Abstract
High-resolution manometry and recently described analysis algorithms, summarized in the Chicago Classification, have increased the recognition of achalasia. It has become apparent that the cardinal feature of achalasia, impaired lower esophageal sphincter relaxation, can occur in several disease phenotypes: without peristalsis, with premature (spastic) distal esophageal contractions, with panesophageal pressurization, or with peristalsis. Any of these phenotypes could indicate achalasia; however, without a disease-specific biomarker, no manometric pattern is absolutely specific. Laboratory studies indicate that achalasia is an autoimmune disease in which esophageal myenteric neurons are attacked in a cell-mediated and antibody-mediated immune response against an uncertain antigen. This autoimmune response could be related to infection of genetically predisposed subjects with herpes simplex virus 1, although there is substantial heterogeneity among patients. At one end of the spectrum is complete aganglionosis in patients with end-stage or fulminant disease. At the opposite extreme is type III (spastic) achalasia, which has no demonstrated neuronal loss but only impaired inhibitory postganglionic neuron function; it is often associated with accentuated contractility and could be mediated by cytokine-induced alterations in gene expression. Distinct from these extremes is progressive plexopathy, which likely arises from achalasia with preserved peristalsis and then develops into type II achalasia and then type I achalasia. Variations in its extent and rate of progression are likely related to the intensity of the cytotoxic T-cell assault on the myenteric plexus. Moving forward, we need to integrate the knowledge we have gained into treatment paradigms that are specific for individual phenotypes of achalasia and away from the one-size-fits-all approach.
Keywords: Achalasia, Esophageal Motility, Dysphagia, Pathogenesis, Esophagus, Swallowing
Although achalasia was first recognized more than 300 years ago, our modern understanding of the disease dates only to 1937, when Lendrum proposed that the syndrome was due to incomplete relaxation of the lower esophageal sphincter (LES) and introduced the name “achalasia,” derived from the Greek word “chalasis” for relaxation.1 Before that and since then, a host of other names have been used, including achalasia cardiae, cardiospasm, and esophageal aperistalsis, reflecting the key physiological abnormalities of the disease.
In the early days, achalasia was diagnosed radiographically with the demonstration of esophageal dilation along with retention of swallowed food and contrast material. Subsequently, esophageal manometry became the method of choice, with the defining characteristics of incomplete LES relaxation and absent peristalsis.2,3 More recently, high-resolution manometry (HRM) has further objectified the diagnosis, using carefully validated metrics to quantify LES relaxation and peristaltic function.4 However, despite these unquestionable technological advances, the diagnosis of achalasia is still grounded in the demonstration of functional abnormalities of the esophagus rather than on histopathologic findings; achalasia is rarely a fatal disease, and the relevant tissue is not easily sampled for investigative or diagnostic purposes. Consequently, because the diagnosis is established on the basis of manometric anomalies, it will never be 100% specific; this is the nature of manometric findings. The same findings can result from benign or malignant distal esophageal obstruction, in which case it is termed “pseudoachalasia”5 or a manifestation of Chagas disease, a parasitic infection targeting autonomic ganglia throughout the body.6
Paralleling improvements in diagnostic testing, our understanding of the physiology of esophageal motility and the pathogenesis of achalasia have also advanced over the past decades. Peristalsis in the distal esophagus is the result of complex interactions between vagal innervation, the myenteric plexus, and contraction of both layers of the muscularis propria. The regulation of LES pressure involves both myogenic properties of the sphincter and myenteric plexus modulation of this. Achalasia is now conceptualized as a “plexitis” of sorts, with immune attack on these controlling neurons leading to dysfunction.7 Clearly, this is a complex system with a multitude of potential mechanisms of failure. Coupled with recent observations from HRM describing distinct functional subtypes of achalasia,8 this raises the issue that perhaps we are not dealing with a single disease but rather a family of closely related diseases. It is from that perspective that we review the implications of recent experimental and clinical data on our understanding of achalasia.
The Physiology of Peristalsis and LES Relaxation
Peristalsis
The target organ for achalasia is the esophagus, specifically the smooth muscle portion of the esophagus. In the human esophagus, the proximal 5% of the muscularis propria, including the upper sphincter, is striated muscle, the next 35% to 40% is mixed with an increasing proportion of smooth muscle moving distally, and the distal 50% to 60% is entirely smooth muscle.9 Along its entire length, the muscularis propria has 2 layers: an inner circular (more accurately, spiral) muscle layer and an outer longitudinal muscle layer. The bundles of the outer longitudinal muscle arise from the cricoid cartilage. Distally, the esophagus is anchored to the diaphragm by the phrenoesophageal ligament at the level of the squamo-columnar junction, with the longitudinal muscle then becoming continuous with the outer muscular layer of the stomach. The entire esophagus contains a nerve network, known as the myenteric plexus, situated between the longitudinal and circular muscle layers.10 However, the function of the myenteric plexus in the striated muscle is unclear because this is directly controlled by somatic motor fibers from lower motor neurons in the nucleus retrofacialis and nucleus ambiguus. Axons of these lower motor neurons course via the recurrent laryngeal nerve of the vagus, forming cholinergic synapses on the muscle cells. On the other hand, the thoracic esophagus receives innervation from preganglionic neurons in the dorsal motor nucleus of the vagus and vagal fibers synapse in the myenteric plexus ganglia, which intervene between the vagus and the smooth muscle. A second nerve network, the submucosal or Meissner plexus, is situated between the muscularis mucosae and the circular muscle layer. The submucosal plexus of the human esophagus is exceedingly sparse.10 The normal esophagus does not exhibit spontaneous contractions and its resting pressure is an approximate reflection of pleural pressure, which is atmospheric during expiration and slightly negative during inspiration.
