Abstract
Seeing the big picture of functional responses within large neural networks in a freely functioning brain is crucial for understanding the cellular mechanisms behind the higher nervous activity, including the most complex brain functions, such as cognition and memory. As a breakthrough toward meeting this challenge, implantable fiber-optic interfaces integrating advanced optogenetic technologies and cutting-edge fiber-optic solutions have been demonstrated, enabling a long-term optogenetic manipulation of neural circuits in freely moving mice. Here, we show that a specifically designed implantable fiber-optic interface provides a powerful tool for parallel long-term optical interrogation of distinctly separate, functionally different sites in the brain of freely moving mice. This interface allows the same groups of neurons lying deeply in the brain of a freely behaving mouse to be reproducibly accessed and optically interrogated over many weeks, providing a long-term dynamic detection of genome activity in response to a broad variety of pharmacological and physiological stimuli.
Optical technologies have long been recognized as a tremendous opportunity for neuroscience. The abilities of laser light to detect and stimulate the activity of neurons, known for more than four decades1,2, have always been particularly intriguing and appealing. In the era of ultrashort-pulse lasers, a breakthrough to deep brain imaging became possible through the use of multiphoton microscopy3,4. Second- and third-harmonic generation microscopy has been shown to detect important morphological features of brain tissues5,6,7. Nonlinear Raman microscopy8 enables chemically selective brain anatomy7,9. Over the past few years, the scene is dominated by optogenetic technologies10,11,12,13, witnessing revolutionary breakthroughs in neuroimaging14,15 and optical control of neuronal activity16,17, as well as promising new solutions for the treatment of brain disorders18.
Fiber-optic technologies provide indispensable tools for optogenetic studies on living brain19,20,21. In a widely used fiber-optic interface for optogenetic work15,22,23, an optical fiber is inserted into a living brain through a guiding cannula right before the experiment. A variety of designs for three-dimensional multiwaveguide arrays24 and arrays of silicon probes25 integrated with optical fibers have been proposed for the delivery of light to multiple targets inside the brain, including distributed neuron circuits. Integration of optical stimulation with multichannel electrophysiological recording has been demonstrated in optogenetic experiments on freely moving mice26. Carefully optimized fiber probes have been shown to enable a precise delivery of stimulation or excitation radiation and simultaneous detection of fluorescent response from circuits of neurons27, including those in living brain28. Advanced fiber solutions have been demonstrated for in vivo detection of sound-evoked network calcium transients in anesthetized mice29 and transient variations in neural activity in direct- and indirect-pathway spiny projection neurons30. These fiber-optic solutions have been of crucial significance for revealing a tremendous potential of optical tools and technologies for neuroscience, enabling the detection of transient changes in neural activity and control of neuron circuits, most notably using the methods of optogenetics22,23.
As the optical methods in brain research are advancing toward challenging goals of studying learning and memory, the standard fiber-optic interface needs to be adapted to the protocols of long-term experiments on multiple sites on deep-brain neuron circuits. Several promising solutions have been recently proposed to address these issues. Implantable optical fibers, developed by Sparta et al.31, have been shown to enable a long-term optogenetic manipulation of neural circuits in freely moving mice with a minimum damage to a brain tissue and minimum changes in light transmission over several weeks32.
In this work, we demonstrate an implantable fiber-optic interface for a simultaneous multisite optical interrogation of distinctly separate deep-brain neuron targets. While most of the earlier studies were concerned with a fiber-optic detection of transient variations in neural activity, the fiber-optic interface demonstrated in this work enables a highly stable long-term optical detection of neuronal activity in freely moving mice. This interface allows the same group of neurons lying deeply in the brain of a freely moving mouse to be reproducibly accessed and optically interrogated over many weeks of experimental work, providing a high-fidelity long-term optical detection of the genome activity in response to a broad variety of pharmacological and physiological stimuli.
Results
In our experiments, the neuronal activity in the brain of a freely moving transgenic mouse is detected (Fig. 1a) using the response of fluorescent protein tags10,11,12,13. The second-harmonic output of a continuous-wave Nd: YAG laser with a wavelength of 473 nm is employed to induce a fluorescence response of the EGFP marker in the brain of a mouse where the EGFP gene expression is controlled by the immediate early gene zif268 (see the Methods section for the details). A specifically designed fiber-optic interface is used to deliver the laser light to the targeted area inside the brain of a freely moving mouse.
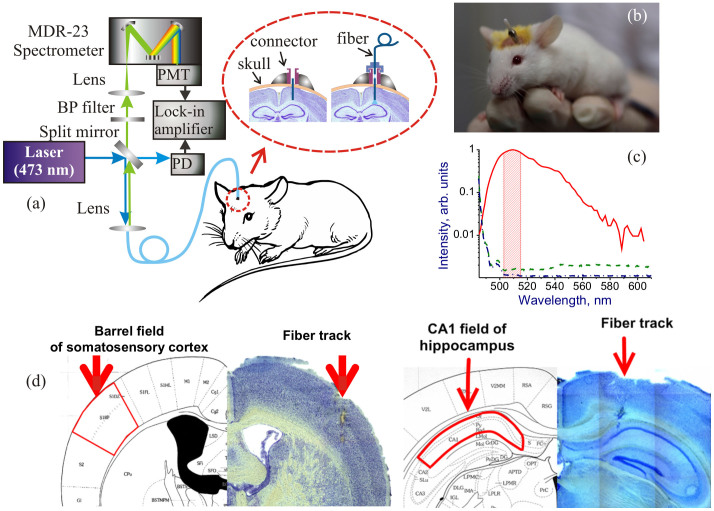
Figure 1. Fiber-based optical interrogation of neuronal activity in the brain of a freely moving animal using an implantable fiber-optic interface.
