Abstract
In the novel object recognition (OR) paradigm, rats are placed in an arena where they encounter two sample objects during a familiarization phase. A few minutes later, they are returned to the same arena and are presented with a familiar object and a novel object. The object location recognition (OL) variant involves the same familiarization procedure but during testing one of the familiar objects is placed in a novel location. Normal adult rats are able to perform both the OR and OL tasks, as indicated by enhanced exploration of the novel vs. the familiar test item. Rats with hippocampal lesions perform the OR but not OL task indicating a role of spatial memory in OL [1]. Recently, these tasks have been used to study the ontogeny of spatial memory but the literature has yielded conflicting results [2, 3]. The current experiments add to this literature by: 1) behaviorally characterizing these paradigms in postnatal day (PD) 21, 26 and 31-day-old rats; 2) examining the role of NMDA systems in OR vs. OL; and 3) investigating the effects of neonatal alcohol exposure on both tasks. Results indicate that normal-developing rats are able to perform OR and OL by PD21, with greater novelty exploration in the OR task at each age. Second, memory acquisition in the OL but not OR task requires NMDA receptor function in juvenile rats. Lastly, neonatal alcohol exposure does not disrupt performance in either task. Implications for the ontogeny of incidental spatial learning and its disruption by developmental alcohol exposure are discussed.
Keywords: Hippocampus, Fetal Alcohol Spectrum Disorders, Spatial Cognition, NMDA Receptor, Ontogeny
1. Introduction
Previous research concerning the developmental emergence of hippocampus-dependent learning and memory has largely focused on reinforcement-driven tasks, such as Pavlovian fear conditioning and appetitive maze learning [4–6]. Typically, performance of the hippocampus-dependent variant emerges later in ontogeny than control tasks that do not require the hippocampus. For example, auditory fear conditioning emerges by postnatal day (PD)16–18, followed by contextual fear conditioning, which emerges around PD23 [4, 7–9] and T-maze delayed alternation develops later than position discrimination [5, 10]. These findings have been attributed to a delay in hippocampal development and function, which causes spatial learning to develop later than non-spatial forms of learning.
Incidental learning encompasses an animal’s natural exploratory tendency when presented with novel environmental stimuli [11, 12]. There are spatial and nonspatial variants of incidental learning. The standard object recognition task (OR) includes exposure to two identical objects (sample phase). Following a delay, the animal is presented with a familiar, as well as a novel object (testing phase). The spatial variant, the object location task (OL) is identical to the object version; however, the testing phase includes a familiar object situated in a novel spatial location [1, 13–18]In both cases, preference for the novel object or the object in a novel location is a result of incidental learning occurring during the sample phase and exploratory behavior during the testing phase. One-trial object recognition paradigms provide an opportunity to examine incidental conjunctive processing functions of the hippocampus without the need for repeated conditioning trials or reinforcement contingencies. Incidental learning is thought to be more sensitive than reinforcement-driven learning to disruptions of hippocampal function [12].
Different brain regions contribute to performance of the OR and OL tasks, at least during adulthood. Perirhinal cortex (PER) is required for OR task performance, while the hippocampus (HPC) is required for OL performance [1, 15–18]. Studies of the OR task and hippocampal NMDA-receptor (NMDAr) function have produced mixed results, depending on the delay interval between the sample and testing phases [19]. For example, NMDAr antagonists administered either systemically or intrahippocampally prior to acquisition of the OR task disrupts performance with delays ranging from 1 to 24hr. [20–23]. However, OR performance remains intact following intra-hippocampal infusions with a 5-min delay between the sample and testing phases [22]. Similarly, intra-perirhinal cortex infusion of AP5 prior to the sample phase impairs OR after a 3 hr but not a 5-min delay [24]. Studies in adult rats implicate the role of NMDArs in the OL task, [25, 26]; however, the effect of NMDAr antagonists in the OR and OL task has, to our knowledge, not been examined during development.
The sensitivity of incidental conjunctive learning to hippocampal injury [12] makes it of great value to study developmental neurobehavioral disorders that involve the hippocampus [27, 28]. For example, developmental alcohol exposure produces teratogenic effects in various brain regions, such as the cerebellum and hippocampus [29, 30] and impairs neurobehavioral function, including spatial memory in humans [31, 32]. Fetal alcohol spectrum disorders (FASDs) affect approximately 2–5 in 100 young children in the U.S. and abroad [33]. In rat models of FASDs, in which alcohol exposure is limited to PD4-9, the third trimester equivalent of human pregnancy, converging neuroanatomical and behavioral evidence reveals hippocampal CA1 [34–36] and CA3 pyramidal cell loss [34]. Ethanol administration restricted to PD7-9 also impairs performance in hippocampus-dependent maze and fear conditioning tasks [37–39], possibly through inhibited induction and transmission of long-term potentiation (LTP) and reduction of hippocampal NMDArs [40–42].
The current report extends previous work from our lab addressing spatial learning and NMDAr function during development and the effect of neonatal alcohol exposure on this function [27, 28, 43–45]. The OR and OL tasks are being developed here as a probe for examining these issues with incidental learning paradigms. Previous research concerning the developmental emergence of various object recognition paradigms has provided mixed results [2, 3] and the developmental role of NMDArs in the non-spatial OR and spatial OL tasks has not been examined. Recent findings from our lab demonstrate that PD7-9 ethanol administration impairs performance of a contextual fear conditioning task that involves incidental learning [27]. Thus, the OR and/or the OL tasks may provide converging evidence for incidental learning deficits as a function of developmental alcohol exposure.
In summary, the purpose of this study was to examine the effects of age, NMDAr antagonists, and neonatal alcohol on OR and OL in juvenile rats. Experiment 1 evaluated the ability of normally-developing weanling and juvenile rats to perform both tasks. The amount of habituation to the empty chamber was manipulated in order to determine how much exposure to the spatial environment was optimal for task performance. Experiment 2 determined whether or not antagonism of NMDArs would impair performance in either or both of these tasks. Based on prior studies with systemically administered MK-801 in spatial learning tasks, we predicted administration prior to acquisition would impair the OL, but not the OR task. Finally, Experiment 3 examined the effects of neonatal alcohol exposure with a dose and window of administration found to be effective in disrupting hippocampal-dependent forms of learning.
1. Experiment 1A: Behavioral determinants of developmental object and spatial location memory
Previous research concerning variations of the OR and OL object recognition paradigms during development has produced mixed results [2, 3, 46–48], highlighting the need for a more systematic approach for examining these tasks. Thus, Experiment 1A sought to compare the OR and OL tasks as a function of empty chamber habituation and sex by using an age during the late stage of postnatal development when performance in these tasks would be expected.
2.1 Materials & Methods
2.1.1 Subjects
The subjects were Long Evans rats bred at the University of Delaware, Office of Laboratory Animal Medicine (OLAM). Time-bred pregnant females were housed in clear polypropylene cages (45×24×21cm) with standard bedding and ad lib access to food and water. Offspring date of birth was determined by checking for births during the light cycle (12:12) and, if newborn pups were found, that day was designated as PD0. On PD2, litters were transported from the breeding facility to the local animal housing rooms in the laboratory. On PD3, litters were culled to 8 pups (typically, 4 males and 4 females) and were paw-marked by a subcutaneous injection with non-toxic black ink. On PD21, pups were weaned and housed with same-sex littermates in 45×24×17cm cages (except where noted). On PD28 (2 days prior to habituation), rats were individually housed in smaller white polypropylene cages (24×18×13cm) with ad lib access to food and water for the remainder of the study. Rats were randomly assigned to the OR or OL task. If same sex littermates were assigned to the same task, they were placed in distinct habituation groups (Group 1 vs. Group 3; Habituation Group), so that no more than one same sex pup from the same litter was assigned to any given experimental condition (Task x Habituation Group). When assigning same-sex littermates to experimental groups was unavoidable, data were averaged together and counted as single observation.
2.1.2 Apparatus
All behavioral procedures were carried out in 1 of two circular arenas measuring 78.7 cm in diameter, with 48.9 cm walls, elevated 26.7 cm from the floor. The arena was constructed of wood with white polyester resin panels constituting the floor and walls. The arena was situated in a well-lit room allowing the rats to see distal visual cues. There were also 2 proximal cues placed inside the top of the wall of the arena; a black ‘X’ made with electrical tape (10.5×9in; north position) and a circular cross-hatching pattern made from contrasting strips of colored paper (8.5in diameter; west position). These cues were situated far enough from the rats to prevent physical contact. Two squares of reusable Velcro (hook component) were attached to the floor of the arena (Velcro USA Inc., Manchester, NH) in order to secure the objects to the arena floor and prevent the rats from displacing them. The relative dimensions and positioning of objects is shown schematically in Figure 1.