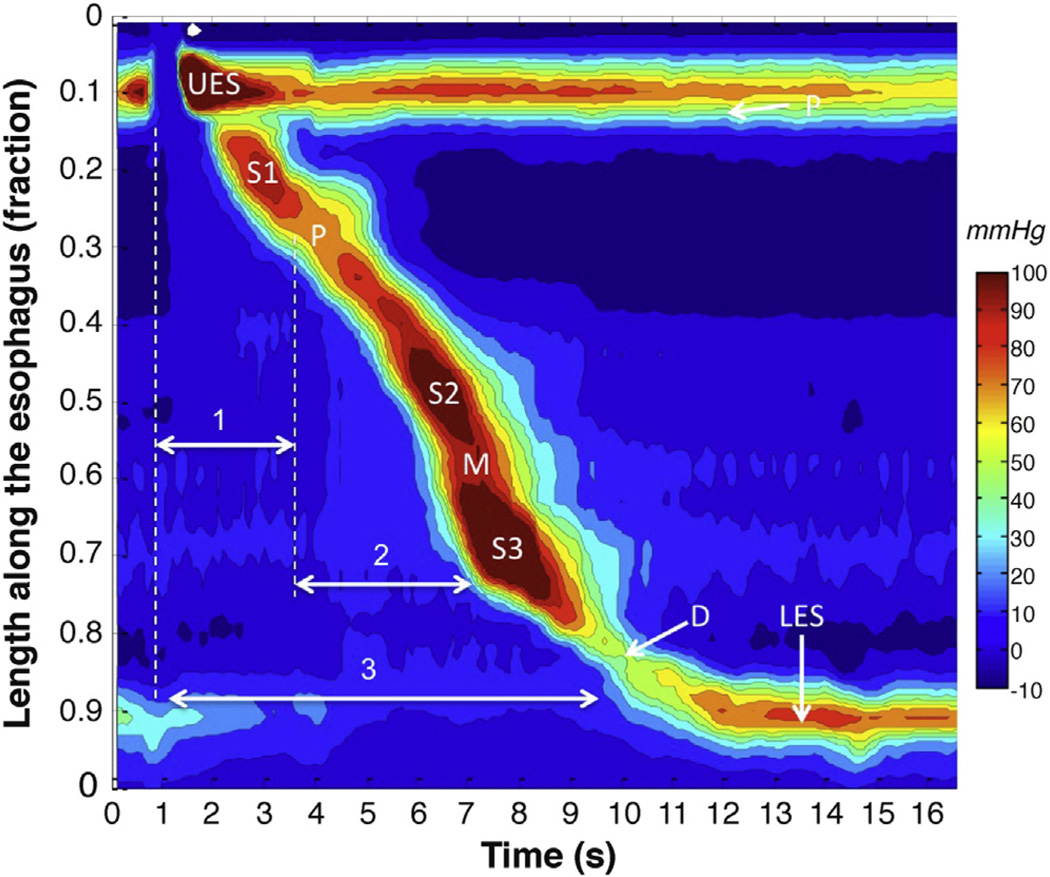
Although swallow-initiated peristalsis progresses from end to end, it is not seamless. Rather, there is a distinct transition zone in the vicinity of the aortic arch characterized by low peristaltic amplitude, a slight delay in progression, and an increased likelihood of failed transmission.11 This transition zone becomes quite evident when peristaltic amplitude and progression are plotted topographically (Figure 1). The topographic analysis also reveals the segmental characteristic of peristaltic progression through the distal esophagus. Two distinct contractile segments are followed by the LES, which contracts with vigor and persistence quite dissimilar to the adjacent esophagus.12
Figure 1.
Averaged pressure topography plot of peristalsis created by superimposing 10 successive swallows of a single healthy subject to illustrate the stereotypic features of peristalsis. The peristaltic contraction is composed of 3 contractile segments (S1, S2, and S3) and the LES contraction separated by 3 relative pressure troughs: proximal (P), middle (M), and distal (D). The latency of contraction at each esophageal locus is a function of centrally mediated deglutitive inhibition (1) persisting for the duration of the S1, centrally programmed contraction and (2) the balance of postganglionic excitation (depolarization) and inhibition (hyperpolarization) from cholinergic and nitrergic myenteric neurons, respectively. LES relaxation (3) is a function of the same myenteric plexus influences, albeit in this case superimposed on a resting myogenic contraction not present in the remainder of the esophagus.
The segmental architecture of peristalsis evident in Figure 1 reflects the unique control mechanisms operational at each locus. The S1 contraction is continuous with the pharyngeal contraction, controlled by the lower motor neurons in the nucleus ambiguus in the brainstem. The nerve fibers are cholinergic and terminate on motor endplates in the striated muscle fibers without intervening autonomic ganglia.13 Thus, the transition zone likely corresponds to the transition from complete central nervous system control to shared central nervous system and autonomic control of peristalsis. The central nervous system still exerts some control over peristalsis distal to the transition zone, but now it depends on the intervening autonomic ganglia to orchestrate contraction of the muscularis propria rather than directly synapsing on the muscular fibers.
Key to understanding the pathogenesis of achalasia is understanding the role of the autonomic ganglia in controlling the contractility of the distal esophagus and LES. Once activated by the vagus, the ensuing esophageal contraction is orchestrated by the postganglionic neurons, precisely the neurons targeted in achalasia. Several control mechanisms come into play. First, there are 2 populations of myenteric plexus neurons: excitatory cholinergic neurons and inhibitory neurons using nitric oxide and vasoactive intestinal polypeptide as a neurotransmitter.14–17 Both populations innervate the circular muscle and LES, but only excitatory fibers are seen innervating the longitudinal muscle of the esophageal body.18 Second, a gradient exists along the circular muscle such that cholinergic postganglionic neurons dominate proximally and inhibitory postganglionic neurons dominate distally.19 Third, the vagus nerve independently activates the inhibitory postganglionic neurons along the entire length of the esophagus with short latency at the onset of the swallow, hyperpolarizing the muscle to a degree that is proportionate to the density of inhibitory postganglionic neurons.20,21 This is followed by excitatory postganglionic nerve activation that is initiated either by local distention in the case of secondary peristalsis or longer latency vagal fibers in the case of primary peristalsis. However, the timing of contraction at each esophageal locus is a function of the balance of inputs from the enteric nervous system, not the vagus. Hence, the distinction between S2 and S3 in Figure 1, while not precise, is reflective of the dominance of cholinergic postganglionic innervation in S2 and nitrergic/VIPergic postganglionic innervation in S3.22
Another cardinal feature of the peristaltic mechanism is deglutitive inhibition. A second swallow, initiated while an earlier peristaltic contraction is still progressing in the proximal esophagus, causes rapid and complete inhibition of the contraction induced by the first swallow.23 If the first peristaltic contraction has reached the distal esophagus, it may proceed distally for a few seconds after the second swallow, but its amplitude diminishes progressively until it disappears.24 With a series of swallows at short intervals, the esophagus remains inhibited and quiescent with the LES relaxed. After the last swallow in the series, a normal peristaltic contraction occurs. Closely grouped swallows can, however, alter the amplitude or velocity of the ending peristaltic contraction or even render it nonperistaltic. These influences can persist for 20 to 30 seconds after the first swallow and are sensitive to bolus size.25,26 Basal LES tone is inhibited with swallowing concurrently with the deglutitive inhibition that traverses the smooth muscle esophagus. Deglutitive inhibition results from hyperpolarization of the circular smooth muscle and is mediated by inhibitory postganglionic neurons.