(a) Optical arrangement: PMT, photomultiplier tube; BP filter, bandpass filter; PD, photodetector. An enlarged view of the fiber-optic interface is shown in the inset. (b) A mouse with an implanted fiber-optic connector. (c) Spectra of EGFP fluorescence (solid line), autofluorescence from the brain cortex in an C57B1-line non-GFP mouse (dashed line), and the Raman background from the fiber probe (dash-dotted line). The detection window is shown by shading. The increase in the spectral intensity on the left is due to the laser line. (d) A stereotaxic atlas vs the Nissl stain image of somatosensory cortex (left) and the CA1 field of hippocampus (right) with a track from the fiber shown.
The design of the fiber-optic interface is the key for a parallel long-term dynamic optical interrogation of brain activity at multiple sites inside the living brain. The interface, sketched in the inset to Fig. 1a, consists of two parts. The first part includes a connector with a plug, implanted in the brain of a mouse, and a short stretch of fiber, which is stripped off of the protective coating and precisely positioned at the targeted site in the brain. The connector is implanted in the brain of an anesthetized mouse and fixed to a skull with an adhesive paste (see Fig. 1b and the Methods section for the details of the surgery and connector implantation procedure).
The second part of the interface consists of a long fiber-optic cable and a fiber-connector counter plug unit (Fig. 1a). The length of the fiber cable was taken long enough to support long-term experiments on a mouse freely moving within a 2 × 2-m2 area. This part of the interface was connected to the short stretch of fiber implanted in the mouse brain through the plug unit. Both the long fiber cable and the short stretch of fiber fixed in the mouse brain had a core diameter of 50 μm and a cladding diameter of 125 μm, which allowed the loss due to fiber connection to be kept below 0.2 dB. The volume of optical interrogation for such a fiber probe, estimated in accordance with the methodology developed in Ref. 33, is on the order of 105 μm3. The fluorescence response from the targeted brain area is collected by the same fiber probe and is transmitted in the backward direction to the detection system, consisting of a photomultiplier and a lock-in amplifier. The fluorescence signal was separated from backscattered scattered pump radiation with a dichroic mirror and a monochromator and was detected within a spectral window from 503 to 515 nm (Fig. 1c), as described in detail in the Methods section.
The long fiber-cable part of the fiber-optic interface is connected to the short stretch of fiber implanted in the mouse brain only during the time when the measurements are performed. During the entire period of experiments, the mice with miniature fiber component implants, whose weight was only 0.17 g were kept under standard conditions in certified laboratory cages with a free access to food and water. This makes it possible to extend optical interrogation experiments to a period of few weeks or even few months. The implanted fiber remains precisely positioned at the targeted site inside the brain of a mouse within the entire experiment. To verify the precision of this positioning, 20-μm-thick frontal brain slices were prepared on a cryostat out of the brain of the mouse studied in experiments. The track from the fiber was visualized on these slices by means of Nissl staining. The images of such Nissl-stained slices are shown in Fig. 1d against the stereotaxic atlas of the mouse brain, visualizing the track from the interrogating fiber positioned in the barrel field of somatosensory cortex (Fig. 1d, left) and the CA1 field of hippocampus (Fig. 1d, right). In approximately 20% of mice, inspection of Nissl-stained images performed after experiments with external stimulation has shown that the fiber has been positioned outside the targeted area inside the brain. The data taken in experiments with such mice have been excluded from the analysis.
In a control series of experiments, we quantified long-term variations in the level of the fluorescence signal from the brain of zif-EGFP mice with an implanted fiber interface kept under otherwise standard conditions without any stimulation. The protocol of this experiment is sketched in the upper panel of Fig. 2a and is explained in detail in the Methods section. The results of such experiments performed on four freely moving mice with a fiber interface positioned in the barrel field of somatosensory cortex (Fig. 2b) within a five-day period and on three freely moving zif-EGFP mice within a one-month period are presented in Figs. 2c–2f. The data acquisition and data averaging procedures adopted for these measurements are described in the Methods section. The individual EGFP fluorescence intensities for all the mice measured within the first five days and averaged over a day of measurements are shown by open circles in Fig. 2c. The bars in Fig. 2c represent the EGFP fluorescence signal averaged over all the mice. The variance σf in the level of fluorescence signal measured in these experiments provides a metrics for uncontrollable factors, which tend to vary from animal to animal on an individual basis and may reflect unsuccessful surgery or anomalies in post-surgery recovery. Only the mice showing σf < 10% in these reference measurements (about 70% of the total number of mice with an implanted fiber interface) were used in experiments with external stimulation.