Figure 1.
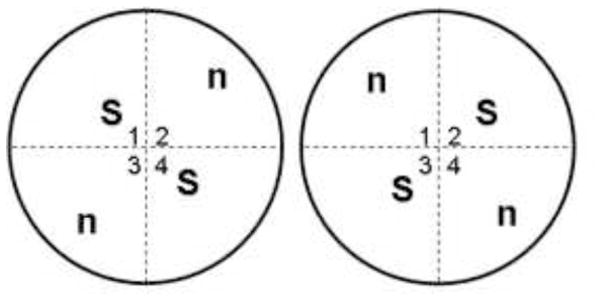
Relative Dimensions. Relative dimensions of the open field arenas for 2 possible combinations of object placement. For the object recognition (OR) task, objects were positioned in standard locations (S) during both the sample and testing phase. For the object location (OL) task, objects were positioned in S for the sample phase. During testing, one object was moved to a novel location (n). Quadrants are labeled 1,2,3,4. Configuration 1 (left); Configuration 2 (right).
Objects used for exploration were obtained from various sources, however, it was necessary that all objects were easily cleaned and made of nonporous material (Figure 2). Objects differed in their surface textures, colors and dimensions, yet maintained relative size. The flat base of each object was fully covered with reusable Velcro (loop component). Object C was found to elicit the highest mean spatial preference in Experiment 1, thus this object was used exclusively for the OL task in subsequent experiments.
Figure 2.

Stimulus Objects. Photographs of objects used (A, B and C) in the OR and OL tasks.
2.1.3 Habituation
Prior to behavioral testing on PD31, animals were habituated to the arena for either 1 or 3 sessions. At the beginning of each habituation session, animals were handled for 3-min in the animal housing room, weighed and carted to the behavioral testing room in their home cage. Each habituation session consisted of placement within the empty arena for 10-min. For all habituation sessions (and behavioral testing), the rats were placed in the center of the arena, facing the north position. Prior to each placement, animals waited in the corridor while the arena was cleaned with 70% ethanol. The possible habituation sessions occurred on the morning and afternoon of PD30 (Sessions 1 and 2, respectively) and during the morning of PD31-32 (Session 3). Animals received either 1 (Group 1) or 3 (Group 3) habituation sessions. Animals in Group 1 were weighed as animals in Group 3, but were not carted to the behavior testing room on P30 (when Group 3 animals received their first two habituation sessions).
2.1.4 Novel Object Recognition (OR)
On the afternoon of PD 31 (Session 4), rats were tested on either the standard novel object recognition (OR) task or on a novel object location recognition task (OL, see Figure 3). For the OR task, each rat was weighed and carted to the animal testing room. Following cleaning, rats were placed in the arena for 5-min where they encountered two identical sample objects (Sample Phase). At the end of the Sample Phase, rats were placed back in their home cages for a 5-min delay (±15 s). For the Testing Phase, animals were returned to the arena for 3-min where one of the familiar objects was replaced with a novel object (Figure 3). For both Sample and Testing phases, exploration was defined as an active event in which the rat was pawing at, sniffing or whisking with its snout directed at the object from a distance of under ~1 cm. Sitting on or next to an object was not counted as active exploration. In addition to the arena, all objects were cleaned with 70% ethanol between each session. Following the completion of Testing, animals were returned to the animal housing room. Object identity, arena configuration and arena number were counterbalanced with each Sex and Habituation grouping variable.
Figure 3.
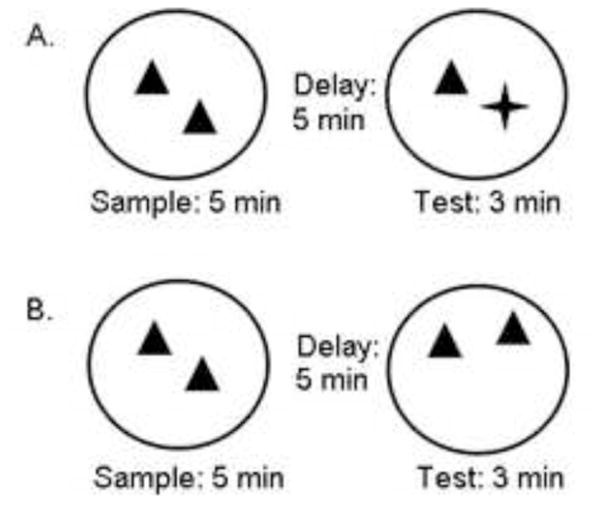
General Procedure. The general procedures used in the standard novel object recognition (OR) and novel object location recognition (OL) tasks. (A) Schematic of the OR task in which 2 identical objects were presented during the sample phase (5-min). Following a 5-min delay, one of the familiar objects was replaced with a novel object at testing (5-min). (B) Schematic of the OL task in which 2 identical objects were presented during the sample phase (5-min). Following a 5-min delay, one of the familiar objects was moved to a novel location at testing (5-min).
2.1.5 Novel Location Recognition (OL)
For the OL task, all procedures were identical to the OR task except that during the Testing phase, rather than presentation of a novel object, rats encountered both familiar objects, with one object located in a different place among the arena (Figure 3).
2.1.6 Data Collection & Analysis
A video camcorder (Panasonic Corporation of North America) was used to record activity and exploration during habituation and testing sessions. Video-recordings of sessions were subsequently scored off-line by an observer blind to the experimental conditions. This was performed manually by running a timing program (Arun Asok, University of Delaware) whereby manual depression of a cursor would activate a timer corresponding to each of the objects (left and right). Data were analyzed in this manner in three successive 1-minute blocks of the test session, as well as the total 3 minute period. A subset of the data were analyzed by a second experimentally blind observer for determination of inter-observer reliability. Correlation analyses showed high observer reliability (r =.75 – 1.0, all p s <.01), and t-tests revealed no significant differences between any rater (all ps >.33).
Raw exploration times for each object were converted to an exploration ratio. This is the proportion of time spent exploring the novel object or novel place during each minute of the 3-min Testing phase divided by the total time spent exploring both objects [tnovel / (tnovel + tsample)[1]]. All statistical analyses were conducted using STATISTICA 12 software. Mixed factorial analysis of variance (ANOVA) was conducted with Habituation (Group 1 vs. Group 3), Task (OR vs. OL) and Sex as between subject factors. The exploration ratios (overall and single-minute) were used as the dependent variables. Post-hoc (Newman-Keuls) analyses were conducted where appropriate. Additionally, one-sample t-tests were used to compare exploration ratios of each group against a ratio (0.5) indicating no preference [1]. Data collected during the Sample phase were also analyzed to examine the amount of time spent exploring between groups.
2.2 Results
2.2.1 Subjects
Subjects were 103 Long-Evans rats derived from 17 litters. Averaging together same-sex littermates that were assigned to the same group, reduced the number of observations from 103 to 78. Data from an additional 9 animals were excluded from the analyses. Two were excluded due to technical failure (Spatial Task, Group 1, F, n=1; Spatial Task, Group 3, M, n=1) and 2 were removed because total object exploration during the testing phase was <1 sec. (Object Task, Group 1, F, n=1; Object Task, Group 1, M, n=1); and data from five animals were excluded from the analyses for meeting the criteria of a statistical outlier (Object Task, Group 1, F, n=1; Spatial Task, Group 1, M, n=1; Spatial Task, Group 1, F, n=1; Spatial Task, Group 3, F, n=2). Outliers were defined by individual session testing data with total exploration ratios that exceeded ± 1.96 standard deviations from the group mean. Analyses were conducted on the remaining 69 subjects (Object Task, Group 1, n=16; Object Task, Group 3, n=18; Spatial task, Group 1, n=15; Spatial Task, Group 3, n=20).
2.2.2 Sample Phase (Figure 4)
Figure 4.