Another property of peristalsis relevant to the pathogenesis of achalasia is its response to distal (outflow) obstruction. Observations in animal models show the development of hypertrophy, excitability, and eventually failed peristalsis by placing calibrated ligatures around the LES, creating increasing degrees of outflow obstruction.27,28 Animal experiments have also shown a prompt return of peristalsis after ligature removal.29 In humans, the gastric Lap-Band might represent an analogous model of esophagogastric junction (EGJ) obstruction. Manometric features of achalasia have been observed in patients after implantation of this device and normal peristalsis has been restored after band deflation or removal,30,31 showing the potential reversibility of the effect of EGJ obstruction on esophageal peristalsis. Finally, observations with HRM have recently showed that patients with EGJ outflow obstruction might exhibit hypercontractility characterized by multipeaked contractions, high distal esophageal amplitude, prolonged duration of contraction, and a jackhammer pattern of contraction.32,33
LES Relaxation
The LES is a short segment of specialized smooth muscle at the distal extreme of the esophagus that normally exhibits a resting tone that varies from 10 to 30 mm Hg relative to the intragastric pressure. Muscle strips from the LES exhibit distinct physiology compared with the adjacent circular muscle, and the most obvious feature is that LES strips develop spontaneous tone.34 This conclusion is also supported by the observation that pressure within the sphincter is minimally affected after the elimination of neural activity by tetrodotoxin.35 Furthermore, the tonic contraction of the sphincter is not wholly associated with electrical transients,36,37 it has a lower resting membrane potential than the adjacent circular muscle,38,39 it exhibits increased passive permeability to potassium,40 and it has a higher intracytosolic concentration of calcium.41 Sphincter tone may be maintained by the inositol phosphate–mediated continuous release of intracellular calcium. Inositol phosphates are found in higher concentrations in the LES than in adjacent circular muscle. Another unique feature of the LES supporting the contention of spontaneous myogenic tone was the observation of inhibitory nerve fibers, not only in the circular muscle but also in the corresponding longitudinal muscle; such fibers are absent in the adjacent esophagus.18 Hence, detailed assessment of postdeglutitive LES opening shows that this is associated with both radial effacement and elongation to form a structure referred to radiographically as the phrenic ampulla.42 It has also been proposed that a primary stimulus for deglutitive LES relaxation is contraction of the more proximal longitudinal muscle43,44 and that the longitudinal muscle within the LES itself then relaxes, serving as a yield zone to accommodate the resultant shortening.45,46
Superimposed on the myogenic LES contraction, input from vagal, adrenergic, and hormonal influences will alter LES pressure. Vagal control is similar to that of the esophageal body, with vagal stimulation activating both excitatory and inhibitory myenteric neurons.17,47 Thus, LES pressure at any instant reflects the balance between excitatory and inhibitory neural input, and altering the pattern of vagal discharge can result in LES relaxation. The excitatory and inhibitory postganglionic neurons are acetylcholine sensitive, with both nicotinic and muscarinic receptors.35 At the LES, the excitatory neurons release acetylcholine,48 whereas the inhibitory neurons mainly use NO as a neurotransmitter.
The crural diaphragm is also a major contributor to EGJ pressure. Even after esophagogastrectomy, with consequent removal of the smooth muscle LES, a persistent EGJ pressure of approximately 6 mm Hg can be shown during expiration.49 A physiological correlate of this tonic activity was the recent demonstration of an excitatory vagal pathway to the crural diaphragm from the dorsal motor nucleus of the vagus that did not exhibit respiratory fluctuation.50 During inspiration, there is substantial augmentation of EGJ pressure attributable to contraction of the crural diaphragm. Experimentally, the inspiratory augmentation of EGJ pressure can be increased with increased respiratory effort or eliminated by manual ventilation.51 Contraction of the crural diaphragm is also augmented during abdominal compression, straining, or coughing.52 On the other hand, during esophageal distention, vomiting, and belching (transient LES relaxation), electrical activity in the crural diaphragm is selectively inhibited despite continued respiration, showing a control mechanism independent of the costal diaphragm.53,54 This reflex inhibition of crural activity is eliminated with vagotomy.
The neural mediation of LES relaxation has been studied extensively. Swallowing induces an initial inhibition of the entire distal esophagus, and LES relaxation is part of this response. Deglutitive LES relaxation is mediated by the vagus nerve, which synapses with inhibitory neurons of the myenteric plexus. Current evidence implicates NO as the main neurotransmitter in the postganglionic neurons responsible for LES relaxation. NO is produced by neuronal NO synthase, a soluble cytosolic enzyme found in neurons of the myenteric plexus coloc-alizing with vasoactive intestinal polypeptide, which may be a second inhibitory neurotransmitter in the LES and distal esophagus.55–57 Multiple in vitro and in vivo studies have shown that NO synthase inhibitors block neurally mediated LES relaxation.
Transient LES Relaxation
Transient lower esophageal sphincter relaxation (TLESR) is a reflex to allow gas venting from the stomach that is also a key mechanism underlying gastroesophageal reflux.58 Distention of the gastric cardia is the primary trigger of this neurally mediated event.58 Gastric distention stimulates vagal afferents that relay this input to the medulla. Medullary nuclei then orchestrate the efferent limb of the reflex via the vagal and phrenic nerves to elicit prolonged LES relaxation, inhibition of the crural diaphragm, and esophageal shortening via vagally activated postganglionic neurons going to distal esophageal longitudinal muscle.58,60,61 Should reflux occur during a TLESR, esophageal pressurization and upper esophageal sphincter relaxation may also be observed.62 Each of these TLESR components has a characteristic signature when imaged in HRM.
Summary
There are several implications of the neuromuscular architecture and physiology of the esophagus regarding the potential effects of plexitis as occurs in achalasia. First of all, the pharyngeal swallow and proximal esophageal peristalsis (S1 in Figure 1) should be relatively spared, because the myenteric plexus does not directly control these. Within the smooth muscle esophagus, the plexitis can selectively target the LES or the LES and smooth muscle esophagus. However, impaired LES relaxation may well lead to secondary effects on the distal esophagus. Within the myenteric plexus, the plexitis could selectively affect inhibitory neurons or all ganglionic neurons. Furthermore, depending on the pattern of involvement, the disease process may selectively involve the circular muscle or involve both circular and longitudinal muscle layers of the muscularis propria. Finally, the sphincteric activity of the crural diaphragm should not be affected because this is mediated centrally.
Subtypes and Variants of Achalasia Evident With HRM
The technology of esophageal manometry has undergone a major evolution during the past decade. Polygraph systems displaying line-tracing recordings from 3 to 8 pressure sensors have been replaced by high-resolution systems, typically using 36 pressure sensors and displaying the data in esophageal pressure topography (EPT) format. Alternatively called isobaric contour plots or, in recognition of the person who pioneered them, Clouse plots,63,64 EPT uses a coordinate system of time on the x-axis, sensor position on the y-axis, and then localized pressure values represented by color within those coordinates (Figure 1). Thus, pressure data are viewed as a continuum along both the time axis and the length of the esophagus. Hence, although not synonymous, HRM is a prerequisite for accurate EPT plots because the sensors must be sufficiently close to each other to allow for interpolation of intermediate pressure values between them without missing anything.
Nowhere has the evolution of HRM had more impact than in the diagnosis of achalasia. Diagnostic criteria have been tightened4 and relevant physiological subtypes identified.8 Particularly instrumental in establishing uniform diagnostic criteria for achalasia was the development of a new metric to quantify EGJ relaxation: the integrated relaxation pressure (IRP).65 Measurement of the IRP uses an “electronic sleeve sensor” initially described by Staiano and Clouse66 and conceptually similar to a Dent sleeve that compensates for potential LES movement by tracking the sphincter within a specified zone. This avoids the artifact of pseudorelaxation (apparent sphincter relaxation caused by elevation of the sphincter above the sensor), which was a fatal flaw in the assessment of LES relaxation with nonsleeve conventional systems. The IRP is calculated from the electronic sleeve as the mean value during 4 seconds of maximal EGJ relaxation after the pharyngeal contraction. The time scored can be continuous or noncontinuous, such as when it is interrupted by contraction of the crural diaphragm. The IRP provides a robust and accurate assessment of deglutitive EGJ relaxation and optimally discriminates normal relaxation from defects of sphincter relaxation characteristic of achalasia.
