + paclitaxel + cetuximab + erlotinib (NCT01316757), cisplatin + docetaxel + erlotinib (NCT00076310), and platinum + docetaxel + erlotinib (NCT01064479) combination therapies. There is also a phase I trial underway to determine the safety of cisplatin + docetaxel + erlotinib + bevacizumab (a VEGF monoclonal antibody) in patients with locally advanced HNSCC (NCT00405405; Table 3).
Gefitinib
Gefitinib is another TKI that has been studied in numerous HNSCC models and trials. In 2003, preclinical studies found gefitinib to have a synergistic effect with cisplatin + 5-FU treatment on inhibition of cellular proliferation, measured via MTT assay.39 Since then, several phase II trials have been completed with RRs ranging 1.4%–10.6% and OS 4.3–8.1 months using different doses and treatment regimens.40–42 While some of these results were similar to previous trials of EGFR inhibitors (RR = 10.6%, OS = 8.1 months),40 most were less promising (Table 2).
Stewart et al compared the efficacy of multiple doses of gefitinib monotherapy to methotrexate treatment in a randomized, phase III trial of 486 patients with R/M HNSCC. In the low dose gefitinib, high dose gefitinib, and methotrexate arms, RR was 2.7%, 7.6%, and 3.9%, respectively, and OS was 5.6 months, 6.0 months, and 6.7 months, respectively. No statistically significant difference was found between any of the 3 groups43 (Table 2).
Additionally, a phase II trial of 60 patients with locally advanced HNSCC receiving cisplatin + 5-FU + gefitinib treatment was completed and compared to a historical cohort of patients receiving only cisplatin + 5-FU. This found no significant increase in survival (69% at 4 years vs. 66% at 4 years, P = 0.84), but triple therapy was found to be difficult to complete and significantly more toxic.44
Altogether, these studies do not favor the development of a R/M HNSCC platinum treatment regimen that includes gefitinib. In addition, gefitinib is not FDA-approved for use in US cancer patients, so it is unlikely to be developed in combination with cisplatin.
Targeting the PI3K/Akt/mTOR pathway
Activation of the PI3K/Akt/mTOR pathway has been demonstrated in HNSCC.45 This pathway is activated by upstream stimulation of EGFR, in addition to EGFR-independent mechanisms. There is some evidence for combination therapy of a PI3K pathway inhibitor with cisplatin, first discovered preclinically in NSCLC. An in vitro study of NSCLC demonstrated that everolimus, an mTOR inhibitor, enhanced effects of cisplatin by 10-fold on A549 cell viability (P < 0.05) as well as potentiated cisplatin’s effect on apoptosis.46 Because of this success, similar studies were carried out in HNSCC preclinical trials. These studies found that when a HNSCC cell line (UM-SCC-23) was induced to become cisplatin-resistant, this line exhibited constitutively activated Akt signaling and became sensitized to cisplatin therapy when treated with temsirolimus, another mTOR inhibitor.47 These results are encouraging for the development of trials examining the efficacy of cisplatin and concomitant PI3K pathway inhibition.
Everolimus
Everolimus is an mTOR inhibitor and a rapalogue of sirolimus (rapamycin). Cisplatin + everolimus combination therapy has demonstrated significantly enhanced efficacy over cisplatin monotherapy in several in vitro and in vivo studies in multiple types of cancer including NSCLC (see above).46 This is evident also in ovarian cancer, in which a xenograft mice model showed decreased tumor burden when mice where treated with cisplatin + everolimus vs. everolimus alone vs. cisplatin alone (92%, 83%, 68%, respectively; P < 0.05 compared to either monotherapy).48
Because of these encouraging results in a multitude of cancers, it was hypothesized that cisplatin + everolimus doublet therapy may be of benefit in R/M HNSCC. Preclinical studies demonstrated that an oral squamous cell carcinoma (OSCC) xenograft model corroborated the results found in ovarian in vivo studies with significant enhancement of cisplatin-directed growth inhibition when adjunctively treated with everolimus.49
These preclinical findings led to a phase I trial studying the safety and efficacy of cisplatin + everolimus doublet therapy. 30 patients with R/M disease from a variety of tumor types were included: 9 of these patients had R/M HNSCC. Cisplatin + everolimus was found to be a safe therapeutic approach with 2 out of 9 patients with R/M HNSCC demonstrating response to therapy.50 2 phase I/II trials are in progress to evaluate the utility of carboplatin + paclitaxel + everolimus (NCT01333085) and carboplatin + cetuximab + everolimus (NCT01283334) combination treatments. Additionally, 2 phase I trials are underway studying cisplatin and everolimus as part of a triple therapeutic regimen in combination with intensity modulated radiation therapy (IMRT) (NCT00858663) or docetaxel (NCT00935961) (Table 3). A phase I/II trial examining cisplatin + everolimus + cetuximab was terminated for toxicity (NCT01009346; Table 2).