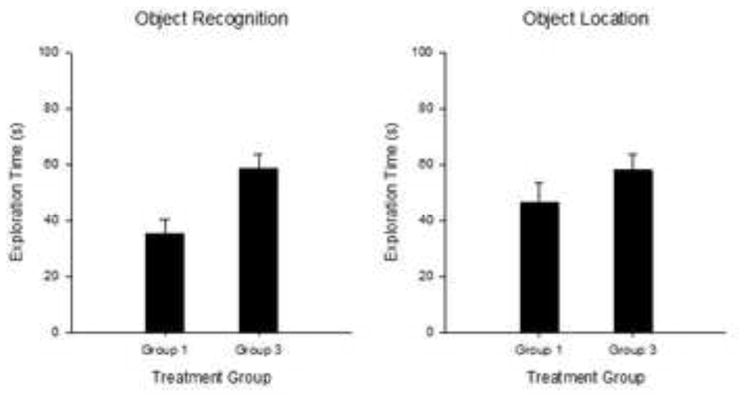
Mean (+/− SE) Object exploration time (s) during the 5-min Sample Phase for Experiment 1A. OR (left panel) and OL tasks (right panel). Group 1 = 1 habituation session to the empty arena; Group 3 = 3 habituation sessions. OR, Group 1 n=16; Group 3 n=15; OL, Group 1 n=18; Group 3 n=20.
In order to determine whether differences in novelty exploration could be explained by variation in baseline exploratory levels during the sample phase, the total amount of exploration during the sample phase for Task, Habituation and Sex were analyzed using a mixed factorial ANOVA. ANOVA revealed a main effect of Group [F(1,61)=8.06, p<0.007], with increased sample time exploration in subjects given 3 compared to 1 habituation sessions.
2.2.3 Testing Phase (Figure 5)
Figure 5.
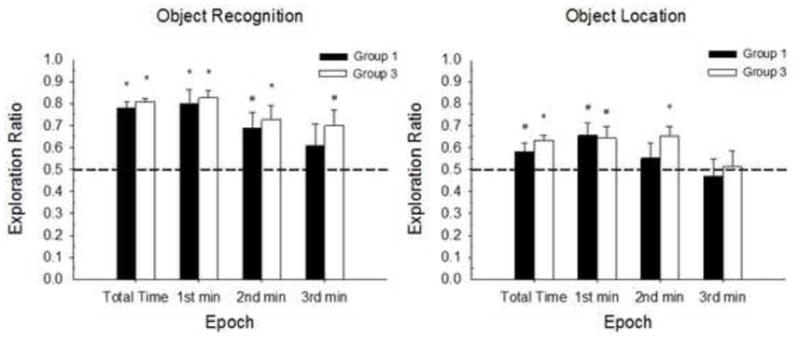
Mean (+/− SE) Exploration ratios during the 3-min Testing Phase for Experiment 1A. OR (left panel) and OL tasks (right panel). From left to right, Epoch includes total testing time, 1st min, 2nd min and 3rd min. Group 1 = 1 habituation session to the empty arena; Group 3 = 3 habituation sessions. Both groups show significant preference for the novel object and novel location. Group n s for the total time, 1st min, 2nd min and 3rd min of testing for OR: Group 1 n=16, 16, 13, 12; Group 3 n=18, 18, 17, 15; OL: Group 1 n=15, 15, 15, 14; Group 3 n=20, 20, 20, 17. Dotted line indicates chance performance. Significant differences from chance performance are indicated for each group (*p < .01; #p < .05).
A factorial ANOVA on the total exploration time ratio with Sex, Habituation and Task as factors, revealed a main effect of Task [F(1,61)=46.69, p<0.001], with significantly greater exploration time ratios for the Object compared to the Spatial task. Exploration ratios (and the number rats showing exploration) declined somewhat across minutes of testing.
One-sample t-tests on exploration ratios compared to chance performance revealed high novel object preference, regardless of habituation group. For both Group 1 and Group 3, object novelty preference decreased throughout the testing session, suggesting the 1st min exploration scores may be a better predictor of novelty preference [Group 1 total time: t(15)=10.13, p<.001; 1st min: t(15)=4.8, p<.001; 2nd min: t(12)=2.74, p<.018; 3rd min: t(10)=1.05, p>.318; Group 3 total time: t(17)=18.48, p<.001; 1st min: t(17)=10.53, p<.001; 2nd min: t(16)=3.54, p<.003; 3rd min: t(14)=2.73, p<.017]. Group 1 and Group 3 displayed significant spatial novelty preference [Group 1 total time: t(14)=2.17, p<.048; 1st min: t(14)=2.82, p<.014; 2nd min: t(14)=0.74, p>.471; 3rd min: t(13)=−.039, p>.704; Group 3 total time: t(19)=5.51, p<.001; 1st min: t(19)=2.69, p<.015; 2nd min: t(19)=3.46, p<.003; 3rd min: t(16)=0.14, p>.887]. However, spatial novelty learning and performance was decreased compared to object novelty, suggesting the spatial novelty assessed here may be a weaker form of learning.
3. Experiment 1B: Ontogeny of object and spatial location memory
The ability of animals to express learning of spatial tasks develops around PD21 in the rat, whereas performance of non-spatial control tasks develops earlier (e.g., [4–6, 49]. Experiment 1B extends the findings of Experiment 1A by examining object vs. spatial novelty in PD21 and 26 rats. This experiment seeks converging evidence on previous developmental studies of spatial learning that examined spatial learning that is “incidental” rather than “reinforcement-driven” [11, 45, 49].
3.1 Materials & Methods
3.1.1 Subjects
The animal care and maintenance were the same as in Experiment 1A. In order to avoid imposing the weaning manipulation during behavioral testing, animals in Group PD21 remained with the dam until completion of testing on PD23. Animals in Group PD25 were weaned on PD21 and individually housed on PD23, as previously described. Weaning did not affect the age comparison (see Results below) and weaning does not affect OL or OR performance on PD25 [50].
3.1.2 Apparatus and Procedure
The apparatus and procedure used for Experiment 1B was the same as in Experiment 1A in every respect except that all subjects received a total of 3 habituation sessions and the home cage for PD21 rats involved group housing with their dam. PD21 rats were transported to and from the behavioral testing rooms in individual white polypropylene cages (the same as those described as the home cages for PD26 rats).
3.1.3 Data Collection & Analysis
All data acquisition and analyses for Experiment 1B were the same as Experiment 1A. Statistical analyses included ANOVA with Age Group (Group PD21 vs. Group PD25), Task (OR vs. OL) and Sex as between subjects factors. As in Experiment 1A, one-sample t-tests were used to compare exploration ratios of each group against chance performance.
3.2 Results
3.2.1 Subjects
Subjects were 78 (37 M; 41 F) Long-Evans rats derived from 14 litters. Instances in which rats in the same litter were represented by the same grouping variables (Task, Age, Sex) were averaged as previously described. This reduced the number of observations to 65. After accounting for same-litter sampling, data from four animals met the criteria as a statistical outlier (previously described) and were excluded from the analyses (Object Task, PD 21, F, n=1; Spatial Task, PD 21, F, n=1; Object Task, PD 26, F, n=1; Spatial Task, PD 26, M, n=1). Data from the remaining 61 subjects were used in the analyses (Object Task, PD 21, n=14; Object Task, PD 26, n=17; Spatial task, PD 21, n=13; Spatial Task, PD 26, n=17).
3.2.2 Sample Phase (Figure 6)
Figure 6.
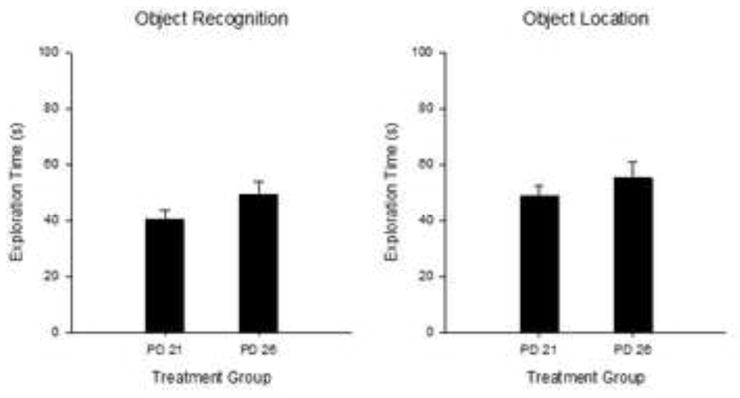
Mean (+/− SE) Object exploration time (s) during the 5-min Sample Phase for Experiment 1B. OR (left panel) and OL tasks (right panel). No significant differences were found between age group for either task. OR, PD21 n=14; PD26 n=17; OL, PD21 n=13; PD26 n=17.
Comparison of the baseline exploration levels in the sample phase between Task, Age and Sex was conducted using a mixed factorial ANOVA. The ANOVA showed no significant main effects or interactions (all ps >0.13), showing that there was no statistical difference in exploration time between rats tested on PD21 (44.63 ± 2.99 sec) or PD26 (51.9 ± 3.5 sec) which could have accounted for the differences in novelty preference at testing.