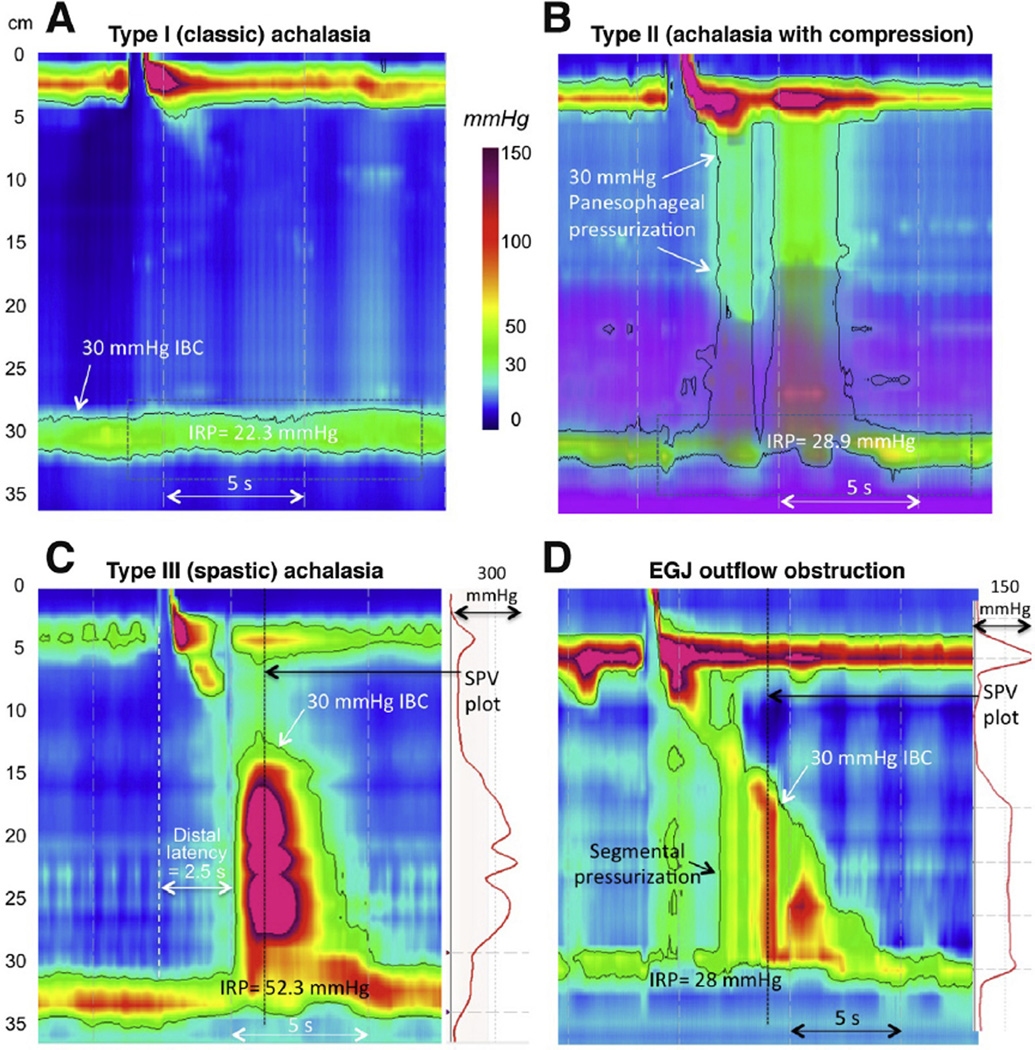
Before the introduction of HRM and EPT, there were no data substantiating the prognostic value of conventional manometric measures in achalasia. Although there were qualitative descriptions of variants, such as vigorous achalasia, achalasia with preserved peristalsis, and cases with complete or partial LES relaxation,67,68 there were no widely accepted conventions for defining these entities. However, with the adoption of EPT, 3 distinct subtypes of achalasia were quantitatively defined using novel validated metrics (Figure 2A–C).8 Additionally, patients with impaired EGJ relaxation but some preserved peristalsis (Figure 2D) are now recognized as a distinct entity that can be a variant phenotype of achalasia. Along the same line, posttreatment fragments of peristalsis are common findings in HRM, suggesting that some preservation of peristalsis is common in achalasia, albeit often masked by esophageal pressurization patterns before treatment.69
Figure 2.
With the adoption of HRM and EPT, 3 distinct subtypes of achalasia were defined using pressure topography metrics. All have impaired EGJ relaxation and absent peristalsis, but the differentiating features are in the patterns of esophageal pressurization; type I has 100% failed peristalsis (aperistalsis), type II exhibits panesophageal pressurization ≥30 mm Hg in ≥20% swallows, and type III exhibits 2 or more premature (spastic) contractions. Note that the impedance data in the type II patient (purple overlay) indicates fluid retention in the esophagus. D is from a patient who did not meet the criterion of absent peristalsis but clearly shows impaired EGJ relaxation with segmental pressurization between the peristaltic contraction and the EGJ; hence, EGJ outflow obstruction was diagnosed. The differentiation between a spastic contraction and segmental pressurization is evident from the spatial pressure variation (SPV) plots to the right of C and D, indicating the top-to-bottom intraluminal pressure profile at the time of the black vertical dotted line. With segmental pressurization, the bolus is trapped between high-pressure zones. The patient in D was treated with a Heller myotomy with relief of dysphagia. Her postmyotomy study showed weak peristalsis. Several recent reports have shown differences in the prognostic value of these achalasia subtypes, supporting the classification scheme
The Pathogenesis of Achalasia
In the absence of any suitable animal model, studies of the pathogeneses of achalasia have faced the fundamental limitation of available tissue specimens. Early histological studies relied on either autopsy material or resection specimens from patients undergoing esophagectomy for end-stage disease. These dilated and grossly dysfunctional specimens mainly showed marked depletion of myenteric ganglia and fibrosis.66,67 Indeed, in end-stage disease, all myenteric neurons had disappeared. A second histopathologic hallmark of achalasia is hypertrophy of the esophageal musculature, most likely secondary to distal esophageal obstruction.70,71
With advances in diagnostic techniques and the widespread adoption of laparoscopic Heller myotomy in the 1980s, tissue samples obtained during myotomy became available from patients in earlier stages of achalasia. These specimens clearly showed persistent myenteric plexus neurons and ganglia surrounded by inflammatory cells, supporting the concept of achalasia as an autoimmune disease that targets the esophageal myenteric plexus.72–78 Further characterization of the myenteric plexus infiltrate found it to consist mainly of T lymphocytes with some eosinophils, plasma cells, B cells, mast cells, and the occasional macrophage. Significantly, most of the mature lymphocytes were cytotoxic CD8+ killer T cells with characteristics of immune activation such as expression of TIA-1 (a cytotoxic T-cell marker associated with the promotion of apoptosis) and granzyme B (an exocytotic granule protease that induces target cell DNA fragmentation and apoptosis).73 Moreover, the T cells in the myenteric infiltrate were tumor necrosis factor positive (a cytokine capable of mediating the killing of a variety of intracellular infectious viruses, bacteria, and parasites) rather than a regulatory phenotype. Examination of the myenteric plexus in tissue collected from patients with achalasia has also shown immunoglobulin M antibodies and evidence of complement activation.79 These antibodies have been proposed to enhance recognition and promote apoptosis of myenteric neurons. Other immune cells such as eosinophils may also contribute to the inflammatory response, as suggested by Tøttrup et al80 and a recent case report.81
The immune attack on the myenteric plexus in patients with achalasia is also associated with the production of serum antineuronal antibodies by plasma cells and B cells.82,83 However, the specificity of antineuronal antibodies for achalasia has subsequently been questioned, because they broadly target enteric neurons rather than selectively targeting the esophageal myenteric plexus and have been detected in similar proportions of patients with achalasia and gastroesophageal reflux disease (51% vs 50%, respectively).83 In fact, only 2 achalasia samples (4.4%) uniquely labeled NO synthase–positive myenteric neurons suggesting any specificity for inhibitory neurons. Thus, in many cases, it seems that serum antineuronal antibodies are an epiphenomenon in achalasia, perhaps indicative of cellular debris from an ongoing immunologic assault on the esophageal myenteric plexus rather than being the cause of it. Of course, there can always be exceptions. For instance, there are paraneoplastic phenomena associated with antineuronal autoantibodies and pseudoachalasia syndromes. These include small cell lung carcinoma, in which anti-Hu antibodies induce intestinal dysmotility.84,85
Taken together, these findings strongly suggest that achalasia is an immune-mediated disease targeting esophageal myenteric neurons, consisting of both a cell-mediated and an antibody-mediated attack directed against an as yet unidentified antigen. Hence, one disease model is that with disease progression, cytotoxic inflammation progressively reduces the myenteric neuron population, eventually leading to aganglionosis, fibrosis, and finally the absence of inflammation when the targets of the immune attack have been exhausted in end-stage disease. In line with that hypothesis, Goldblum et al reported that 3 of 3 specimens taken from patients with “vigorous” achalasia had myenteric inflammation but a normal number of ganglion cells. This led those investigators to propose that vigorous achalasia may be the earliest phase of achalasia with preservation of the motor response of the esophageal body, thereafter progressing to more typical achalasia.72 However, there are several arguments against that hypothesis, the most obvious being that one of these patients with vigorous achalasia had symptoms for 15 years without obvious progression before treatment. Similarly, when subtyped according to the Chicago Classification, type III (spastic) patients enrolled in the recent European Achalasia Trial all had persistent esophageal spasm at follow-up.86 Hence, although prospective studies would be required to definitively address this question, neither the available published data nor the bulk of clinical experience suggest progression from type III to type I or II achalasia.