Temsirolimus
Another mTOR inhibitor that is currently being studied in R/M HNSCC is temsirolimus, only differing from everolimus in pharmacokinetic and pharmacodynamics profiles. It is currently being used for treatment of renal cell carcinoma. Similar to everolimus, it has shown synergistic effects on cisplatin action in a multitude of cancer types, both in vitro (small cell lung cancer [SCLC])51 and in vivo xenograft models (melanoma).52 A phase II trial studying treatment with cisplatin or carboplatin + etoposide or irinotecan (topoisomerase 1 inhibitors) + temsirolimus in 87 patients with extensive stage SCLC did not show significant prolongation of PFS or OS as compared to results from previous literature.53
As described above, temsirolimus exhibited enhancement of cisplatin effects on HNSCC cell growth inhibition in vitro47 as well as in xenograft models.54 While no clinical studies have been completed of temsirolimus in HNSCC at this point, currently, a phase I/II trial studying the safety and efficacy of carboplatin + paclitaxel ± temsirolimus is recruiting participants (NCT01016769; Table 3). A phase II trial of cisplatin + cetuximab ± temsirolimus was recently terminated (NCT01015664; Table 2).
Inhibition of VEGF and angiogenesis
Blood vessel formation is an essential step in the transformation of tumors to invasive, metastatic malignancies. In 1971, it was first proposed that angiogenesis inhibition could be an effective therapeutic approach to cancer.55 VEGF is a signaling protein that stimulates angiogenesis. Specifically in HNSCC, a meta-analysis of 12 studies found that VEGF overexpressing patients had a 1.88-fold higher risk of death in 2 years than their non-overexpressing counterparts (P < 0.001),56 making VEGF-targeted therapies a logical addition to the treatment of R/M HNSCC. Similar to inhibition of EGFR, VEGF can be inhibited via either monoclonal antibodies directly targeting VEGF or TKIs with selectivity for the VEGF receptor.
Bevacizumab
Bevacizumab is a humanized monoclonal antibody that targets VEGF and was originally designed for the treatment of colorectal cancer. Since, it also been approved for the treatment of NSCLC, renal cell carcinoma, HER2 negative breast cancer, and glioblastoma.
An in vivo study using an SCC-tumor bearing C3H mouse allograft model measured the antiproliferative effects of cisplatin, cetuximab, and bevacizumab either alone or in various combinations. While bevacizumab monotherapy did not significantly abrogate tumor growth, cisplatin + bevacizumab did in comparison to control (untreated) mice and treatment with each monotherapy as well as improved OS (P < 0.05). Surprisingly, this doublet therapy was significantly more effective than the triple therapy (P < 0.05). Similarly, treatment with bevacizumab + cisplatin resulted in increased levels of apoptosis when compared to control and triple therapy arms (31.6% ± 12%, 1.2% ± 0.6%, 9.7% ± 2.8%),57 further indicating that bevacizumab may have additional therapeutic advantage when combined with cisplatin, but that the addition of cetuximab may not be beneficial.
Several phase I and II trials have reported encouraging results with bevacizumab. A phase I trial to determine the safety and feasibility of bevacizumab + cisplatin + IMRT triple therapy treated 10 patients with locally advanced HNSCC with bevacizumab monotherapy for 3 weeks, followed by bevacizumab + cisplatin + IMRT. Inhibition of tumor growth was significant both during bevacizumab monotherapy and at mid-course (P < 0.05;86 Table 2).
Fury et al performed a phase II trial of 42 patients with locally advanced HNSCC without distant metastasis. These patients were treated with cisplatin + bevacizumab + IMRT. The two year PFS rate was 75.9% and OS rate was 88.0%58 (Table 2). This study is difficult to compare to past studies due to the changing demographic of the HNSCC population—93% of patients in this study had OSCC, and the sample contained many non-smoking, human papillomavirus (HPV) positive tumors. These tumors have a much better prognosis than their historic tobacco- and alcohol-related counterparts, and make a comparison of the studies misleading.
For this review, a drawback of these studies is the inclusion of only patients with locally advanced HNSCC free of metastasis. This makes results possibly inapplicable to the R/M population. Furthermore, these studies included IMRT, which is not of clinical use in the majority of patients with R/M HNSCC. A phase III trial of cisplatin + docetaxel or 5-FU ± bevacizumab in patients with R/M HNSCC is currently underway, which will hopefully better elucidate the effects of this combination therapy in the R/M population (NCT00588770). Additionally, a phase I trial is in progress to determine the safety of cisplatin + docetaxel + erlotinib + bevacizumab combination treatment (NCT00405405) (Table 3).
Tyrosine kinase inhibitors
Several TKIs of the VEGF receptor have been developed and are approved for use in an array of malignancies, such as sorafenib in renal cell and hepatocellular carcinoma and vandetanib in medullary thyroid cancer. Numerous preclinical and clinical studies have been and are currently evaluating the utility of these agents in R/M HNSCC.