3.2.3 Testing Phase (Figure 7)
Figure 7.
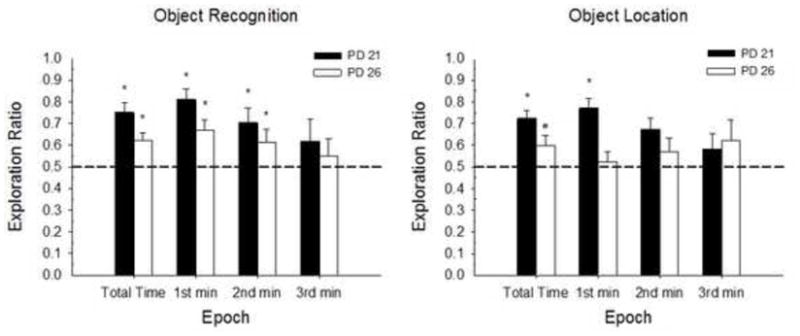
Mean (+/− SE) Exploration ratios during the 3-min Testing Phase for Experiment 1B. OR (left panel) and OL tasks (right panel). From left to right, Epoch includes total testing time, 1st min, 2nd min and 3rd min. Both age groups show significant preference for the novel object, as well as novel location. Group n s for the total time, 1st min, 2nd min and 3rd min of testing for OR: PD21 n=14, 14, 14, 13; PD26 n=17, 17, 17, 17; OL: PD21 n=13, 13, 13, 11; PD26 n=17, 17, 16, 15. Dotted line indicated chance performance. Significant differences from chance performance are indicated for each group (*p < .01; #p < .05).
A factorial ANOVA conducted on the total exploration time ratio with Sex, Age, and Task as factors, revealed a main effect of Task [F(1, 53)=8.46, p<0.006], with significantly greater exploration time ratios for the OR compared to the OL task. No other main or interaction effects were significant (ps > 0,48).
One-sample t-tests on exploration ratios compared to chance performance (0.5) revealed high novel object preference, regardless of age [PD21 total time: t(13)=5.96, p<.001; 1st min: t(13)=6.63, p<.001; 2nd min: t(12)=3.07, p<0.10; 3rd min: t(13)=1.16, p>0.26; PD26 total time: t(16)=6.31, p<.001; 1st min: t(16)=6.32, p<.001; 2nd min: t(16)=3.42, p<.004; 3rd min: t(16)=1.13, p>.274]. Both ages, PD21 and PD26, showed significant spatial novelty preference as well. However, the spatial novelty learning and performance was not as high as that of object novelty [PD21 total time: t(12)=3.16, p<.009; 1st min: t(12)=3.3, p<.007; 2nd min: t(12)=1.91, p>.08; 3rd min: t(10)=0.66, p>.523; PD26 total time: t(16)=2.13, p<.049; 1st min: t(16)=0.47, p>.64; 2nd min: t(15)=1.14, p>.271; 3rd min: t(14)=1.32, p>.206].
Taken together, Experiments 1A and 1B indicate that both incidental object and spatial learning are present as early as PD21. Performance on OR was higher than on OL at all ages but performance on neither task changed significantly in strength across the weanling/juvenile period. We discuss this finding in relation to other developmental studies of incidental learning below (Discussion).
4. Experiment 2: The effect of NMDA receptor antagonism on object vs. spatial novelty
NMDA receptor function in areas such as the hippocampus and prefrontal cortex has been implicated in the postnatal development of various forms of contextual and spatial memory (e.g., [45, 51]. In Experiment 2, PD31 rats received systemic administration of an NMDA receptor antagonist or vehicle prior to acquisition in the novel object and spatial recognition tasks in order to determine whether learning and performance of these tasks (especially spatial novelty) is NMDA-dependent in juveniles, as seen in adult rats [25, 26].
4.1 Materials & Methods
4.1.2 Subjects and Apparatus
Animal care, maintenance, and apparatus were all the same as in Experiment 1A.
4.1.3 Handling
All handling procedures for Experiment 2 were the same as in Experiment 1A. However, a subset of subjects (n=21) were inadvertently not handled prior to placement in the arena for Sessions 1–3 (experimenter error). Because data from these subjects did not differ from their handled counterparts, they were included in the analysis.
4.1.4 Habituation
All habituation procedures were the same as Experiment 1B (the 3-exposure condition from Experiment 1A).
4.1.5 Drug Treatment
MK-801 (dizocilpine maleate) was commercially purchased from Tocris (Ellisville, Missouri) and dissolved in 0.9% sterile saline prior to the experiment. MK-801 was given via intraperitoneal injection of 0.06 mg/kg in a volume of 1.0 ml/kg. Control rats received an intraperitoneal injection of 0.9% sterile saline in a 1.0 ml/kg volume. This dose was taken from our previous studies of weanling-juvenile rats [52].
4.1.6 Novel Object and Location Recognition
Both task procedures were completed identically to Experiment 1A except that animals were given MK-801 or saline injections 30–32 min prior to the Sample phase of Session 4. All rats waited in the animal housing room between drug treatment and behavioral testing.
4.1.7 Data Collection & Analysis
All data acquisition and analyses for Experiment 2 were the same as in the previous experiments. Statistical analyses included ANOVA with Treatment Group (MK-801 vs. Saline), Task (OR vs. OL) and Sex as between subjects factors. All other statistical analyses were conducted as in Experiments 1A and 1B.
4.2 Results
4.2.1 Subjects
Subjects were 58 (30M; 28F) Long-Evans rats derived from 9 litters. Data from a total of 3 animals were excluded from the analyses. Data from 1 animal was removed due to a total of <1 sec. sample object exploration (Spatial Task, SAL, F). Data from 2 animals were excluded from the analyses for meeting the criteria as a statistical outlier, as previously defined (Spatial Task, SAL, F, n=1; Object Task, MK, M, n=1). Analyses were conducted on the remaining 55 subjects (Object Task, MK, n=13; Object Task, SAL, n=15; Spatial task, MK, n=15; Spatial Task, SAL, n=12).
4.2.2 Sample Phase (Figure 8)
Figure 8.
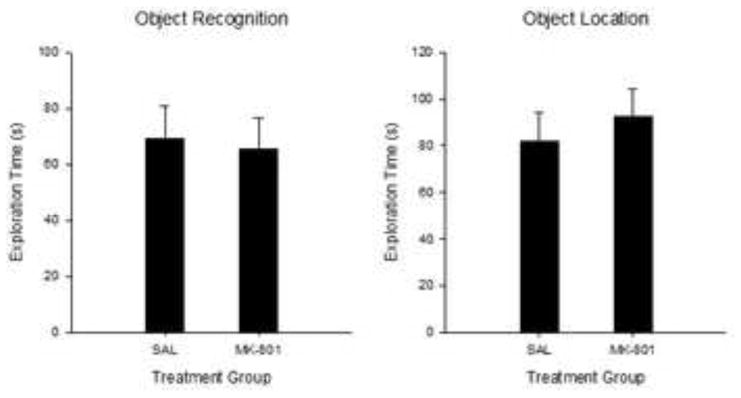
Mean (+/− SE) Exploration time (s) during the 5-min Sample Phase for Experiment 2. OR (left panel) and OL tasks (right panel). No significant differences were found between treatment groups for either task. OR, SAL n=15; MK n=13; OL, SAL n=12; MK n=15.
In order to determine whether differences in novelty exploration could be explained by variation in baseline exploratory levels between drug groups, the total amount of exploration during the sample phase for Task, Treatment and Sex were analyzed using a mixed factorial ANOVA. ANOVA revealed no main or interaction effects of any grouping factor (all ps > .10)
4.2.3 Testing Phase (Figure 9)
Figure 9.