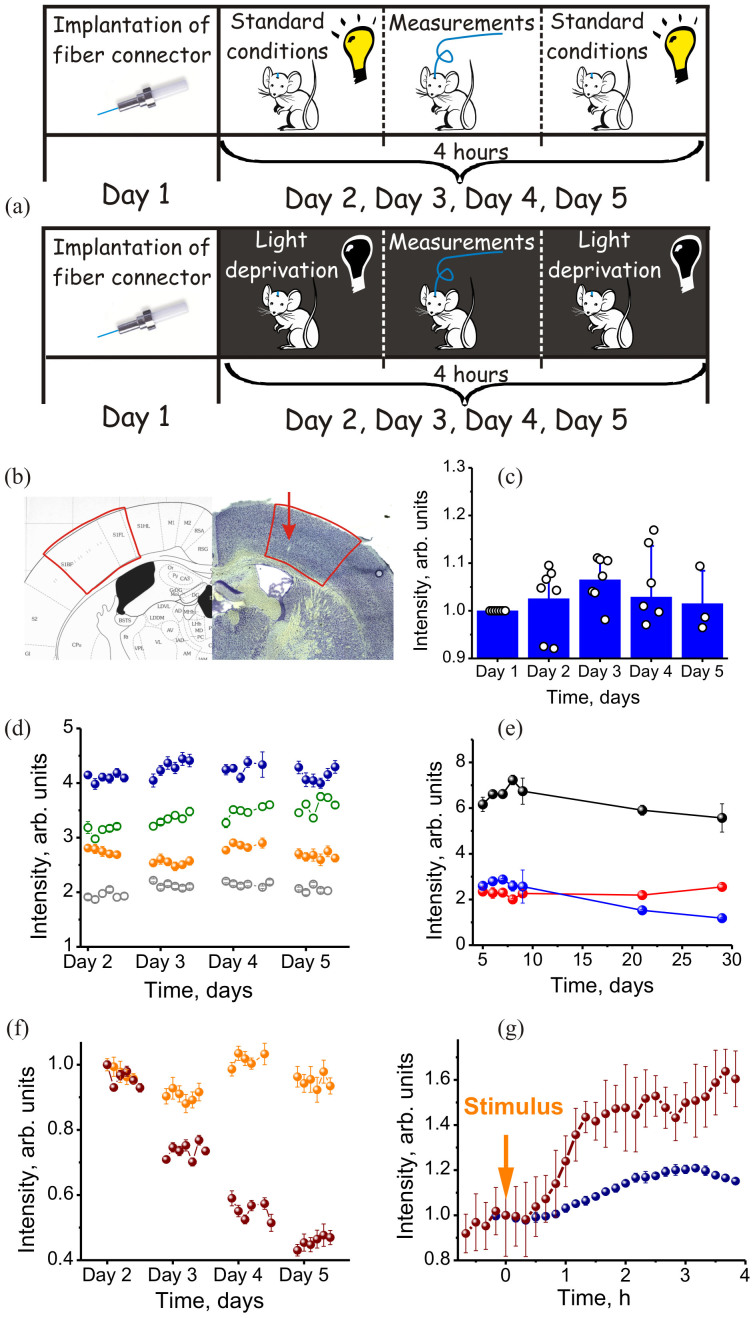
Figure 2. Long-term optical examination of transcription factor induction in the brain of freely moving zif-EGFP mice.
(a) Protocols of experiments with freely moving zif-EGFP mice under standard conditions (the upper panel) and with light deprivation (the lower panel). (b) A fragment of stereotaxic atlas vs the Nissl stain image of somatosensory cortex with a track from the fiber shown. (c) EGFP fluorescence intensity from the brain of seven freely moving zif-EGFP mice kept under standard conditions without any external stimulation measured on five consecutive days: (circles) EGFP fluorescence intensities for individual mice averaged over one day of measurements, (bars) result of averaging over all the mice. The signal intensity for each mouse is normalized to the fluorescence intensity measured on the first day. (d, e) Fluorescence response from EGFP in zif-EGFP mice kept under standard conditions performed on (d) four zif-EGFP mice within a five-day period and (e) three zif-EGFP mice within a one-month period following the implantation of the fiber-optic connector into the hippocampal CA1 field (open circles) and the barrel fields in the somatosensory cortex (filled circles). Each data point represents averaging over (d) 1 minutes and (e) 1 day of measurement in accordance with the procedure described in the Methods section. (f) Fluorescence response from EGFP in a zif-EGFP mouse (orange circles) kept under standard conditions and (purple circles) after light deprivation performed within four days after the implantation of the fiber-optic connector into the hippocampal CA1 field. (g) Fluorescence response from EGFP in a zif-EGFP mouse within four hours following (purple circles) electrical skin stimulation and (blue circles) pentylenetetrazole convulsant. The error bars are for the statistics of (c) averaging over all the mice used for measurements within a specific day (i.e., over seven mice on days 1, 2, and 3; six mice on day 4; and three mice on day 5), with the results of measurements for individual mice shown by open circles; (d, e, f, g) averaging over 30 measurements repeated with the same mouse within (d, f, g) 1 minute and (e) one day.
Figure 2d displays fluorescent studies of zif-EGFP expression performed on four mice within four days in a row. The mice were kept in certified cages during the entire period of the experiment without any stimulation or stress. In two of these mice, the fiber-optic connector was implanted into the hippocampal CA1 field (open circles). In the other two, the fiber-optic interface was immersed into the barrel fields in the somatosensory cortex (filled circles). As can be seen from the results presented in Fig. 2d, although the basal level of zif-EGFP expression can strongly vary from mouse to mouse, it remains constant within a variance of about 10% for each individual mouse, yielding a reproducible stable EGFP signal within both a full day of in vivo work and across a period of at least four days. In Fig. 2e, we present the results of zif-EGFP expression studies performed with three zif-EGFP mice within a one month period. Each data point in this plot represents the result of averaging over the entire day of in vivo work. The animals used in these experiments were kept in their cages during the entire period of the experiment with a fiber-optic connector implanted into the somatosensory cortex under otherwise regular conditions.