Sorafenib
Two phase II trials examining the efficacy of sorafenib monotherapy in 2759 and 4160 patients with R/M HNSCC have been completed. Both exhibited a poor RR (3.7% and 2%) but a modest effect on PFS (1.8 months and 4 months) and OS (4.2 months and 9 months). The difference between these 2 trials may be due to the difference in patient population, as Williamson et al excluded patients treated with chemotherapy in the past 6 months or that had a PS of 2 or higher. The results from this trial are encouraging as they are comparable to cytotoxic monotherapy. The low RR is less worrisome as sorafenib monotherapy has exhibited similarly low RR but significant improvement in PFS or OS in other kinds of malignancies.60
Currently, a phase II trial of carboplatin + paclitaxel + sorafenib is underway to examine both the efficacy and safety of this combination treatment (NCT00494182).
Vandetanib
Vandetanib has been examined in a preclinical study looking at its effects on combination therapy with cisplatin and radiation therapy (RT). These effects were studied both in vitro, using OSC-19 and HN5 cell lines, and in vivo with an orthotopic nude mouse model. Triple therapy, when compared to cisplatin + RT, resulted in significantly decreased tumor volume (P = 0.0451) and increased in vivo survival (42.5 days vs. 34.0 days, P = 0.014). In addition, a decreased percentage of mice were found to have lymph node metastases (15.3% and 53.8%), but this did not meet statistical significance.61 2 clinical trials examining cisplatin + radiation therapy + vandetanib are underway (NCT00720083, NCT00450138; Table 3). Further clinical trials in R/M HNSCC of cisplatin + vandetanib combination therapy may be of benefit.
Future Directions
While many targeted therapies are currently being studied with cisplatin treatment for R/M HNSCC, these chemotherapeutic agents do not specifically attack the known mechanisms of cisplatin resistance, such as increased DNA repair or independent inhibition of apoptosis (Fig. 2). Galluzzi et al have reviewed mechanisms of cisplatin resistance, classifying them into 4 categories: pre-target resistance (targeting steps before cisplatin binds DNA), on-target resistance (directly related to cisplatin binding DNA), post-target resistance (cisplatin-directed lethality after cisplatin binds DNA), and off-target resistance (via not obviously related molecular pathways).88 Targeting such pathways represents a rational approach to overcome cisplatin resistance and improve treatment responses. 2 of these pathways, the nucleotide excision repair pathway (NER) and survivin inhibition of apoptosis, are further explored in this section as evidence suggests targeting these pathways may be effective.
Figure 2.
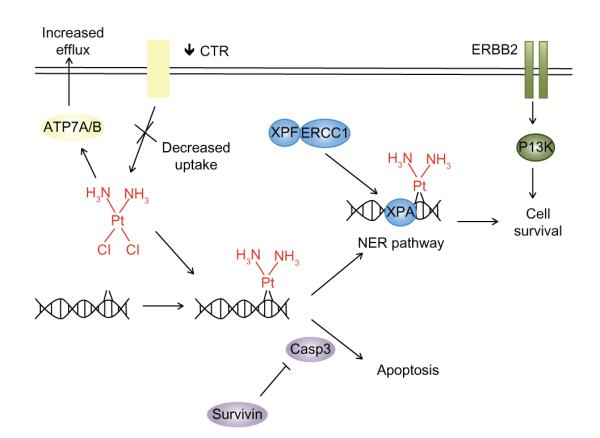
Mechanisms of cisplatin resistance.
Notes: Cisplatin creates DNA adducts and activates the apoptotic pathway, thus killing rapidly dividing cells. Resistance to cisplatin can be classified into 4 categories. Examples of each of these are depicted: pre-target resistance before cisplatin interacts with DNA (yellow), on-target resistance directly related to the cisplatin-DNA interaction (blue), post-target resistance after cisplatin binds to DNA (purple), and off-target resistance using remote molecular pathways (green).
Targeting components of DNA repair
Sensitivity to cisplatin can be a precarious balance. DNA repair pathways must be sufficiently activated to induce apoptosis of the malignant cells. On the other hand, overstimulation of these pathways may simply result in repair of cisplatin-DNA adducts, causing cisplatin resistance (Fig. 2). The DNA repair mechanism most frequently implicated in repair of DNA adducts is the NER pathway.62
Briefly, the NER pathway first recognizes that DNA damage is present, and then uses a complex of proteins to remove a short single-stranded section of DNA that includes that damage. DNA polymerase then fills in this section of DNA and seals it with ligase. Specific DNA-binding protein complexes are made throughout this process such as the ERCC1-XPF (excision repair cross-complementing rodent repair deficiency, complementation group 1-xeroderma pigmentosum, complementation group F) heterodimer binding to XPA (xeroderma pigmentosum, complementation group A), an identifier of DNA damage. This is crucial for the initial cut that induces removal of the DNA damage-including section.63,64
Several researchers have described increased NER as a mechanism of cisplatin resistance.62,65 Evidence for this has been found in various types of cancer. Testicular cancer is a particularly cisplatin-sensitive malignancy with 80% of advanced metastatic testis tumors being cured with cisplatin-based combination therapy.66 This is significant in light of the dismal cure rates found for most metastatic malignancies. The physiology behind this response to cisplatin has been associated with intrinsically low levels of NER proteins in the majority of testis tumors. Specifically, when compared to prostate, bladder, breast, lung, cervical, and ovarian cell lines, testicular cancer cell lines have been determined to express significantly lower levels of ERCC1, XPA, and XPF proteins (P = 0.004, P = 0.001, P = 0.001, respectively).67 Furthermore, in ovarian and gastric cancer, increased ERCC1 expression has been correlated with impaired response to platinum-based chemotherapy,68,69 and in ovarian cancer cell lines, enhanced response to cisplatin treatment is induced when the NER pathway is impaired using siRNA.70
Similar findings have been reported in HNSCC. A study of tumor samples from 103 patients with stage IV HNSCC found that when polymorphisms in DNA repair enzymes are present, such as ERCC1, a significant survival advantage is conferred (decreased probability of dying by a factor of 2 for each polymorphism, P < 0.001). Additionally, these patients also have an increased likelihood of achieving a complete response to cisplatin therapy (2.94x, P = 0.041).71
Furthermore, analysis of XPF expression in tumors from 80 patients with newly diagnosed HNSCC demonstrated that high XPF expression was associated with decreased PFS (HR = 1.83, P = 0.05). While response to cisplatin treatment was not exclusively analyzed in this study, 88% of these patients did receive platinum based chemotherapy.72 Altogether, these results indicate that overexpression of DNA repair enzymes may induce DNA repair via the NER pathway and lead to cisplatin resistance with poorer clinical prognosis in R/M HNSCC.