If not representative of an early stage in disease progression, patients with type III achalasia likely represent a distinct entity characterized by a less “aggressive” immune response affecting neuronal function but not causing apoptosis. Supportive of that hypothesis, incubation of resected human gastric fundus tissue with serum of patients with achalasia induced down-regulation of NO synthase expression and increased cholinergic sensitivity without affecting the number of neurons.87 Circulating cytokines such as interleukin-8 may mediate this response, suggesting that local cytokine release could induce an imbalance between inhibitory and excitatory postganglionic neuronal function, thereby explaining the preserved number of neurons but reduced inhibition and enhanced excitation observed in patients with type III achalasia. One could even speculate that, depending on the regional localization of the inflammatory response, this mechanism may be involved in other motility disorders of the esophagus. In patients with nutcracker esophagus, an increased choline acetyltransferase/NO synthase ratio has indeed been reported with evidence of myenteric inflammation in 3 of 13 patients.88 These data suggest that in these patients and perhaps also in patients with diffuse esophageal spasm, the inflammatory response may result from an inflammatory process confined to the esophageal body, leaving the LES functionally intact. Taken one step further, sporadic reports of progression to achalasia89–91 could then theoretically be explained by subsequent involvement of the LES.
Whereas type III achalasia may occur by a distinct immunologic mechanism, type I and type II achalasia likely both result from a cytotoxic immune attack leading to progressive myenteric plexus neuronal apoptosis without selectivity among subsets of myenteric plexus neurons.77 Rather, variability among patients is reflective of the rapidity with which this occurs. Demonstrative of this variability, Goldblum et al reported that patients with no myenteric plexus infiltrate had a shorter duration of symptoms and more severe loss of nerve fibers compared with patients with myenteric infiltrate; they even observed complete aganglionosis in some patients who were symptomatic for less than a year.72 These findings suggest that the type of immune response and the intensity of the cytotoxic T-cell attack are the most relevant determinants of the clinical presentation of the disease.
Most fundamental among the unknowns regarding the autoimmune attack leading to achalasia is the exact target. How this process begins and why its domain is functionally limited to the esophagus also remain unknown. Unquestionably, antibodies to myenteric neurons can be found in the serum of patients with achalasia,82,92 especially in patients with HLA-DQA1*0103 and -DQB1*0603 alleles.82 Because HLA proteins are crucial in the process of antigen recognition, this suggests an aberrant immune response to an as yet unidentified antigen. Viruses such as herpes simplex virus 1 (HSV-1), measles, and human papillomavirus have all been proposed as potential antigens without consensus among investigators.93–96 However, the most recent evidence has strongly implicated HSV-1 with the demonstration of viral DNA in esophageal tissue from patients with achalasia. Most importantly, isolated esophageal T cells from that tissue were oligo-clonal, specifically proliferating and releasing cytokines on exposure to HSV-1 antigens.93,97 Because HSV-1 is a neurotropic virus with a predilection for squamous epithelium, this hypothesis fits with the selective esophageal involvement in achalasia. However, achalasia is not an inevitable consequence of HSV-1 infection. Facco et al found HSV-1 DNA just as frequently in the esophagus of control subjects as in patients with achalasia, suggesting that it causes autoimmunity against esophageal enteric neurons only when triggered by a still unknown factor and only in genetically susceptible individuals.7
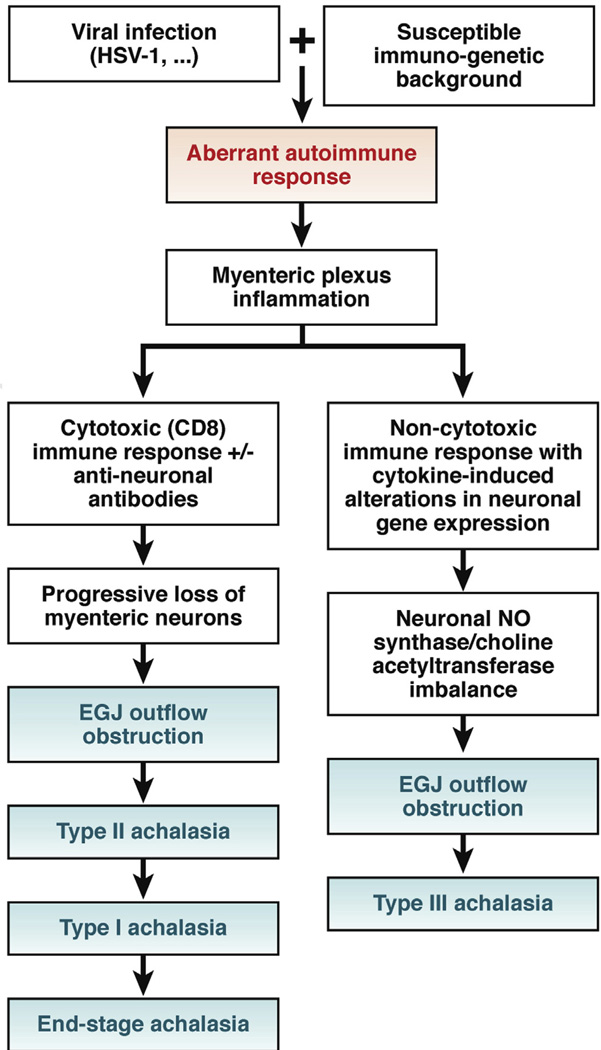
In summary, accumulating evidence overwhelmingly supports the concept that achalasia is an autoimmune disease directed against the esophageal myenteric plexus. However, the details of the immunologic response vary and may explain the observed heterogeneity in disease manifestations. It could be hypothesized that depending on the immunogenetic background, prior exposure to HSV-1, and associated immune response, patients may alternatively present with chronic inflammation in the absence of neuronal loss (Chicago Classification type III) or a predominantly cytotoxic immune response with progressive loss of enteric neurons (Chicago Classification type I and II). The time course of progression that may ultimately lead to aganglionosis and fibrosis in these patients depends on the intensity of the initial and sustained immune response, and localization of the inflammatory response may determine the specific manometric abnormalities. This hypothesis is diagrammed in Figure 3. Clearly, future prospective studies are needed to prove this.