In experiments with freely behaving mice subjected to unilateral tactile stimulation, the fiber-optic interface was positioned in the somatosensory cortex in one of the hemispheres in the brain of zif-EGFP mouse (Fig. 3a) as described in the Methods section. The induction of zif regulatory proteins in the somatosensory cortex was continuously monitored by measuring the growth of the EGFP fluorescence signal in response to unilateral whisker stimulation (Figs. 3b, 3c). The zif-EGFP mice prepared as described in the Methods section were divided into two groups. For the five mice from the first group, the tactile stimulation was contralateral, while the six mice of the second group were subject to ipsilateral stimulation. The kinetics of the EGFP fluorescence signal following tactile stimulation at t = 20 min for all the mice used in experiments is presented in Figs. 3c, 3d. The time dependence of the EGFP fluorescence signals averaged over all the mice within each group is shown in Fig. 3e.
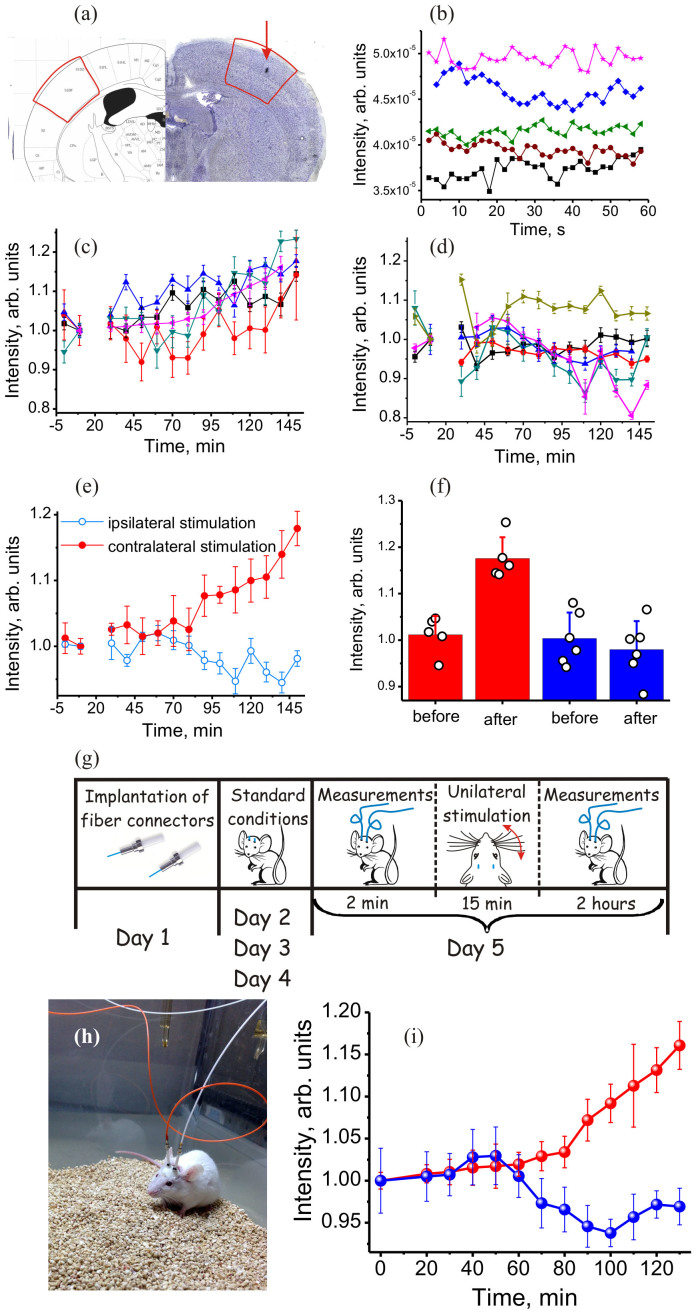
Figure 3. Fiber-based online monitoring of tactile-stimulation-induced transcription factor activation in the brain of zif-EGFP mice.
(a) A fragment of stereotaxic atlas vs the Nissl stain image of somatosensory cortex with a track from the fiber shown. (b) Time-resolved EGFP fluorescence intensity from the brain of freely moving mice within a 1-minute time interval at t = 0 (black rectangles), 10 min (brown circles), 50 min (green triangles) 120 min (blue diamonds), and 150 min (pink asterisks) after contralateral whisker stimulation. (c, d) Dynamics of the EGFP fluorescence response from the brain of freely moving mice following (a) contralateral and (b) ipsilateral whisker stimulation at t = 20 min. (e) Dynamics of the EGFP fluorescence signals averaged over (filled circles) five and (open circles) six mice following (filled circles) contralateral and (open circles) ipsilateral whisker stimulation at t = 20 min. (f) EGFP fluorescence intensity from the brain of freely moving mice following (red) contralateral and (blue) ipsilateral whisker stimulation before and 2 h after the stimulation: (circles) EGFP fluorescence intensity for individual mice and (bars) result of averaging over all the mice involved in the experiment. (g) Protocol of simultaneous optical interrogation of neuronal activity in the left and right hemispheres of somatosensory cortex in a freely moving mouse in response to a tactile unilateral whisker stimulation. (h) A transgenic mouse during a multisite optical brain interrogation experiment. (i) Fluorescence response from EGFP measured simultaneously for (blue circles) the ipsilateral and (red circles) the contralateral hemisphere following a tactile unilateral whisker stimulation. The error bars are for the statistics of (c, d, e, i) averaging over 30 measurements repeated with the same mouse within 1 minute and (f) averaging over five mice subjected to contralateral whisker stimulations (red bars) and six mice subjected to ipsilateral whisker stimulations (blue bars).