It is therefore logical that decreased levels of NER pathway components such as ERCC1 would enhance response to cisplatin and improve outcomes. One study in HNSCC has explored this. Sorafenib, an aforementioned inhibitor of VEGF, had antiproliferative effects on HNSCC cell lines both in vitro and in vivo. When used with cisplatin and RT, synergistic effects on growth inhibition were observed in the triple therapy arm (70%, 81%, and 75% for UM-SCC-74A, CAL27, and HDMEC cell lines, respectively) in comparison to cisplatin and RT alone (1%, 43%, and 56% for UM-SCC-74A, CAL27, and HDMEC cell lines, respectively). Sorafenib also had significant effects on cell motility, invasion, and colony formation.73
While sorafenib is traditionally considered a VEGF inhibitor, in this study HNSCC cell lines treated with sorafenib demonstrated decreased expression of ERCC1 via Western blotting.73 Sorafenib’s antiproliferative effects and synergy with cisplatin may be due to decreased ERCC1 levels and DNA repair, indicating this molecule and/or pathway as an attractive therapeutic target for R/M HNSCC. One group is currently screening for and characterizing inhibitors of the ERCC1/XPA complex, demonstrating that one of these inhibitors confers enhanced ultraviolet radiation sensitivity to colon cancer cells.74 NER pathway inhibitors could be a novel and promising class of therapeutic agents for cisplatin combination therapy in R/M HNSCC.
Survivin
Survivin is a member of the inhibition of apoptosis (IAP) family, facilitating this inhibition via physical binding to caspases (Fig. 2).75 It also indicated in regulation of cell division and mitosis.76 Survivin levels have been assessed in HNSCC tumors using immunohistochemical staining. One study analyzed 37 laryngeal squamous cell carcinoma tumors measuring survivin expression as mean percentage of cells with positive survivin stain. This study found that high levels of survivin expression were correlated with significantly increased likelihood of lymph node metastasis at the time of presentation (28.3% lymph node positive vs. 18.8% with no lymph node metastases, P = 0.017). These lymph node metastases expressed notably higher levels of survivin compared to their primary counterparts (65.5% vs. 28.3%, P = 0.002).77
Survivin expression was significantly increased in tumors that recurred vs. tumors with no recurrence after surgical resection with a mean follow-up time of 39.3 months (28.0% vs. 19.1%, P = 0.039), indicating a prognostic value of survivin expression.77 While no causal relationship can be concluded, the fact that tumors with decreased expression of survivin experience less metastasis and recurrence leads to the hypothesis that inhibition of survivin may lead to better clinical outcomes. Additionally, the level of survivin is minimal in terminally differentiated tissues, but is found to be increased in most human cancers (including HNSCC),78 especially in cisplatin-resistant cell lines and tumors.79,80 Altogether, this evidence indicates survivin as an appealing target for chemotherapeutic agents as well as combination therapy with cisplatin.
High throughput screening identified YM155 as a small molecule inhibitor of survivin and was confirmed to suppress survivin expression and induce apoptosis in hormone refractory prostate cancer cell lines. Furthermore, YM155 completely inhibited tumor growth in orthotopic xenograft mice models,81 propelling it to clinical studies. A phase I trial has proven safety of treatment with YM155 treatment82 while a multicenter phase II trial found modest monotherapy activity in 37 patients with NSCLC (RR = 5.4%, PFS = 1.7 months, OS = 6.6 months).83
Additional in vivo studies using tumor xenografts demonstrated that when YM155 therapy was added to cisplatin treatment, there was a delay in repair of DNA breaks, apoptosis and activation of caspases was increased synergistically, and growth inhibition of NSCLC xenograft tumors was greater than when treated with cisplatin monotherapy (P < 0.0001).84 These preclinical findings of cisplatin-synergy in conjunction with clinical data demonstrating YM155 as a safe and active chemotherapeutic agent warrant clinical trials of cisplatin + YM155 combination therapy.