Figure 3.
Proposed model of immunotoxicity patterns leading to distinct achalasia phenotypes. Although other possibilities have been proposed, the model assumes that spastic achalasia is an immune-mediated functional aberration that is not part of the progression to aganglionosis. It is also proposed that the specifics of the antibody response vary among patients and may participate in the cytotoxic attack and/or result in cytokine-mediated alterations in neuron function. The pathogenesis of muscle hypertrophy is unknown, but this is consistently observed to accompany hypercontractility.
Integrating HRM Findings With The Pathophysiology of Plexitis
The single unifying concept among all HRM achalasia subtypes is impaired LES relaxation in the absence of mechanical obstruction. Physiologically, this likely equates inflammatory compromise or apoptosis of the postganglionic neurons controlling the LES. Herein lies the most fundamental heterogeneity among patients with achalasia; in many cases, the LES contracts vigorously after a swallow, suggesting total loss of inhibitory input with preserved cholinergic excitatory innervation, quite likely from hypertrophy of the extrinsic vagal fibers controlling the LES.98,99 In other cases, there is partial EGJ relaxation followed by contraction, suggesting an imbalance between inhibitory and excitatory modulation, and in still other cases there is no apparent deglutitive response, excitatory or inhibitory. Translated into nerve function, this implies cases of complete loss of neural modulation, partial or complete loss of postganglionic control, and even accentuated excitatory nerve function. This heterogeneity is also demonstrable using the multiple rapid swallow challenge during the manometry protocol wherein subjects swallow five 5-mL water boluses in rapid succession. The normal response is of prolonged LES relaxation with an augmented peristaltic contraction after the final swallow. When challenged with multiple rapid boluses, type I patients exhibit no LES response, type II patients may have augmented LES pressure, and type III patients exhibit LES relaxation to a degree approaching normal.100,101
Similar heterogeneity can be inferred among achalasia subtypes from the EPT patterns observed in the distal esophagus. Type III (spastic) achalasia, as exemplified in Figure 2C, likely has loss of postganglionic nerve function with preservation or even augmentation of vagal extrinsic cholinergic modulation along the entire length of the smooth muscle esophagus. The cholinergic nerves are triggering premature contractions on account of reduced to absent inhibitory input. On the other hand, type I (classic) achalasia, as in Figure 2A, with no postdeglutitive contractility is likely the consequence of nearly complete aganglionosis. This would be consistent with the classic reports on the neuropathology of achalasia, which predominantly examined end-stage disease.102,103 Between these extreme patterns are type II achalasia and cases of achalasia exhibiting EGJ outflow obstruction (Figure 2B and 2D), where there is at least some preserved function of both excitatory and inhibitory postganglionic nerves. Note that in this circumstance, with balanced compromise of both excitatory and inhibitory postganglionic function, it is proposed that the residual weak contraction maintains normal latency. The panesophageal pressurization pattern itself is likely the consequence of some preserved contraction of proximal striated muscle, longitudinal muscle, and even nonocclusive circular muscle contraction in the distal esophagus against augmented EGJ outflow obstruction.104,105
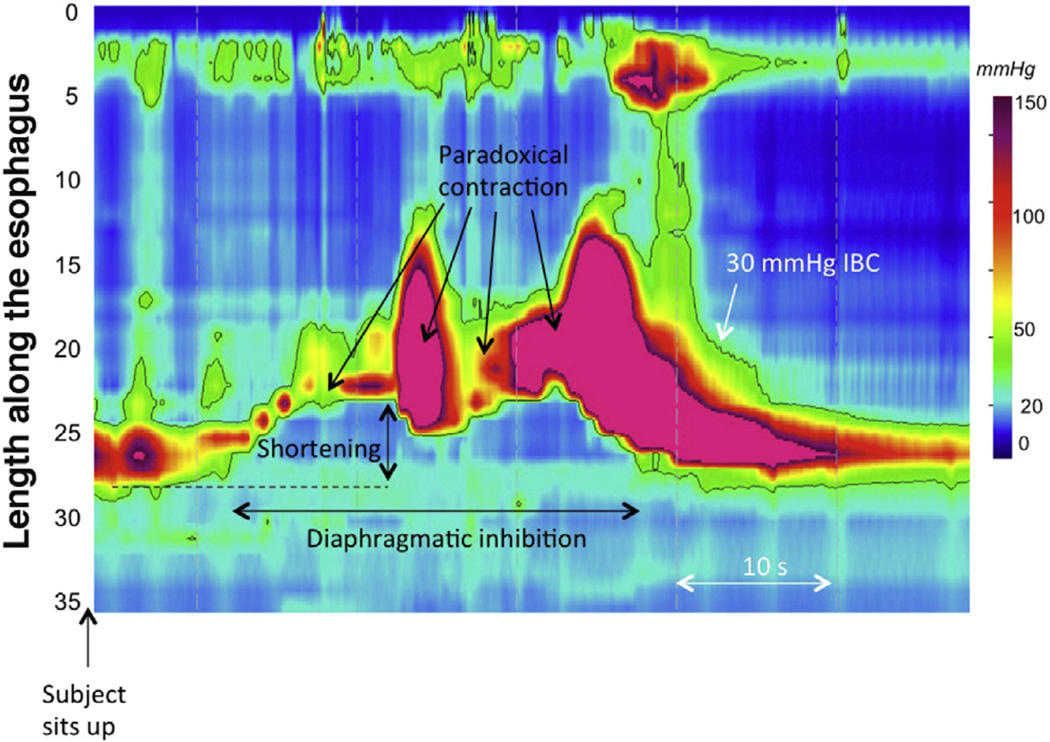
Another line of evidence supporting some degree of preserved postganglionic neuron function in achalasia comes from the observation that patients with achalasia exhibit TLESRs.106 The premise is that, despite having immune-mediated damage to the myenteric plexus leading to impaired deglutitive LES relaxation and absent peristalsis, patients with achalasia can still have TLESRs, albeit incomplete ones. This observation was prompted by the serendipitous observation that TLESRs were often triggered by sitting up after performing a series of 12 test swallows in a supine position during a routine clinical HRM protocol and that patients with achalasia exhibited a pressure topography response with many similarities to a TLESR at that same time point in that manometry protocol (Figure 4). On further analysis, these did indeed appear to be incomplete TLESRs: they occurred with similar frequency to TLESRs in patients without achalasia, they had a similar degree and duration of associated distal esophageal shortening, and they were similarly always associated with concomitant inhibition of the crural diaphragm. The only consistent differences were the absence of associated prolonged LES relaxation and the absence of manometric evidence of gastroesophageal reflux. The implications are that depending on the specifics of the incomplete TLESR elicited, the patient has preserved postganglionic excitatory nerve function to the LES and distal esophagus and uniformly exhibits preserved postganglionic innervation to the longitudinal muscle. One limitation of this study was that too few patients with type I (classic) achalasia were included. However, Mittal et al reported analogous observations using intraluminal ultrasonography and suggested that longitudinal muscle function was vastly attenuated or absent in patients with type I achalasia.104,105
Figure 4.