Discussion
We now examine the results of experiments described in the previous section from the perspective of the central goal of this work – demonstration of the capability of the implantable fiber-optic interface developed here to chronically record optical signals from awake behaving animals, including a parallel long-term optical interrogation of distinctly separate, functionally different sites in the brain of freely moving mice. To this end, we focus on experiments demonstrating that our fiber-optic neurointerface can detect transcription factor induction in EGFP mice in response to tactile unilateral whisker stimulation. As earlier studies on rats have shown34,35, this type of stimulation activates transcription regulatory proteins, including neuronal zinc finger proteins, in somatosensory cortex. However, the induction of these proteins could only be visualized in those earlier studies by means of immunohistochemical analysis in vitro, yielding postmortem maps of positively stained cells at a fixed time following stimulation, with no access to protein dynamics in living brain. The fiber-optic neurointerface developed in this work offers a unique tool for continuous online quantitative monitoring of transcription factor dynamics in the brain of freely behaving mice over many hours (Fig. 2g), days (Figs. 2c, 2d, 2f), and even months (Fig. 2e) of experimental in vivo work. With the basal level of EGFP expression in somatosensory cortex strongly varying from one mouse to another (Figs. 2c–2e; see also Refs. 34, 35), a histological examination of fluorescent images from mice subjected to stimulations versus native fluorescence from other mice does not enable a reliable validation of zif-EGFP expression. In view of this difficulty, experiments with control groups of mice, which were kept under standard conditions, as well as experiments with various types of stimulations or deprivation, which either induce or suppress zif-EGFP expression in different brain areas, all performed in combination with a careful statistical analysis (see the Methods section), were used for such a validation.
With this perspective in mind, we first examine the results of long-term measurements on the EGFP fluorescence level in freely moving zif-EGFP mice kept under standard conditions with no stimulation and compare these results with similar measurements performed on a freely moving mouse subjected to light deprivation four days after the implantation of the fiber-optic connector into the CA1 field of hippocampus (the protocol of this experiment is sketched in the lower panel of Fig. 2a). The level of zif268 transcription factor gene expression in rodent hippocampus is known to depend on the environment, increasing with the enrichment of the environment36. Experimental results presented in Figs. 2e and 2f show that the developed fiber-optic interface can reliably detect a suppression of zif expression in the hippocampus of freely moving zif-EGFP mice subjected to light deprivation. Indeed, the difference between the kinetics of the EGFP fluorescence signal from intact and light-deprived mice, seen in Fig. 2f, is striking. While for mice kept under standard conditions, variations in the fluorescence signal fall within a usual 10% variance, the fluorescence response from the hippocampus in light-deprived mice is seen to monotonically decrease as a function of time, decreasing by approximately 60% on the fifth day of experiments. Electrical skin stimulation and an intraperitoneally injected convulsan, on the other hand, induce a long-term monotonic growth in the EGFP fluorescence response (Fig. 2g), indicating the induction of transcription factors in the CA1 field of hippocampus. These experiments clearly demonstrate the capability of the developed fiber-optic interface to chronically interrogate deep subcortical brain structures, including the hippocampus, which are inaccessible for in vivo optical studies unless a dedicated fiber probe is used.
In the lack of a better reference to quantify the basal level of EGFP expression, which may strongly vary, as mentioned above, from one mouse to another (Figs. 2c–2e), the signal from the ipsilateral hemisphere (open circles in Fig. 3e, blue bars in Fig. 3f) provides a valuable measure of the natural background in the EGFP fluorescence response and helps assess its variations. The variance of the EGFP fluorescence signal from the ipsilateral hemisphere in our experiments (10% in Figs. 3e, 3f) is well within the limits of variations in basal EGFP expression, suggesting no definitive judgment as to whether a unilateral tactile stimulation may give rise to an actual reduction in zif expression in the ipsilateral hemisphere or the observed variations are solely due to fluctuations in the basal level of EGFP expression, inevitable in experiments on freely behaving animals.
The averaged EGFP fluorescence signal from the contralateral hemisphere after whisker stimulation is 16% higher than the level of this signal before the stimulation (filled circles in Fig. 3e, red bars in Fig. 3f). This change in the EGFP response before and after contralateral tactile stimulation is appreciably larger than the 10% variance of the EGFP signal from the ipsilateral hemisphere (cf. red and blue bars in Fig. 3f). This correlates well with the Mann–Whitney U test37 parameter, which was found to be p > 0.5 in the case of ipsilateral stimulation (see the details in the Methods section), indicating no statistically significant changes in the EGFP signal. This result is in striking contrast with p values observed in the case of contralateral stimulation, p < 0.005, revealing a statistically significant systematic change in the EGFP signal, which indicates the induction of zif transcription factors in the brain of freely behaving mice. Furthermore, a 16% increase in the EGFP fluorescence signal in response to contralateral whisker stimulation is consistent with the results of the earlier in vitro immunohistochemical studies of transcription protein induction in somatosensory cortex34,35, performed under similar tactile stimulation conditions.