These promising results have translated to preclinical HNSCC trials. HNSCC cell lines treated with YM155 demonstrated decreased survivin levels and enhanced antiproliferative and apoptotic effects of cisplatin (P < 0.05) in vitro and in SCID xenograft models.85 This indicates that clinical trials combining cisplatin and YM155 treatment could have significant impact on future therapy for R/M HNSCC. While currently there are no HNSCC-exclusive clinical trials to determine YM155 safety and efficacy, a phase I trial is underway to determine the safety of YM155 treatment in patients with recurrent and/or advanced solid tumors, which would include R/M HNSCC (NCT01100931).
Conclusion
Despite extensive clinical research over the past 40 years, R/M HNSCC still has a poor prognosis. Since the discovery of cisplatin in 1977, the only significant improvement in treatment has been introduction of cisplatin + 5-FU + cetuximab combination therapy. Much of the active research for improvement of R/M HNSCC treatment relies on combination of traditional cytotoxic approaches with novel molecular targeted therapies. Currently, EGFR, PI3K/Akt/mTOR, and VEGF directed agents are being studied, with several clinical trials underway. Intriguing findings have emerged that identify novel therapeutic targets such as the NER pathway and survivin as possible and promising future directions for the management of R/M HNSCC.
Acknowledgments
Funding Author(s) disclose no funding sources.
Footnotes
Author Contributions Conceived and designed the review: KPP. Analyzed the data: KPP. Wrote the first draft of the manuscript: KPP. Made critical revisions and approved final version: JRG. All authors reviewed and approved of the final manuscript.
Competing Interests Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemo-therapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 4.Jennette KW, Lippard SJ, Vassiliades GA, Bauer WR. Metallointercalation reagents. 2-hydroxyethanethiolato(2,2′,2′-terpyridine)-platinum(II) mono-cation binds strongly to DNA by intercalation. Proc Natl Acad Sci U S A. 1974;71(10):3839–43. doi: 10.1073/pnas.71.10.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wittes RE, Cvitkovic E, Shah J, Gerold FP, Strong EW. CIS-Dichlorodiammineplatinum(II) in the treatment of epidermoid carcinoma of the head and neck. Cancer Treat Rep. 1977;61(3):359–66. [PubMed] [Google Scholar]
- 6.Morton RP, Rugman F, Dorman EB, et al. Cisplatinum and bleomycin for advanced or recurrent squamous cell carcinoma of the head and neck: a randomised factorial phase III controlled trial. Cancer Chemother Pharmacol. 1985;15(3):283–9. doi: 10.1007/BF00263902. [DOI] [PubMed] [Google Scholar]
- 7.Amer MH, Al-Sarraf M, Vaitkevicius VK. Factors that affect response to chemotherapy and survival of patients with advanced head and neck cancer. Cancer. 1979;43(6):2202–6. doi: 10.1002/1097-0142(197906)43:6<2202::aid-cncr2820430607>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Kish J, Drelichman A, Jacobs J, et al. Clinical trial of cisplatin and 5-FU infusion as initial treatment for advanced squamous cell carcinoma of the head and neck. Cancer Treat Rep. 1982;66(3):471–4. [PubMed] [Google Scholar]
- 9.Decker DA, Drelichman A, Jacobs J, et al. Adjuvant chemotherapy with cis-diamminodichloroplatinum II and 120-hour infusion 5-fluorouracil in Stage III and IV squamous cell carcinoma of the head and neck. Cancer. 1983;51(8):1353–5. doi: 10.1002/1097-0142(19830415)51:8<1353::aid-cncr2820510805>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Kish JA, Weaver A, Jacobs J, Cummings G, Al-Sarraf M. Cisplatin and 5-fluorouracil infusion in patients with recurrent and disseminated epidermoid cancer of the head and neck. Cancer. 1984;53(9):1819–24. doi: 10.1002/1097-0142(19840501)53:9<1819::aid-cncr2820530903>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Dasmahapatra KS, Citrin P, Hill GJ, Yee R, Mohit-Tabatabai MA, Rush BF., Jr A prospective evaluation of 5-fluorouracil plus cisplatin in advanced squamous-cell cancer of the head and neck. J Clin Oncol. 1985;3(11):1486–9. doi: 10.1200/JCO.1985.3.11.1486. [DOI] [PubMed] [Google Scholar]
- 12.Rowland KM, Jr, Taylor SGt, Spiers AS, et al. Cisplatin and 5-FU infusion chemotherapy in advanced, recurrent cancer of the head and neck: an Eastern Cooperative Oncology Group Pilot Study. Cancer Treat Rep. 1986;70(4):461–4. [PubMed] [Google Scholar]
- 13.Jacobs C, Lyman G, Velez-Garcia E, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992;10(2):257–63. doi: 10.1200/JCO.1992.10.2.257. [DOI] [PubMed] [Google Scholar]
- 14.Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol. 1992;10(8):1245–51. doi: 10.1200/JCO.1992.10.8.1245. [DOI] [PubMed] [Google Scholar]
- 15.Clavel M, Vermorken JB, Cognetti F, et al. Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol. 1994;5(6):521–6. doi: 10.1093/oxfordjournals.annonc.a058906. [DOI] [PubMed] [Google Scholar]