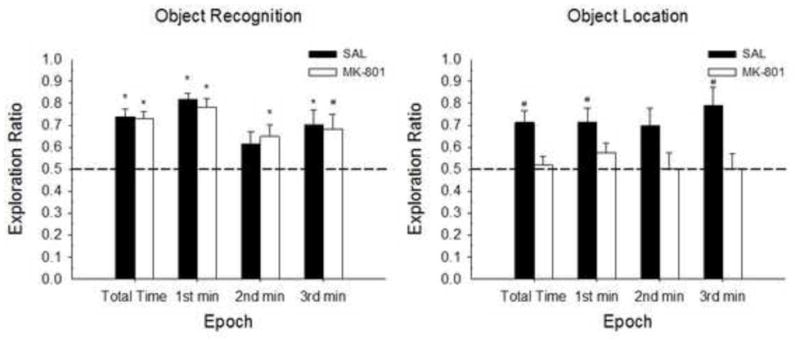
Mean (+/− SE) Exploration ratios during the 3-min Testing Phase for Experiment 2. OR (left panel) and OL tasks (right panel). From left to right, Epoch includes total testing time, 1st min, 2nd min and 3rd min. MK-801 impaired performance of the OL task but not the OR task. Group n s for the total time, 1st min, 2nd min and 3rd min of testing for OR: SAL n=15, 15, 15, 15; MK n=13, 13, 13, 13 and OL: SAL n=15, 15, 15, 15; MK n=12, 12, 12, 10. Dotted line indicated chance performance. Significant differences from chance performance are indicated for each group (*p < .01; #p < .05).
A factorial ANOVA on the total exploration time ratio with Sex, Treatment and Task as factors, revealed a main effect of Task [F(1,47)=7.11, p<0.02], with significantly greater exploration time ratios for the Object compared to the Spatial task and a main effect of treatment [F(1,47)=7.02, p<0.011] with greater exploration times for Group SAL compared to Group MK. A significant Task X Treatment interaction was also found [F(1,47)=5.55, p<0.023]. Newman-Keuls tests indicated significantly greater total spatial novelty scores for Group SAL compared to Group MK (p<.002) and significantly higher scores for the Object compared to the Spatial Task for Group MK (p<.003). No other main or interaction effects were significant (all ps>0.21).
One-sample t-tests on exploration ratios compared to chance performance revealed high novel object preference, regardless of Treatment group. As in Experiment 1A, object novelty preference decreased throughout the testing session, suggesting the 1st min exploration scores may be a better predictor of novelty preference; and this was evident for both MK and SAL groups. [Group SAL total time: t(14)=6.77, p<.001; 1st min: t(14)=12.33, p<.001; 2nd min: t(14)=1.61, p>.0.13; 3rd min: t(14)=3.07, p<.009; Group MK total time: t(12)=20.86, p<.001; 1st min: t(12)=18.72, p<.001; 2nd min: t(12)=12.26, p<.001; 3rd min: t(12)=9.39, p<.001]. In contrast to the OR task, drug treatment significantly impaired performance on the OL task. Group SAL displayed significant spatial novelty preference; however, Group MK indicated no difference in spatial preference scores compared to chance performance [Group SAL total time: t(11)=3.93, p<.003; 1st min: t(11)=3.23, p<.001; 2nd min: t(11)=2.45, p<.033; 3rd min: t(9)=3.41, p<.008; Group MK total time: t(14)=0.41, p>.687; 1st min: t(14)=1.7, p>.111; 2nd min: t(14)=0.04, p>.97; 3rd min: t(14)=0.07, p>.946]. The lack of novel spatial recognition for Group MK, but not Group SAL, highlights the influence of NMDAr function in this spatial task variant. That this effect is not evident in the object task suggests that the drug did not impair OL performance by interfering with sensory, motor, and motivational processes that are common to both the OR and OL tasks.
5. Experiment 3: The effect of neonatal alcohol exposure on object and spatial location memory
Neonatal alcohol exposure has been found to disrupt a variety of spatial learning tasks, including context representations incidentally acquired during the Context Preexposure Facilitation Effect (CPFE) [27, 28]. Using an identical alcohol dose and window of exposure (PD7–9), Experiment 3 asked if these findings would extend to another incidental form of spatial learning, novel location recognition.
5.1 Materials & Methods
5.1.1 Subjects
The animal care and maintenance were the same as in previous experiments.
5.1.2 Alcohol Dosing
Pups were given a single binge dose of alcohol (5.25g/kg; Group 5.25g) or were sham intubated (SI; Group SI) for 3 days from PD7 through PD9. All procedures were completed following those previously reported from this lab [27]. Beginning on PD7, pups were placed onto plastic containers resting on a heating pad, set to the lowest setting. Alcohol was mixed with a custom milk formula prior to administration [53] at a dose of 5.25g/kg/day (23.93% v/v). All pups were weighed to determine the solution volume to be administered (0.0278 mL/g). Single binge-like dose administration occurred via intragastric intubation. A 1 mL syringe used to infuse the solution was attached to a thin tube (PE10), which was lubricated with commercially available corn oil. The tube was gently passed down the esophagus and into the stomach where the solution was infused over a 5–10s interval. Alcohol administration was followed 2h later by an equal volume of the milk formula only. A second formula infusion was administered only on PD7. Sham intubations involved the identical process, except that no solution was infused.
5.1.2 Blood Alcohol Concentration Analysis
Two hours after the first alcohol/sham administration (PD7) a blood sample was collected in a heparinized capillary tube from Group 5.25g following a small tail clip. Blood samples from Group SI were collected then disposed. Samples from Group 5.25g were centrifuged and plasma was collected and stored at −20°C. Blood alcohol concentrations (BACs) were determined using an Analox GL5 Analyzer (Analox Instruments, Luneburg, MA) as previously described [54].
5.1.2 Apparatus & Procedure
All equipment, handling, habituation, and task procedures were identical to those used previously, including the 3-habituation-session condition used in Experiments 1A, 1B, and 2.
5.1.3 Data Collection & Analysis
Weight gain over the dosing period (PD7–9) was analyzed with a repeated measures ANOVA with the between subjects factors of Sex and Treatment Group and the within-subjects factors of age. Additionally, body weights during testing (PD31) were examined with a factorial ANOVA with Sex and Treatment Group as factors. A one-way ANOVA assessed differences between group BACs and Sex.
Statistical analyses included ANOVA with Treatment Group (5.25g vs. SI), Task (OR vs. OL) and Sex as between subjects factors. All data acquisition and analyses were also the same as described previously.
5.2 Results
5.2.1 Subjects
Subjects were 96 (47M, 49F) Long-Evans rats derived from 14 litters. Averaged data from same litter oversampling of Task, Treatment, Sex occurred on 2 instances (Spatial Task, SI, F, n=2). Data from 1 animal were excluded due to experimenter error (Object Task, SI, M, n=1). Data from a total of 6 animals were excluded from the analyses. Data from 2 animals were removed due to a total of <1 sec. testing object exploration (Object Task, 5.25g, M, n=1; Spatial Task, 5.25g, M, n=1). Data from 5 animals were excluded from the analyses for meeting the criteria as a statistical outlier (Object Task, 5.25g, M, n=1; Object Task, SI, M, n=1; Spatial Task, 5.25g, F, n=1; Spatial Task, 5.25g, M, n=2). Analyses were conducted on the remaining 86 subjects (Object Task, 5.25g, n=19; Object Task, SI, n=23; Spatial task, 5.25g, n=19; Spatial Task, SI, n=25).
5.2.2 Blood Alcohol Concentration Analysis and Body Weights
A 2 (Treatment Group) X 2 (Sex) X 2 (Days) repeated measures ANOVA revealed significant main effects of Sex [F(1, 80)=4.01, p<0.05], of Treatment Group [F(1, 80)=22.17, p<0.01], and of Days [F(1, 80)=525.3, p<0.01], in addition to a significant Days X Dosing Condition interaction [F(1, 80)=510.1, p<0.01]. The main effect of Sex demonstrated that females had reduced body weights compared to males (15.7g ± 0.31 vs. 16.5g ± 0.32, respectively). Newman-Keuls post-hoc tests of the Days X Dosing Condition interaction showed that weights did not differ across groups (p>0.36) on PD7 (Group SI, 14.7g ± 0 .24; Group 5.25g and 15.1 ± 0.28). On PD9, Group 5.25g (14.7g ± .29), weighed significantly less than Group SI (18.9g ± .34; p<0.01).
A 2 (Treatment Group) X 2 (Sex) factorial ANOVA performed on PD31–32 weights revealed significant main effects of Treatment Group [F(1, 79) = 10.03, p< 0.01] and Sex [F(1, 79) = 16.04, p< 0.01]. Group 5.25g weighed less than Group SI (89.6 ± 2.1g vs. 96 ± 1.6g, respectively) and females showed reduced body weights compared to males (89.1g ± 1.6 vs. 98g ± 2.0g, respectively).
BACs taken from blood samples of Group 5.25g on PD7 was not influenced by Sex (p> 0.69). Samples taken from Group 5.25g (n=37 out of 38) showed an average BAC of 412.0 ± 7.56 mg/dl.
5.2.3 Sample Phase (Figure 10)
Figure 10.