For optical interrogation of neuronal activity simultaneously at two sites inside the brain of freely moving mice, two identical fiber-optic interfaces of the above-described design have been implanted in the left and right hemispheres of somatosensory cortex of transgenic EGFP mice (Figs. 3g, 3h) using the procedures described in the Methods section. Such mice were then used in experiments studying variations in the EGFP fluorescence signal in response to a tactile unilateral whisker stimulation (Fig. 3g). Due to the two-component design of the developed fiber-optic interface, we were able to start optical interrogation experiments few days after the surgery, reducing the post-surgery stress and helping recover the natural behavior of the mice to a maximum extent. The measurements presented below were carried out four days after the fiber implantation, when the normal physical parameters and the normal behavior of the mice, as well as a stable fluorescence response corresponding to the basal level of EGFP gene expression were fully recovered after the surgery needed for fiber implantation.
The EGFP fluorescence response was measured following a sedative injection of 20 mg/kg of zoletil, reference measurement of the basal level of EGFP gene expression, and 15-min tactile unilateral whisker stimulation (Fig. 3g), in accordance with the protocol of Ref. 31. The sensory information from whiskers is known to be processed by the contralateral hemisphere of somatosensory cortex, leading, as shown by earlier in vitro immunohistochemical studies34 to enhanced transcription factor activation in the contralateral hemisphere in response to unilateral whisker stimulation. Accordingly, our measurements reveal the growth of the EGFP fluorescence signal from the contralateral hemisphere (red circles in Fig. 3i) in response to this stimulus. This is observed to be in a striking contrast with the behavior of the EGFP fluorescence response from the ipsilateral hemisphere (blue circles in Fig. 3i), which provides a measure of uncontrolled fluctuations in the EGFP fluorescence response, inevitable for experiments on freely behaving animals.
To summarize, we have shown that a specifically designed implantable fiber-optic interface provides a powerful tool for parallel long-term optical interrogation of distinctly separate, functionally different sites in the brain of freely moving transgenic mice. This interface allows the same group of neurons lying deeply in the brain of a freely behaving mouse to be optically interrogated over many weeks of experimental work, providing a high-fidelity long-term dynamic optical detection of the genome activity in response to a broad variety of pharmacological and physiological stimuli. Such a fiber-optic neurointerface offers a unique tool for continuous online quantitative monitoring of transcription factor dynamics in the brain of freely behaving mice, suggesting new ways toward understanding neuron plasticity, learning, and memory.
Methods
Animal subjects
Transgenic male mice of the Tg(Egr1-EGFP)60Gsat/Mmcd line three to eight months of age were used in all the optical interrogation experiments. Expression of the EGFP gene in these mice was driven by the immediate early gene zif268 (early growth response protein 1). During the entire period of experiments, the mice were kept under standard conditions in certified laboratory cages with a free access to food and water. All the experiments on live vertebrates were performed in accordance with the relevant guidelines and regulations and approved by the scientific board of Kurchatov Institute.
Fiber-optic neurointerface
The fiber-optic interface consists of a short, a few millimeter long stretch of optical fiber and a connector. The distal end of the fiber is stripped off the protective coating and immersed in the area of interest inside mouse brain. The other end of this fiber stretch is fixed to the skull as described below. The outer diameter of the fiber cladding in our fiber-optic neurointerface is 125 μm. The core diameter of the fiber-optic interface controls the optically interrogated volume33. In the experiments presented here, a fiber with a core diameter of 50 μm was chosen (ThorLabs cat. no. AFS50/125Y or Precision Fiber Products cat. no. MMBF-50-AQU-100), confining optical interrogation to a volume of about 105 μm3. Elsewhere, similarly designed fiber probes using a single-mode fiber with a core diameter of 4 μm were employed (ThorLabs cat. no. S405-XP), to confine optical interrogation to a volume less than 103 μm3, allowing the optical response from fewer neurons to be detected.
One end of the fiber is pasted into a ceramic ferrule with a hole diameter of 126 μm (ThorLabs cat. no. CFLC126-10 or Precision Fiber Products cat. no. MM-FER2007C-1260), cleaved and polished with a fiber-optic termination/connectorization kit (e.g., ThorLabs cat. no. CK03). The other end of the fiber is cleaved with a diamond or ruby fiber scribe (e.g., ThorLabs cat. no. S90R). The length of the fiber stretch is chosen, with the help of a stereotaxic atlas, in such a way as to make sure that the distal end of the fiber is immersed into the brain region of interest. Specifically, a 2-mm fiber stretch was employed to probe the CA1 hippocampal region in a mouse brain. The somatosensory cortex was probed with a fiber stretch shorter than 1 mm. The fiber in a ferrule is immersed into the brain and is fixed to the skull by successively applying a cyanoacrylate adhesive paste and tooth cement. Precise positioning of the fiber in the brain of the mouse was implemented using the stereotaxic atlas.
The second part of the interface consists of a replaceable fiber whose structure is identical to the structure of the fiber stretch implanted into the mouse brain. This replaceable part of the fiber interface was also pasted into a ceramic ferrule, which was connected to its counterpart holding the other part of the fiber interface, fixed in the brain of a mouse with a ceramic split sleeve (e.g., ThorLabs cat. no. ADAL1 or Precision Fiber Products cat. no. SM-CS125S). The length of the fiber in this part of the interface is taken large enough (about 1 m in the experiments presented in this paper) to allow a mouse to freely move within a sufficiently large area. For fiber lengths exceeding 3 m, additional measures are typically needed to discriminate the signal from the Raman background from the fiber38.