- 16.Rozencweig M, Dodion P, Bruntsch U, et al. Combination chemotherapy with cisplatin, methotrexate, bleomycin, and vincristine (CABO) in advanced squamous cell carcinoma of the head and neck. Cancer. 1984;54(8):1499–503. doi: 10.1002/1097-0142(19841015)54:8<1499::aid-cncr2820540804>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Clavel M, Cognetti F, Dodion P, et al. Combination chemotherapy with methotrexate, bleomycin, and vincristine with or without cisplatin in advanced squamous cell carcinoma of the head and neck. Cancer. 1987;60(6):1173–7. doi: 10.1002/1097-0142(19870915)60:6<1173::aid-cncr2820600603>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Forastiere AA, Shank D, Neuberg D, Taylor SG, 4th, DeConti RC, Adams G. Final report of a phase II evaluation of paclitaxel in patients with advanced squamous cell carcinoma of the head and neck: an Eastern Cooperative Oncology Group trial (PA390) Cancer. 1998;82(11):2270–4. [PubMed] [Google Scholar]
- 19.Dreyfuss AI, Clark JR, Norris CM, et al. Docetaxel: an active drug for squamous cell carcinoma of the head and neck. J Clin Oncol. 1996;14(5):1672–8. doi: 10.1200/JCO.1996.14.5.1672. [DOI] [PubMed] [Google Scholar]
- 20.Forastiere AA, Leong T, Rowinsky E, et al. Phase III comparison of high-dose paclitaxel + cisplatin + granulocyte colony-stimulating factor versus low-dose paclitaxel + cisplatin in advanced head and neck cancer: Eastern Cooperative Oncology Group Study E1393. J Clin Oncol. 2001;19(4):1088–95. doi: 10.1200/JCO.2001.19.4.1088. [DOI] [PubMed] [Google Scholar]
- 21.Adamo V, Ferraro G, Pergolizzi S, et al. Paclitaxel and cisplatin in patients with recurrent and metastatic head and neck squamous cell carcinoma. Oral Oncol. 2004;40(5):525–31. doi: 10.1016/j.oraloncology.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Basaran M, Bavbek SE, Gullu I, et al. A phase II study of paclitaxel and cisplatin combination chemotherapy in recurrent or metastatic head and neck cancer. J Chemother. 2002;14(2):207–13. doi: 10.1179/joc.2002.14.2.207. [DOI] [PubMed] [Google Scholar]
- 23.Gibson MK, Li Y, Murphy B, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23(15):3562–7. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 24.Taylor EC, Kuhnt D, Shih C, et al. A dideazatetrahydrofolate analogue lacking a chiral center at C-6, N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid, is an inhibitor of thymidylate synthase. J Med Chem. 1992;35(23):4450–4. doi: 10.1021/jm00101a023. [DOI] [PubMed] [Google Scholar]
- 25.Pivot X, Raymond E, Laguerre B, et al. Pemetrexed disodium in recurrent locally advanced or metastatic squamous cell carcinoma of the head and neck. Br J Cancer. 2001;85(5):649–55. doi: 10.1054/bjoc.2001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 27.Urba S, van Herpen CM, Sahoo TP, et al. Pemetrexed in combination with cisplatin versus cisplatin monotherapy in patients with recurrent or metastatic head and neck cancer: final results of a randomized, double-blind, placebo-controlled, phase 3 study. Cancer. 2012;118(19):4694–705. doi: 10.1002/cncr.27449. [DOI] [PubMed] [Google Scholar]
- 28.Baselga J, Trigo JM, Bourhis J, et al. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23(24):5568–77. doi: 10.1200/JCO.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 29.Herbst RS, Arquette M, Shin DM, et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23(24):5578–87. doi: 10.1200/JCO.2005.07.120. [DOI] [PubMed] [Google Scholar]
- 30.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 31.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23(34):8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 32.Bourhis J, Rivera F, Mesia R, et al. Phase I/II study of cetuximab in combination with cisplatin or carboplatin and fluorouracil in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24(18):2866–72. doi: 10.1200/JCO.2005.04.3547. [DOI] [PubMed] [Google Scholar]
- 33.Fury MG, Pfister DG. Current recommendations for systemic therapy of recurrent and/or metastatic head and neck squamous cell cancer. JNCCN. 2011;9(6):681–9. doi: 10.6004/jnccn.2011.0056. [DOI] [PubMed] [Google Scholar]
- 34.Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90(11):824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 35.Machiels JP, Subramanian S, Ruzsa A, et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol. 2011;12(4):333–43. doi: 10.1016/S1470-2045(11)70034-1. [DOI] [PubMed] [Google Scholar]
- 36.Pollack VA, Savage DM, Baker DA, et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and anti-tumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291(2):739–48. [PubMed] [Google Scholar]
- 37.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22(1):77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 38.Siu LL, Soulieres D, Chen EX, et al. Phase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a Princess Margaret Hospital phase II consortium and National Cancer Institute of Canada Clinical Trials Group Study. J Clin Oncol. 2007;25(16):2178–83. doi: 10.1200/JCO.2006.07.6547. [DOI] [PubMed] [Google Scholar]