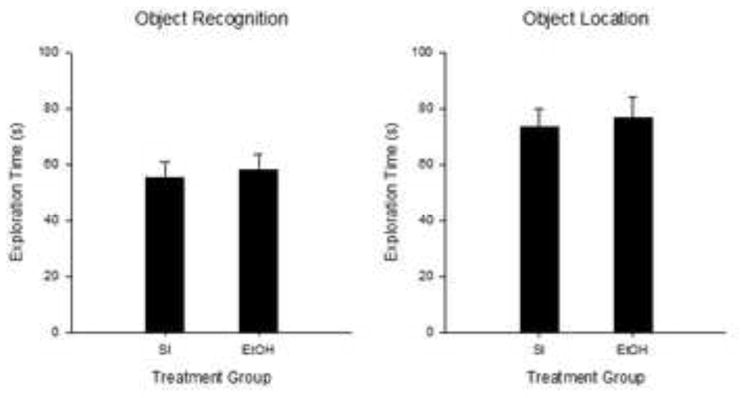
Mean (+/− SE) Exploration time (s) during the 5-min Sample Phase for Experiment 3. OR (left panel) and OL tasks (right panel). No significant differences were found between treatment groups for either task. OR, SI n=23; 5.25g n=19; OL, SI n=25; 5.25g n=19.
In order to ensure developmental alcohol exposure did not affect baseline exploratory levels, the total amount of exploration during the sample phase for Task, Treatment, and Sex were analyzed using a mixed factorial ANOVA. ANOVA revealed only a main effect of Task [F(1,78)=8.0, p<0.006] and no interaction effects involving any other grouping variable (Fs<0.77).
5.2.4 Testing Phase (Figure 11)
Figure 11.
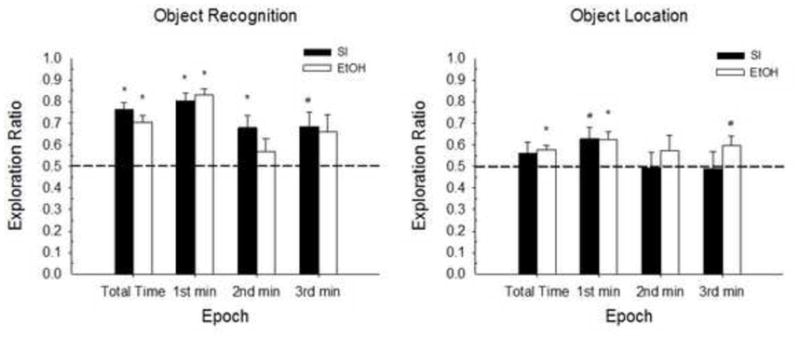
Mean (+/− SE) Exploration ratios during the 3-min Testing Phase for Experiment 2. OR (left panel) and OL tasks (right panel). From left to right, Epoch includes total testing time, 1st min, 2nd min and 3rd min. Both treatment groups show significant preference for the novel object and novel location. Group n s for the total time, 1st min, 2nd min and 3rd min of testing for OR: SI n=23, 23, 20, 21; 5.25g n=19, 19, 18, 17 and OL: SI n=25, 23, 23, 23; 5.25g n=19, 19, 18, 19. Dotted line indicated chance performance. Significant differences from chance performance are indicated for each group (*p < .01; #p < .05).
A factorial ANOVA on the total exploration time ratio with Sex, Treatment and Task as factors, revealed a main effect of Task [F(1,78)=17.49, p<0.001], with significantly greater exploration time ratios for the Object compared to the Spatial task. There were no other main or interaction effects involving any grouping variable (Fs<0.83).
One-sample t-tests on exploration ratios compared to chance performance revealed high novel object preference, regardless of treatment [Group SI total time: t(22)=8.16, p<.001; 1st min: t(22)=8.56, p<.001; 2nd min: t(19)=3.19, p<.005; 3rd min: t(20)=2.81, p<.02; Group 5.25g total time: t(18)=6.2, p<.001; 1st min: t(18)=10.82, p<.001; 2nd min: t(17)=1.09, p>.292; 3rd min: t(16)=1.98, p>.066]. Both Group SI and Group 5.25g indicated significant spatial novelty preference [Group SI total time: t(24)=1.20, p>.241; 1st min: t(22)=2.54, p<.019; 2nd min: t(22)=−0.09, p>.929; 3rd min: t(22)=−.138, p>.891; Group 5.25g total time: t(18)=4.11, p<.001; 1st min: t(18)=3.37, p<.004; 2nd min: t(18)=1.04, p>.31; 3rd min t(17)=2.23, p<.04]. Intact novel spatial recognition for Group 5.25g suggests that this task is not sensitive to neonatal alcohol during this treatment window (PD7–9) and with our current spatial task parameters.
6. General Discussion
Four experiments behaviorally characterized the non-spatial OR (novel object recognition) task and the spatial OL (object location) task during development in the rat. Experiment 1A demonstrated the ability of normal-developing juvenile rats to perform both the OR and OL tasks. Experiment 1B extended these findings to pups as young as PD21. Experiment 2 determined that performance of the OL but not OR task requires NMDA receptor function. Lastly, Experiment 3 found that neonatal alcohol exposure does not disrupt performance in either task.
Experiment 1 showed that the ability of rats to perform both the OR and OL tasks is apparent by PD31. Previous reports have demonstrated performance of the OR task by PD18–24, with delays ranging from 1min to 1hr between the sample and testing phases [2, 3, 47, 48, 55, 56]. However, data concerning when spatial object recognition emerges is conflicting [2, 3, 48]. Based on these reports, Experiment 1 tested a juvenile age (PD31) which was expected to show evidence of novelty exploration in the OL task, in order to confirm test parameters in our lab. Subsequently, the optimal conditions (3 total habituation sessions, object identity for the OL task) were used to examine the ontogeny of these tasks (Experiment 1B).
The present report shows that the OL task can be performed in pups tested as young as PD21. Recently, Ainge & Langston reported the emergence of an object-in-place (OiP;[16]) task by PD30; however this ability was not seen in pups at PD24 [2]. In comparison, Kruger et al. demonstrated performance in the OL task by PD15–17 [3]. This discrepancy may be due to the variation in task demands. Kruger et al. incorporated an OL task similar to the one used in the current report, in which a familiar object was moved to a different spatial location which was never experienced during the sample phase. Ainge & Langston, however, presented two distinct objects during the sample phase and two identical objects during the testing phase. In this manner, one of the testing objects was presented in the same location as in the sample phase, but the other object was situated in the location that was previously occupied by the different object. The OL task used in the current report differs from the OiP task used by Ainge and Langston in two ways. First, in the latter task, processing both object identity and object location information is necessary whereas processing object identity information is not required in the OL task. Secondly, in order to make their task hippocampal dependent, Ainge & Langston used different start locations on different trials or between the sample and test phase of a trial. This is thought to promote the association between objects and their locations in an allocentric spatial framework [57]. In support of Kruger et al., then, the current findings extend this previous developmental work by demonstrating the ability of developing rats tested on PD21, 26 and 31 to distinguish familiar objects that have changed spatial location. Further research is needed to determine how differences between the paradigms used by Ainge & Langston versus Kruger et al., and the present study may account for the variation in developmental onset of spatial processing of objects.
The ability of PD21 rats to recognize familiar objects presented in a novel location indicates that incidental spatial learning has emerged during the weanling period of development. Another form of incidental learning, the context preexposure facilitation effect (CPFE) is a variant of contextual fear conditioning in which acquisition of the context representation, association of the representation with shock and retrieval of that association occur on separate days [58]. Typically, rats given exposure to the shock context on the first day display increased levels of freezing to the same context during testing on the final day. The contextual memory acquired on the first day is incidental due to the absence of reinforcement. The CPFE develops around PD21 [7, 49] and requires HPC function during acquisition of the context on PD24 [45]. The finding that neonatal hippocampal lesions disrupt OL performance by PD15–17 [3] contrasts with the absence of the CPFE at this age. One difference between these two incidental learning paradigms is the addition of a long-term consolidation component in the CPFE, compared to the OL task. It may be that pups as young as PD15–17 would not be able to perform the OL task if a longer delay were imposed between the sample and testing phases (e.g., 24-hr). In general, though, the ontogenetic profiles of the incidental learning OL task presented here are consistent with other reinforcement-driven and incidental hippocampal- dependent tasks [5, 59–62]
The NMDA glutamate receptor subtype has been widely implicated in synaptic plasticity and various forms of behavioral learning and memory [63, 64]. Since the OR and OL tasks recruit brain areas containing a high density of NMDArs [65], Experiment 2 sought to determine the role of these receptors in both tasks. Experiment 2 showed that NMDAr function is necessary for performance in the OL but not OR task. However, other studies report impairment of OR following NMDAr antagonist administration depending on the retention interval. In contrast to the 5-min delay used here, systemic and intrahippocampal NMDA antagonists administered prior to acquisition in the OR task disrupt performance with delays ranging from 1 to 24hr [20–23, 25, 66]. It may be that the OR task becomes increasingly difficult as the delay increases, possibly requiring greater NMDAr involvement and/or additional brain structures (e.g., HPC and/or PFC; but see, [67]).