Fiber implantation
For the implantation of a fiber-optic interface, a transgenic mouse was anesthetized with a mixture of 40 mg/kg of zoletil and 5 mg/kg of xylazine. The skin on the head of the mouse was disinfected with iodine solution and dissected with a scalpel or scissors. Following the cleaning of the surface with 3% solution of hydrogen peroxide and ethyl alcohol, a 1-mm hole was drilled in the skull. A stretch of optical fiber with a connector plug unit was inserted into the brain through this hole and fixed to the skull by successively applying a cyanoacrylate adhesive paste and tooth cement. Precise positioning of the fiber in the brain of the mouse was implemented using the stereotaxic atlas. Already on the eighth day after fiber implantation, the open-field39 and rotarod40 tests, performed with 10 male zif-EGFP mice with implanted fiber connectors and 8 intact male zif-EGFP mice, revealed no statistically significant (according to the Mann–Whitney U test37) difference in locomotor activity, coordination and anxiety between these two groups of mice (9% difference in the overall path in the open-field test and 3 – 6% difference in the time latency to fall off the rotarod, depending on the rotation speed in the rotarod test were well within the statistical variance, 14 and 20% for the open-field and rotarod tests, respectively).
Light source and fluorescence detection
The second-harmonic output of a continuous-wave Nd: YAG laser with a wavelength of 473 nm was used to induce a fluorescence response of the EGFP marker. In experiments on freely moving animals, the power of laser radiation was reduced to 0.2 μW, in order to avoid EGFP bleaching effects, by using neutral density filters and by utilizing only 3% of the laser output, reflected off a silica wedge beam splitter (Fig. 1a) with a wedge angle of 10°. The same fiber was used to deliver laser radiation to a targeted brain area and to collect the fluorescence response from this area. The full spectrum of the EGFP fluorescence recorded from the brain of a zif-EGFP mouse is shown in Fig. 1c. The silica-wedge beam splitter gave rise to a 10% attenuation of the fluorescence signal due to reflection from the wedge surfaces but did not cause any distortions in the spectrum of this signal. In experiments with freely behaving mice, only the central, most intense part of this spectrum, was selected by letting the fluorescence signal pass through a monochromator set to transmit a spectral band from 503 to 515 nm (Fig. 1c).
To enhance low-power signal detection sensitivity, 473-nm laser radiation was modulated at 364 Hz with a duty cycle of 50%, delivering a sequence of pulses with a pulse width of 1.4 ms. The photomultiplier output voltage was synchronously read out and digitized using an SRS 830 lock-in amplifier with a moving average variable from 0.1 to 1 s within a time interval of 1 minute. The readings were stored in a computer memory every 2 s. The results of measurements were then averaged over the 1-min measurement time. The entire procedure was controlled with homemade software in LabView 8.2 and was repeated every 10 minutes, returning the statistical means and variances. A typical example of raw data is provided in Fig. 3b. Normalization to the photodiode signal was performed automatically, which allowed high-frequency noise to be filtered out.
To understand the role of autofluorescence from the brain cortex in EGFP fluorescence measurements using the developed fiber-optic probe, spectra of autofluorescence were measured for the brain cortex in C57B1-line non-GFP mice. As can be seen from Fig. 1c, the autofluorescence signal can be easily discriminated against the autofluorescence, which has a distinctly different spectrum (shown by the dashed line in Fig. 1c) and a factor of 500 less intense than the EGFP signal within the 503–515-nm detection window (the shaded area in Fig. 1c). The dash–dotted line in Fig. 1c shows the spectrum of the Raman background from the fiber probe, measured in experiments with a fiber probe tip not connected to any fluorescent sample. The intensity of this signal is seen to be almost three orders of magnitude lower than the intensity of EGFP fluorescence.
Statistical analysis
The Mann–Whitney U test37 was used for the statistical analysis of experimental data. When applied to the results of control experiments, where long-term variations in the level of the EGFP fluorescence signal from the brain of mice with implanted fiber connectors was quantified without any stimulation, this test reveals no statistically significant difference in EGFP fluorescence signals measured on different days (Fig. 2c). Specifically, the U test applied to the experimental data for days 2 and 4 returns a U-test parameter p > 0.9. The U-test analysis of the data acquired on days 4 and 5 yields p ≈ 0.7. The minimum value of the p parameter obtained for all the measurements performed with no external stimulation is 0.27.
Application of the Mann–Whitney U test to the results of experiments performed on zif-EGFP mouse before and 2 h after ipsilateral whisker stimulation (blue bars in Fig. 3f) gives p > 0.5. In a striking contrast, when applied to the results of measurements on zif-EGFP mouse before and 2 h after contralateral whisker stimulation (red bars in Fig. 3f), the U test returns p < 0.005, providing a strong statistical evidence of a systematic change in the EGFP signal, indicating the induction of zif transcription factors in the brain of freely behaving mice.
Author Contributions
L.V.D.-A. designed and performed the experiments, performed fiber implantation, analyzed the data, prepared the graphic material, and wrote the paper. I.V.F. designed and performed the experiments. O.I.I. and M.A.Z. contributed to sample preparation and participated in experiments, A.B.F. participated in project planning and discussions of the results and oversaw the experiments, K.V.A. designed and oversaw the experiments, analyzed the data, and oversaw manuscript preparation A.M.Z. designed the experiments, analyzed the data, and wrote the paper.