- 39.Magne N, Fischel JL, Tiffon C, et al. Molecular mechanisms underlying the interaction between ZD1839 (‘Iressa’) and cisplatin/5-fluorouracil. Br J Cancer. 2003;89(3):585–92. doi: 10.1038/sj.bjc.6601131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen EE, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21(10):1980–7. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 41.Cohen EE, Kane MA, List MA, et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11(23):8418–24. doi: 10.1158/1078-0432.CCR-05-1247. [DOI] [PubMed] [Google Scholar]
- 42.Kirby AM, A’Hern RP, D’Ambrosio C, et al. Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or metastatic head and neck cancer. Br J Cancer. 2006;94(5):631–6. doi: 10.1038/sj.bjc.6602999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected] J Clin Oncol. 2009;27(11):1864–71. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez CP, Adelstein DJ, Rybicki LA, et al. Single-arm phase II study of multiagent concurrent chemoradiotherapy and gefitinib in locoregionally advanced squamous cell carcinoma of the head and neck. Head Neck. 2012;34(11):1517–23. doi: 10.1002/hed.21971. [DOI] [PubMed] [Google Scholar]
- 45.Molinolo AA, Hewitt SM, Amornphimoltham P, et al. Dissecting the Akt/mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13(17):4964–73. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 46.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120(6):747–59. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 47.Aoki K, Ogawa T, Ito Y, Nakashima S. Cisplatin activates survival signals in UM-SCC-23 squamous cell carcinoma and these signal pathways are amplified in cisplatin-resistant squamous cell carcinoma. Oncol Rep. 2004;11(2):375–9. [PubMed] [Google Scholar]
- 48.Mabuchi S, Altomare DA, Cheung M, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13(14):4261–70. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 49.Hirashima K, Baba Y, Watanabe M, et al. Aberrant activation of the mTOR pathway and anti-tumour effect of everolimus on oesophageal squamous cell carcinoma. Br J Cancer. 2012;106(5):876–82. doi: 10.1038/bjc.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fury MG, Sherman E, Haque S, et al. A phase I study of daily everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;69(3):591–8. doi: 10.1007/s00280-011-1734-5. [DOI] [PubMed] [Google Scholar]
- 51.Wu C, Wangpaichitr M, Feun L, et al. Overcoming cisplatin resistance by mTOR inhibitor in lung cancer. Molecular Cancer. 2005;4(1):25. doi: 10.1186/1476-4598-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thallinger C, Poeppl W, Pratscher B, et al. CCI-779 plus cisplatin is highly effective against human melanoma in a SCID mouse xenotranplantation model. Pharmacology. 2007;79(4):207–13. doi: 10.1159/000101008. [DOI] [PubMed] [Google Scholar]
- 53.Pandya KJ, Dahlberg S, Hidalgo M, et al. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the Eastern Cooperative Oncology Group (E1500) J Thorac Oncol. 2007;2(11):1036–41. doi: 10.1097/JTO.0b013e318155a439. [DOI] [PubMed] [Google Scholar]
- 54.Ekshyyan O, Rong Y, Rong X, et al. Comparison of radiosensitizing effects of the mammalian target of rapamycin inhibitor CCI-779 to cisplatin in experimental models of head and neck squamous cell carcinoma. Mol Cancer Ther. 2009;8(8):2255–65. doi: 10.1158/1535-7163.MCT-08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 56.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11(4):1434–40. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Dong L, Bi Q, et al. Investigation of the efficacy of a bevacizumab-cetuximab-cisplatin regimen in treating head and neck squamous cell carcinoma in mice. Targeted Oncol. 2010;5(4):237–43. doi: 10.1007/s11523-010-0164-3. [DOI] [PubMed] [Google Scholar]
- 58.Fury MG, Lee NY, Sherman E, et al. A phase 2 study of bevacizumab with cisplatin plus intensity-modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer. 2012;118(20):5008–14. doi: 10.1002/cncr.27498. [DOI] [PubMed] [Google Scholar]
- 59.Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25(24):3766–73. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 60.Williamson SK, Moon J, Huang CH, et al. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group Study S0420. J Clin Oncol. 2010;28(20):3330–5. doi: 10.1200/JCO.2009.25.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sano D, Matsumoto F, Valdecanas DR, et al. Vandetanib restores head and neck squamous cell carcinoma cells’ sensitivity to cisplatin and radiation in vivo and in vitro. Clin Cancer Res. 2011;17(7):1815–27. doi: 10.1158/1078-0432.CCR-10-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reed E. Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev. 1998;24(5):331–44. doi: 10.1016/s0305-7372(98)90056-1. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad A, Robinson AR, Duensing A, et al. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol Cell Biol. 2008;28(16):5082–92. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park CH, Sancar A. Formation of a ternary complex by human XPA, ERCC1, and ERCC4(XPF) excision repair proteins. Proc Natl Acad Sci U S A. 1994;91(11):5017–21. doi: 10.1073/pnas.91.11.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14(5):1291–5. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 66.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337(4):242–53. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- 67.Welsh C, Day R, McGurk C, Masters JR, Wood RD, Koberle B. Reduced levels of XPA, ERCC1 and XPF DNA repair proteins in testis tumor cell lines. Int J Cancer. 2004;110(3):352–61. doi: 10.1002/ijc.20134. [DOI] [PubMed] [Google Scholar]