In adult animals, NMDAr antagonists disrupt performance on variants of the OL task when administered both systemically [25] or via localized AP5 infusions into dentate gyrus, CA3 and CA1 [26]. Conversely, Ozawana et al. report enhanced performance following systemic administration of D-cycloserine (DCS; an NMDAR agonist) either before or after encoding or prior to testing [68]. The role of NMDArs has also been implicated in other hippocampal-dependent incidental learning tasks during adulthood, including spontaneous alternation and the CPFE [69, 70]. However, the current report is the first to administer an NMDAr antagonist during performance of both the OR and OL tasks in juvenile rats. The sensitivity of the OL task to NMDA disruption at this age confirms previous studies from our lab involving various reinforcement-driven spatial tasks that engage a variety of brain regions [43, 51, 71]. The present findings extend this developmental work from our lab by highlighting the function of NMDArs in the OL versus OR tasks.
To our knowledge, the current report is the first to examine the developmental effects of neonatal ethanol exposure in either the OR or OL task in juvenile rats. When testing occurs in adulthood, prenatal alcohol exposure has no effect in a reinforced delayed non-match to sample (DNMS) object recognition task, with delays of up to 5-min between the sample and testing phase [72]. Intact OR performance has also been demonstrated during adulthood following ethanol injections administered on PD7, with a 30-min delay [73]. On the other hand, one study using mice reported impaired performance following PD7 ethanol injections in the OL task during adulthood, using a 24hr retention interval [74]. Similar alcohol-induced deficits are evident in the CPFE which requires that incidental spatial learning is retrieved after a 24-hr retention interval [27, 28], see above. In light of the current findings, then, it may be that neonatal alcohol exposure would impair the OL task if a longer delay was imposed between the sample and testing phase.
The current set of experiments sheds light onto various mechanisms involved in memory of object identity and object location during development. The finding that performance of the OL task is present by PD21, along with our previous reports demonstrating the CPFE by this same age, provides converging evidence for the development of incidental spatial learning around the time of weaning. Experiment 2 extends previous studies of adult rats by demonstrating a role of NMDArs in the OL but not OR task during the juvenile period of development. Finally, the current report is the first to examine the effect of neonatal alcohol exposure on performance in these tasks in the juvenile rat. While results from Experiment 3 indicate a lack of an alcohol effect at a short retention interval, it is possible that alcohol would disrupt performance if a longer delay is imposed between the sample and testing phases (e.g., 24hr [74]). The results reported here provide a foundation for additional empirical work characterizing the ontogenetic emergence, the role of NMDARs and neonatal ethanol exposure in memory for object identity and their spatial locations.
Highlights.
Object and spatial novelty recognition is evident around the time of weaning
Spatial recognition requires NMDA receptor function in juvenile rats
Neonatal alcohol exposure does not impair novel object or spatial recognition
Acknowledgments
The authors would like to thank Lisa B. Dokovna for assistance with behavioral data collection and the University of Delaware Office of Laboratory Medicine (OLAM) for the care of the animals. The study was supported by 1-R21-HD070662-01.
Abbreviations
- BAC
blood alcohol concentration
- CPFE
context preexposure facilitation effect
- FASDs
fetal alcohol spectrum disorders
- GD
gestational day
- NMDAr
NMDA-receptor
- OiP
object-in-place task
- OL
object location task
- OR
object recognition task
- PD
postnatal day
- SI
sham intubated
Footnotes
Conflict of Interest
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mumby DG, Glenn MJ, Nesbitt C, Kyriazis DA. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learning and Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ainge JA, Langston RF. Ontogeny of neural circuits underlying spatial memory in the rat. Frontiers in Neural Circuits. 2012:6. doi: 10.3389/fncir.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krüger H, Brockmann MD, Salamon J, Ittrich H, Hanganu-Opatz IL. Neonatal hippocampal lesion alters the functional maturation of the prefrontal cortex and the early cognitive development in pre-juvenile rats. Neurobiol Learn Mem. 2012;97:470–481. doi: 10.1016/j.nlm.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behavioral Neuroscience. 1993;107:877–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- 5.Green RJ, Stanton ME. Differential ontogeny of working memory and reference memory in the rat. Behavioral Neuroscience. 1989;103:98–105. doi: 10.1037//0735-7044.103.1.98. [DOI] [PubMed] [Google Scholar]
- 6.Rudy JW, Paylor R. Development of interocular equivalence of place learning in the rat requires convergence sites established prior to training. Behavioral Neuroscience. 1987;101:732–734. doi: 10.1037//0735-7044.101.5.732. [DOI] [PubMed] [Google Scholar]
- 7.Burman MA, Murawski NJ, Schiffino FL, Rosen JB, Stanton ME. Factors governing single-trial contextual fear conditioning in the weanling rat. Behavioral Neuroscience. 1009;123:1148–1152. doi: 10.1037/a0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumas TC, Rudy JW. Development of the hippocampal memory system: Creating networks and modifiable synapses. In: Blumberg MS, Freeman JR, Robinson SR, editors. Handbook of Developmental Behavioral Neuroscience. Oxford University Press; 2010. pp. 587–606. [Google Scholar]
- 9.Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behavioral Neuroscience. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- 10.Freeman JH, Jr, Stanton ME. Fimbria-fornix transections disrupt the ontogeny of delayed alternation but not position discrimination in the rat. Behavioral Neuroscience. 1991;105:386–395. doi: 10.1037//0735-7044.105.3.386. [DOI] [PubMed] [Google Scholar]
- 11.Douglas RJ. The development of hippocampal function: implications for theory and for therapy. In: Isaacson RL, Pribram KH, editors. The Hippocampus. 2. Plenum Press; 1975. pp. 327–361. [Google Scholar]
- 12.Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learning and Memory. 2009;107:887–891. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 14.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 15.Aggleton JP, Albasser MM, Aggleton DJ, Poirier GL, Pearce JM. Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behavioral Neuroscience. 2010;124:55–68. doi: 10.1037/a0018320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MW, Barker GRI, Aggleton JP, Warburton EC. What pharmacological interventions indicate concerning the role of the perirhinal cortex in recognition memory. Neuropsychologia. 2012;50:3122–3140. doi: 10.1016/j.neuropsychologia.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? Journal of Neuroscience. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good MA, Barnes P, Staal V, McGregor A, Honey RC. Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behavioral Neuroscience. 2007;121:218–223. doi: 10.1037/0735-7044.121.1.218. [DOI] [PubMed] [Google Scholar]
- 19.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience & Biobehavioral Reviews. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 20.de Lima MNM, Laranja DC, Bromberg E, Roesler R, Schröder N. Pre- or post-training administration of the NMDA receptor blocker MK-801 impairs object recognition memory in rats. Behav Brain Res. 2005;156:139–143. doi: 10.1016/j.bbr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 21.van der Staay FJ, Rutten K, Erb C, Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav Brain Res. 2011;220:215–229. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 22.Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning and Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Winters BD, Bussey TJ. Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. Journal of Neuroscience. 2005;25:4243–4251. doi: 10.1523/JNEUROSCI.0480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larkin AE, Fahey B, Gobbo O, Callaghan CK, Cahill E, O’Mara SM, Kelly ÁM. Blockade of NMDA receptors pre-training, but not post-training, impairs object displacement learning in the rat. Brain Res. 2008;1199:126–132. doi: 10.1016/j.brainres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behavioral Neuroscience. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- 27.Murawski NJ, Stanton ME. Effects of Dose and Period of Neonatal Alcohol Exposure on the Context Preexposure Facilitation Effect. Alcoholism: Clinical and Experimental Research. 2011;35:1160–1170. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4–9. Behav Brain Res. 2010;212:133–142. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuropsychological Society. 