Acknowledgments
Valuable help of T. Kunitsyna in performing open-field and rotarod tests is gratefully acknowledged. This research was supported in part by the Russian Foundation for Basic Research (projects nos. 12-02-33161, 12-02-31225, 13-04-40335, and 13-02-01465), the Welch Foundation (grant no. A-1801), and Skolkovo Foundation (grant no. 78).
References
- Cohen L. B., Keynes R. D. & Hille B. Light scattering and birefringence changes during nerve activity. Nature 218, 438–441 (1968). [DOI] [PubMed] [Google Scholar]
- Fork R. L. Laser stimulation of nerve cells in Aplysia. Science 171, 907–908 (1971). [DOI] [PubMed] [Google Scholar]
- Denk W., Strickler J. & Webb W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990). [DOI] [PubMed] [Google Scholar]
- Helmchen F. & Denk W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005). [DOI] [PubMed] [Google Scholar]
- Debarre D. et al. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat. Methods 3, 47–53 (2006). [DOI] [PubMed] [Google Scholar]
- Campagnola P. J. & Loew L. M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 21, 1356–1360 (2003). [DOI] [PubMed] [Google Scholar]
- Doronina-Amitonova L. V. et al. Nonlinear-optical brain anatomy by harmonic-generation and coherent Raman microscopy on a compact femtosecond laser platform. Appl. Phys. Lett. 99, 231109 (2011). [Google Scholar]
- Freudiger C. W. et al. Label-free biomedical imaging with high sensitivity by stimulated raman scattering microscopy. Science 322, 1857–1861 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin A. A. et al. Ionization penalty in nonlinear Raman neuroimaging. Opt. Lett. 36, 508–510 (2011). [DOI] [PubMed] [Google Scholar]
- Boyden E. S., Zhang F., Bamberg E., Nagel G. & Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005). [DOI] [PubMed] [Google Scholar]
- Diester I. et al. An optogenetic toolbox designed for primates. Nat. Neurosci. 14, 387–397 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nat. Methods 8, 26–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G. The optogenetic catechism. Science 326, 395–399 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang F. et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protocols 5, 439–456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin J. A. et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protocols 5, 247–254 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Wang L.-P., Boyden E. S. & Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 3, 785–792 (2006). [DOI] [PubMed] [Google Scholar]
- Gradinaru V., Mogri M., Thompson K. R., Henderson J. M. & Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flusberg B. A. et al. Fiber-optic fluorescence imaging. Nat. Methods 2, 941–950 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flusberg B. A. et al. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat. Methods 5, 935–938 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronina L. V. et al. Tailoring the soliton output of a photonic crystal fiber for enhanced two-photon excited luminescence response from fluorescent protein biomarkers and neuron activity reporters. Opt. Lett. 34, 3373 (2009). [DOI] [PubMed] [Google Scholar]
- Aravanis A. M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural. Eng. 4, S143–S156 (2007). [DOI] [PubMed] [Google Scholar]
- Adamantidis A. R., Zhang F., Aravanis A. M., Deisseroth K. & de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzos A. N., Boyden E. S. & Fonstad C. G. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt. Lett. 35, 4133–4135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S. et al. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur. J. Neurosci. 31, 2279–2291 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anikeeva P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashaie R. & Falk R. Single Optical Fiber Probe for Fluorescence Detection and Optogenetic Stimulation. IEEE Transactions on Biomedical Engineering 60, 268–280 (2013). [DOI] [PubMed] [Google Scholar]
- Doronina-Amitonova L. V. et al. Photonic-crystal-fiber platform for multicolor multilabel neurophotonic studies. Appl. Phys. Lett. 98, 253706 (2011). [Google Scholar]
- Grienberger C. et al. Sound-evoked network calcium transients in mouse auditory cortex in vivo. The Journal of Physiology 899–917 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G. et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta D. R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nature protocols 7, 12–23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronina-Amitonova L. V. et al. Enhancing the locality of optical interrogation with photonic-crystal fibers. Appl. Phys. Lett. 101, 021114 (2012). [Google Scholar]
- Mack K. J. & Mack P. A. Induction of transcription factors in somatosensory cortex after tactile stimulation. Brain Res. Mol. Brain Res. 12, 141–147 (1992). [DOI] [PubMed] [Google Scholar]
- Knapska E. & Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 74, 183–211 (2004). [DOI] [PubMed] [Google Scholar]
- Olsson T., Mohammed A. H., Donaldson L. F., Henriksson B. G. & Seckl J. R. Glucocorticoid receptor and NGFI-A gene expression are induced in the hippocampus after environmental enrichment in adult rats. Molecular brain research 23, 349–353 (1994). [DOI] [PubMed] [Google Scholar]
- Lehmann E. L. Nonparametrics: statistical methods based on ranks. (Springer, 2006). [Google Scholar]
- Doronina-Amitonova L. V. et al. Raman detection of cell proliferation probes with antiresonance-guiding hollow fibers. Opt. Lett. 37, 4642–4644 (2012). [DOI] [PubMed] [Google Scholar]
- Lipkind D. New replicable anxiety-related measures of wall vs. center behavior of mice in the open field. Journal of Applied Physiology 97, 347–359 (2004). [DOI] [PubMed] [Google Scholar]
- Bogo V., Hill T. A. & Young R. W. Comparison of accelerod and rotarod sensitivity in detecting ethanol- and acrylamide-induced performance decrement in rats: review of experimental considerations of rotating rod systems. Neurotoxicology 2, 765–787 (1981). [PubMed] [Google Scholar]