Incomplete TLESR in a patient with type III achalasia. Similar to patients without achalasia, patients with achalasia frequently exhibit TLESR in the course of a manometry protocol after performing a series of swallows in the supine position and then sitting up. In the case of achalasia, the resultant motor response, shown in this figure, contains all elements of a TLESR (prolonged esophageal shortening with concomitant inhibition of the crural diaphragm and strong after-contraction) except LES relaxation. In fact, the LES paradoxically contracts, consistent with the hypothesis that type III achalasia is a consequence of immune-mediated dysregulation of postganglionic inhibitory nerve function, often with an accentuation of excitatory postganglionic nerve function.
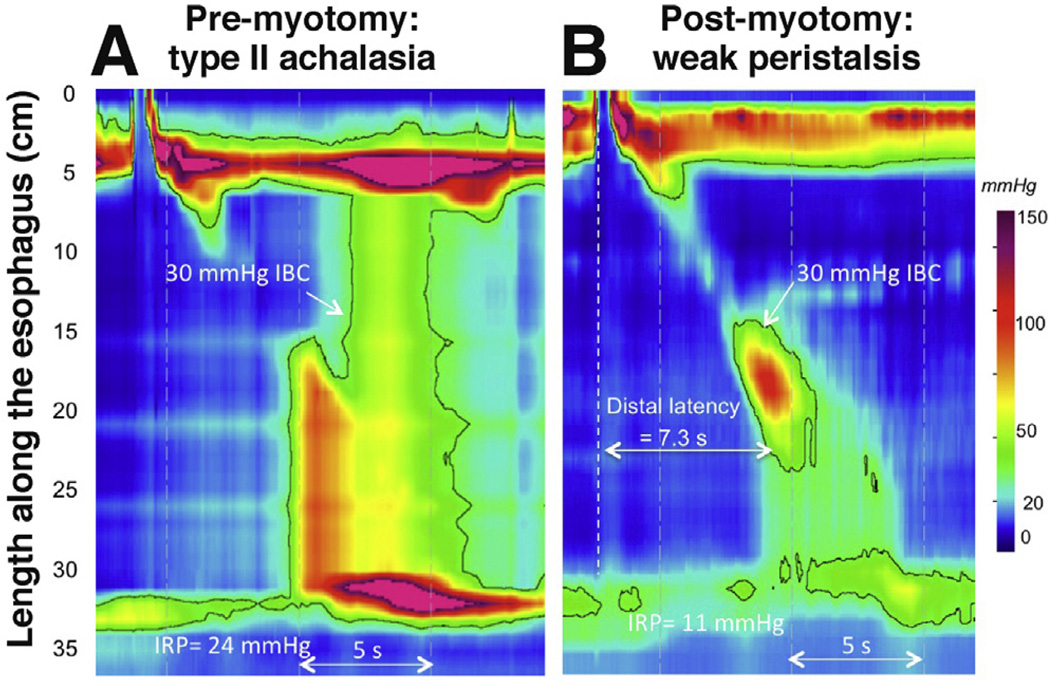
Another clue to the extent of plexopathy in achalasia comes from reexamining patients after effective elimination of outflow obstruction, whether by surgical myotomy, endoscopic myotomy, or pneumatic dilation. The issue of return of peristalsis in achalasia after surgical myotomy has long been controversial, with some arguing that return is seen because absent peristalsis is a consequence of outflow obstruction rather than a primary abnormality.107–109 A recent series examined 30 patients with HRM before and after treatment and found remnants of peristalsis or “recovery” of peristalsis in about half.69 Certainly, it would be anticipated that markedly reducing EGJ outflow obstruction by myotomy would modify achalasia subtyping in some cases and result in patients no longer meeting diagnostic criteria for achalasia in others. The demonstration of type II achalasia, for instance, depends on the existence of an EGJ outflow resistance of at least 30 mm Hg, a condition that should not often occur after myotomy. Furthermore, once that outflow obstruction is eliminated, patterns of contraction previously masked by panesophageal pressurization are now revealed. Consistent with this construct, postmyotomy peristalsis was usually characterized as weak with large breaks in the 30–mm Hg isobaric contour and having a low distal contractile integral (Figure 5). Posttreatment data also support the hypothesis that patients with type III achalasia likely had preserved myenteric plexus in that all but one of the patients exhibited either some intact peristalsis or premature contractions after myotomy.
Figure 5.
A patient with type II achalasia before and after laparoscopic Heller myotomy. After myotomy, a remnant of peristalsis with normal latency and likely extending to the proximal margin of the myotomy is clearly evident. This is a weak contraction based on the large break in the isobaric contour (IBC) between the first and second segments.
In summary, although all HRM subtypes of achalasia exhibit compromised inhibitory postganglionic neuron function in the LES, the overall pattern of neuronal dysfunction in the distal esophagus varies considerably, leading to widely discrepant patterns of esophageal contraction and pressurization. This became quite evident with the widespread use of HRM, leading to the subclas-sification of achalasia into 3 (really 4) subtypes. Table 1 is an attempt at characterizing the patterns of specific neuronal dysfunction characteristic of each HRM subtype. Note that neuronal dysfunction does not equate to neuronal loss, consistent with the experimental observations of cytokine-mediated modified neuronal function without neuronal loss.
Table 1.
Proposed Patterns of Neuronal Function (Postganglionic and Vagal) Among Achalasia Subgroups
| LES |
Distal (smooth muscle) esophagus |
||||
|---|---|---|---|---|---|
| Achalasia subgroup |
Inhibitory | Excitatory | Circular muscle inhibitory |
Circular muscle excitatory |
Longitudinal muscle excitatory |
| Type I (classic) | − − | − − | − − | − − | − − to − |
| Type II (with compression) |
− | − to++ | − | − | + to ++ |
| Type III (spastic) | − | + to++ | − | + to++ | + to++ |
| EGJ outflow obstructiona |
− | − to++ | − to + | − to++ | + to++ |
NOTE. ++, accentuation; +, preservation; −, reduction; − −, elimination. The inferences regarding preserved neural function are based on TLESRs with achalasia, the distal latency of peristalsis premyotomy and postmyotomy, recovery of peristalsis after treatment, and postdeglutitive LES contractions. The table is conceptual, indicative of the “classic case” of each subtype. Clinically, it is sometimes hard to distinguish type I from type II, type II from EGJ outflow obstruction, and type III from type II in borderline cases.
Some, but not all, cases of EGJ outflow obstruction are achalasia.