- 68.Dabholkar M, Bostick-Bruton F, Weber C, Bohr VA, Egwuagu C, Reed E. ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J Natl Cancer Inst. 1992;84(19):1512–7. doi: 10.1093/jnci/84.19.1512. [DOI] [PubMed] [Google Scholar]
- 69.Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16(1):309–16. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- 70.Selvakumaran M, Pisarcik DA, Bao R, Yeung AT, Hamilton TC. Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res. 2003;63(6):1311–6. [PubMed] [Google Scholar]
- 71.Quintela-Fandino M, Hitt R, Medina PP, et al. DNA-repair gene polymorphisms predict favorable clinical outcome among patients with advanced squamous cell carcinoma of the head and neck treated with cisplatin-based induction chemotherapy. J Clin Oncol. 2006;24(26):4333–9. doi: 10.1200/JCO.2006.05.8768. [DOI] [PubMed] [Google Scholar]
- 72.Vaezi A, Wang X, Buch S, et al. XPF expression correlates with clinical outcome in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2011;17(16):5513–22. doi: 10.1158/1078-0432.CCR-11-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yadav A, Kumar B, Teknos TN, Kumar P. Sorafenib enhances the antitumor effects of chemoradiation treatment by downregulating ERCC-1 and XRCC-1 DNA repair proteins. Mol Cancer Ther. 2011;10(7):1241–51. doi: 10.1158/1535-7163.MCT-11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barakat KH, Jordheim LP, Perez-Pineiro R, Wishart D, Dumontet C, Tuszynski JA. Virtual Screening and Biological Evaluation of Inhibitors Targeting the XPA-ERCC1 Interaction. PloS One. 2012;7(12):e51329. doi: 10.1371/journal.pone.0051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58(23):5315–20. [PubMed] [Google Scholar]
- 76.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22(53):8581–9. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 77.Marioni G, Bertolin A, Giacomelli L, et al. Expression of the apoptosis inhibitor protein Survivin in primary laryngeal carcinoma and cervical lymph node metastasis. Anticancer Res. 2006;26(5B):3813–7. [PubMed] [Google Scholar]
- 78.Sharma H, Sen S, Mathur M, Bahadur S, Singh N. Combined evaluation of expression of telomerase, survivin, and anti-apoptotic Bcl-2 family members in relation to loss of differentiation and apoptosis in human head and neck cancers. Head Neck. 2004;26(8):733–40. doi: 10.1002/hed.20059. [DOI] [PubMed] [Google Scholar]
- 79.Nomura T, Yamasaki M, Nomura Y, Mimata H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep. 2005;14(4):993–7. [PubMed] [Google Scholar]
- 80.Tirro E, Consoli ML, Massimino M, et al. Altered expression of c-IAP1, survivin, and Smac contributes to chemotherapy resistance in thyroid cancer cells. Cancer Res. 2006;66(8):4263–72. doi: 10.1158/0008-5472.CAN-05-3248. [DOI] [PubMed] [Google Scholar]
- 81.Nakahara T, Kita A, Yamanaka K, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67(17):8014–21. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 82.Tolcher AW, Mita A, Lewis LD, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26(32):5198–203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giaccone G, Zatloukal P, Roubec J, et al. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27(27):4481–6. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- 84.Iwasa T, Okamoto I, Takezawa K, et al. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103(1):36–42. doi: 10.1038/sj.bjc.6605713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar B, Yadav A, Lang JC, et al. YM155 reverses cisplatin resistance in head and neck cancer by decreasing cytoplasmic survivin levels. Mol Cancer Ther. 2012;11(9):1988–98. doi: 10.1158/1535-7163.MCT-12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harari PM, Khuntia D, Traynor AM, et al. Phase I trial of bevacizumab combined with concurrent chemoradiation for squamous cell carcinoma of the head and neck: Preliminary outcome results. ASCO Annual Meeting; Chicago: 2011. [Google Scholar]
- 87.Eisenberger M, Hornedo J, Silva H, et al. Carboplatin (NSC-241-240): an active platinum analog for the treatment of squamous cell carcinoma of the head and neck. J Clin Oncol. 1986;4(10):1506–9. doi: 10.1200/JCO.1986.4.10.1506. [DOI] [PubMed] [Google Scholar]
- 88.Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–83. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]