2008;14:1022. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 32.Uecker A, Nadel L. Spatial locations gone awry: Object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia. 1996;34:209–223. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- 33.May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- 34.Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 35.Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol Teratol. 2003;25:519–528. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 36.Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. International Journal of Developmental Neuroscience. 2004;22:363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Goodlett CR, Johnson TB. Neonatal Binge Ethanol Exposure Using Intubation: Timing and Dose Effects on Place Learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- 38.Goodlett CR, Peterson SD. Sex Differences in Vulnerability to Developmental Spatial Learning Deficits Induced by Limited Binge Alcohol Exposure in Neonatal Rats. Neurobiol Learn Mem. 1995;64:265–275. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- 39.Murawski NJ, Jablonski SA, Brown KL, Stanton ME. Effects of neonatal alcohol dose and exposure window on long delay and trace eyeblink conditioning in juvenile rats. Behav Brain Res. 2013;236:307–318. doi: 10.1016/j.bbr.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Puglia MP, Valenzuela CF. Ethanol Acutely Inhibits Ionotropic Glutamate Receptor-Mediated Responses and Long-Term Potentiation in the Developing CA1 Hippocampus. Alcoholism: Clinical and Experimental Research. 2010;34:594–606. doi: 10.1111/j.1530-0277.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puglia MP, Valenzuela CF. Repeated third trimester-equivalent ethanol exposure inhibits long-term potentiation in the hippocampal CA1 region of neonatal rats. Alcohol. 2010;44:283–290. doi: 10.1016/j.alcohol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savage DD, Queen SA, Sanchez CF, Paxton LL, Mahoney JC, Goodlett CR, West JR. Prenatal ethanol exposure during the last third of gestation in rat reduces hippocampal NMDA agonist binding site density in 45-day-old offspring. Alcohol. 1992;9:37–41. doi: 10.1016/0741-8329(92)90007-w. [DOI] [PubMed] [Google Scholar]
- 43.Watson DJ, Stanton ME. Intrahippocampal administration of an NMDA-receptor antagonist impairs spatial discrimination reversal learning in weanling rats. Neurobiol Learn Mem. 2009;92:89–98. doi: 10.1016/j.nlm.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson DJ, Stanton ME. Medial prefrontal administration of MK-801 impairs T-maze discrimination reversal learning in weanling rats. Behav Brain Res. 2009;205:57–66. doi: 10.1016/j.bbr.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. 2011;95:190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reger ML, Hovda DA, Giza CC. Ontogeny of Rat Recognition Memory measured by the novel object recognition task. Dev Psychobiol. 2009;51:672–678. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson MJ, Barnes GW, Briggs JF, Ashton KM, Moody EW, Joynes RL, Riccio DC. Effects of ontogeny on performance of rats in a novel object-recognition task. Psychological Reports. 2004;94:437–443. doi: 10.2466/pr0.94.2.437-443. [DOI] [PubMed] [Google Scholar]
- 48.Ricceri L, Colozza C, Calamandrei G. Ontogeny of spatial discrimination in mice: A longitudinal analysis in the modified open-field with objects. Dev Psychobiol. 2000;37:109–118. doi: 10.1002/1098-2302(200009)37:2<109::aid-dev6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 49.Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Dev Psychobiol. 2012;54:714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westbrook SR. Using Object vs. Spatial Novelty Tasks to Study the Ontogeny of Spatial Learning. University of Delaware Undergraduate Honors Thesis; 2013. [Google Scholar]
- 51.Jablonski SA, Watson DJ, Stanton ME. Role of medial prefrontal NMDA receptors in spatial delayed alternation in 19-, 26-, and 33-day-old rats. Dev Psychobiol. 2010;52:583–591. doi: 10.1002/dev.20465. [DOI] [PubMed] [Google Scholar]
- 52.Chadman KK, Watson DJ, Stanton ME. NMDA receptor antagonism impairs reversal learning in developing rats. Behavioral Neuroscience. 2006;120:1071–1083. doi: 10.1037/0735-7044.120.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly SJ, Lawrence CR. Intragastric intubation of alcohol during the perinatal period. Methods in Molecular Biology. 2008;447:101–110. doi: 10.1007/978-1-59745-242-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown KL, Calizo LH, Stanton ME. Dose-Dependent Deficits in Dual Interstimulus Interval Classical Eyeblink Conditioning Tasks Following Neonatal Binge Alcohol Exposure in Rats. Alcoholism: Clinical and Experimental Research. 2008;32:277–293. doi: 10.1111/j.1530-0277.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 55.Heyser CJ, Ferris JS. Object exploration in the developing rat: Methodological considerations. Dev Psychobiol. 2013;55:373–381. doi: 10.1002/dev.21041. [DOI] [PubMed] [Google Scholar]
- 56.Krüger H, Hanganu-Opatz IL. Neonatal cholinergic lesion alters the acoustic structure of infant rat vocalization but not the early cognitive development. Dev Psychobiol. 2013;55:294–308. doi: 10.1002/dev.21029. [DOI] [PubMed] [Google Scholar]
- 57.Langston RF, Wood ER. Associative recognition and the hippocampus: Differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–1153. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- 58.Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning and Behavior. 1990;110:73–81. [Google Scholar]
- 59.Akers KG, Candelaria-Cook FT, Rice JP, Johnson TE, Hamilton DA. Cued platform training reveals early development of directional responding among preweanling rats in the Morris water task. Dev Psychobiol. 2011;53:1–12. doi: 10.1002/dev.20480. [DOI] [PubMed] [Google Scholar]
- 60.Stanton ME, Jensen KF, Pickens CV. Neonatal exposure to trimethyltin disrupts spatial delayed alternation learning in preweanling rats. Neurotoxicol Teratol. 1991;13:525–530. doi: 10.1016/0892-0362(91)90060-a. [DOI] [PubMed] [Google Scholar]
- 61.Rudy JW, Paylor R. Development of interocular equivalence of place learning in the rat requires convergence sites established prior to training. Behavioral Neuroscience. 1987;101:732–734. doi: 10.1037//0735-7044.101.5.732. [DOI] [PubMed] [Google Scholar]
- 62.Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- 63.Shapiro M. Plasticity, hippocampal place cells, and cognitive maps. Archives of Neurology. 2001;58:874–881. doi: 10.1001/archneur.58.6.874. [DOI] [PubMed] [Google Scholar]
- 64.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford University Press; 1978. [Google Scholar]
- 65.Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nilsson M, Hansson S, Carlsson A, Carlsson ML. Differential effects of the N-methyl-d-aspartate receptor antagonist MK-801 on different stages of object recognition memory in mice. Neuroscience. 2007;149:123–130. doi: 10.1016/j.neuroscience.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 67.Gaskin S, Gamliel A, Tardif M, Cole E, Mumby DG. Incidental (unreinforced) and reinforced spatial learning in rats with ventral and dorsal lesions of the hippocampus. Behav Brain Res. 2009;202:64–70. doi: 10.1016/j.bbr.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 68.Ozawa T, Yamada K, Ichitani Y. Long-term object location memory in rats: Effects of sample phase and delay length in spontaneous place recognition test. Neurosci Lett. 2011;497:37–41. doi: 10.1016/j.neulet.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 69.Hliňák Z, Krejčíi I. Spontaneous alternation behaviour in rats: Kynurenic acid attenuated deficits induced by MK-801. Behav Brain Res. 2006;168:144–149. doi: 10.1016/j.bbr.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. Journal of Neuroscience. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watson DJ, Stanton ME. Medial prefrontal administration of MK-801 impairs T-maze discrimination reversal learning in weanling rats. Behav Brain Res. 2009;205:57–66. doi: 10.1016/j.bbr.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim CK, Kalynchuk LE, Kornecook TJ, Mumby DG, Dadgar NA, Pinel JP, Weinberg J. Object-recognition and spatial learning and memory in rats prenatally exposed to ethanol. Behavioral Neuroscience. 1997;111:985–995. doi: 10.1037//0735-7044.111.5.985. [DOI] [PubMed] [Google Scholar]
- 73.Röskam S, Koch M. Effects of neonatal and peripubertal ethanol treatment on various aspects of adult rat behavior andbrain anatomy. International Journal of Developmental Neuroscience. 2009;27:249–256. doi: 10.1016/j.ijdevneu.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Wilson DA, Peterson J, Basavaraj BS, Saito M. Local and Regional Network Function in Behaviorally Relevant Cortical Circuits of Adult Mice Following Postnatal Alcohol Exposure. Alcoholism: Clinical and Experimental Research. 2011;35:1974–1984. doi: 10.1111/j.1530-0277.2011.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