Conclusions: Putting It All Together
Achalasia is defined by a set of manometric phenomena rather than by a specific marker of pathogenesis. The accepted criteria are dysphagia in the absence of obstruction with impairment of LES relaxation as well as peristalsis. New manometric technology and recently described analysis algorithms summarized in the Chicago Classification4 have greatly improved the clinical recognition of achalasia. However, in so doing, it became evident that impaired LES relaxation can occur in association with absent peristalsis, premature (spastic) distal esophageal contractions, panesophageal pressurization, or preserved peristalsis, all of which are potentially achalasia. Paralleling these developments, laboratory observations have shown that achalasia is an autoimmune disease targeting esophageal myenteric neurons with both a cell-mediated and an antibody-mediated attack directed against an as yet unidentified antigen; it is quite possible that this is related to HSV-1 infection in genetically predisposed patients. However, again there is substantial heterogeneity. At one end of the spectrum, there is complete aganglionosis, as in end-stage disease or rapidly progressing recent-onset cases.72 At the opposite extreme is type III (spastic) achalasia, in which case there has been no demonstrated neuronal loss, only impairment of inhibitory postganglionic neuron function, often associated with accentuated contractility, and quite possibly mediated by cytokine-induced alterations in gene expression. Distinct from these extremes there is a progressive plexopathy, likely evolving from achalasia with preserved peristalsis, to type II achalasia and then to type I achalasia. Isolated examples showing this progression over time have been observed with HRM.110 The variability in extent and rate of progression are likely related to the intensity of the cytotoxic T-cell assault on the myenteric plexus.
Moving forward, there are several challenges. There is always room for more research to reveal the remaining secrets of pathogenesis. Clinically, we need to spread the word; clinging to outdated concepts of achalasia diagnostics is hindering the delivery of appropriate care to many patients who can be helped. Therapeutically, we need to integrate the knowledge we have gained into treatment paradigms and evolve away from the one-size-fits-all approach. Within the realm of currently available therapies, are there disease phenotypes optimally treated with one modality or another? In the future, might there not be instances in which antiviral, immunologic, or even stem cell therapies111,112 might be advantageous? If there is one thing learned and relearned in this recent journey, it is the wisdom of keeping an open mind.
Acknowledgments
Funding
Dr Kahrilas was supported by grant R01 DK56033 from the Public Health Service. Dr Boeckxstaens was supported by grants from the Research Foundation - Flanders (FWO) (Odysseus program, G.0905.07) and the Agency for Innovation by Science and Technology (IWT).
Abbreviations used in this paper
- EGJ
esophagogastric junction
- EPT
esophageal pressure topography
- HSV-1
herpes simplex virus 1
- IRP
integrated relaxation pressure
- LES
lower esophageal sphincter
- TLESR
transient lower esophageal sphincter relaxation
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Lendrum FC. Anatomic features of the cardiac orifice of the stomach with special reference to cardiospasm. Arch Intern Med. 1937;59:474–451. [Google Scholar]
- 2.Pandolfino JE, Kahrilas PJ. The second American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209–229. doi: 10.1053/j.gastro.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Pandolfino JE, Kahrilas PJ. American Gastroenterological Association medical position statement: clinical use of esophageal manometry. Gastroenterology. 2005;128:207–208. doi: 10.1053/j.gastro.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Bredenoord AJ, Fox M, Kahrilas PJ, et al. Chicago Classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Kishk SM, Helm JF, et al. A comparison of pseudoachalasia and achalasia. Am J Med. 1987;82:439–446. doi: 10.1016/0002-9343(87)90443-8. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira RB, Rezende Filho J, Dantas RO, et al. The spectrum of esophageal motor disorders in Chagas’ disease. Am J Gastroenterol. 1995;90:1119–1124. [PubMed] [Google Scholar]
- 7.Boeckxstaens GE. Achalasia: virus-induced euthanasia of neurons? Am J Gastroenterol. 2008;103:1610–1612. doi: 10.1111/j.1572-0241.2008.01967.x. [DOI] [PubMed] [Google Scholar]
- 8.Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer GW, Austin RM, Brady CE, III, et al. Muscle anatomy of the human esophagus. J Clin Gastroenterol. 1986;8:131–134. doi: 10.1097/00004836-198604000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Christensen J, Rick GA, Robison BA, et al. Arrangement of the myenteric plexus throughout the gastrointestinal tract of the opossum. Gastroenterology. 1983;85:890–899. [PubMed] [Google Scholar]
- 11.Brasseur JG. Mechanical studies of the esophageal function. Dysphagia. 1993;8:384–386. doi: 10.1007/BF01321782. [DOI] [PubMed] [Google Scholar]
- 12.Clouse RE, Staiano A. Topography of normal and high-amplitude esophageal peristalsis. Am J Physiol. 1993;265:G1098–G1107. doi: 10.1152/ajpgi.1993.265.6.G1098. [DOI] [PubMed] [Google Scholar]
- 13.Roman C, Car A. Déglutitions et contractions oesophagiennes réflexes obtenues par la stimulation des nerfs vague et laryngé supérieur. Exp Brain Res. 1970;11:48–74. doi: 10.1007/BF00234201. [DOI] [PubMed] [Google Scholar]
- 14.Murray J, Du C, Ledlow A, et al. Nitric oxide: mediator of non-adrenergic noncholinergic responses of opossum esophageal muscle. Am J Physiol. 1991;261:G401–G406. doi: 10.1152/ajpgi.1991.261.3.G401. [DOI] [PubMed] [Google Scholar]
- 15.Biancani P, Walsh JH, Behar J. Vasoactive intestinal polypeptide. A neurotransmitter for lower esophageal sphincter relaxation. J Clin Invest. 1984;73:963–967. doi: 10.1172/JCI111320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guelrud M, Rossiter A, Souney PF, et al. The effect of vasoactive intestinal polypeptide on the lower esophageal sphincter in achalasia. Gastroenterology. 1992;103:377–382. doi: 10.1016/0016-5085(92)90824-i. [DOI] [PubMed] [Google Scholar]
- 17.Kuramato H, Kadowaki M, Yoshida N. Morphological demonstration of a vagal inhibitory pathway to the lower esophageal sphincter via nitrergic neurons in the rat esophagus. Neurogastroenterol Motil. 2013;25:e485–e494. doi: 10.1111/nmo.12146. [DOI] [PubMed] [Google Scholar]
- 18.Christensen J, Fang S, Rick GA. NADPH-diaphorase-positive nerve fibers in smooth muscle layers of opossum esophagus: gradients in density. J Auton Nerv Syst. 1995;52:99–105. doi: 10.1016/0165-1838(94)00149-e. [DOI] [PubMed] [Google Scholar]
- 19.Crist JR, Gidda JS, Goyal RK. Intramural mechanism of esophageal peristalsis: roles of cholinergic and noncholinergic nerves. Proc Natl Acad Sci U S A. 1984;81:3595–3599. doi: 10.1073/pnas.81.11.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gidda JS, Goyal RK. Regional gradient of initial inhibition and refractoriness in esophageal smooth muscle. Gastroenterology. 1985;89:843–851. doi: 10.1016/0016-5085(85)90582-7. [DOI] [PubMed] [Google Scholar]
- 21.Gidda JS, Goyal RK. Swallow-evoked action potentials in the vagal preganglionic efferents. J Neurophysiol. 1984;52:1169–1180. doi: 10.1152/jn.1984.52.6.1169. [DOI] [PubMed] [Google Scholar]
- 22.Staiano A, Clouse RE. The effects of cisapride on the topography of oesophageal peristalsis. Aliment Pharmacol Ther. 1996;10:875–882. doi: 10.1046/j.1365-2036.1996.94266000.x. [DOI] [PubMed] [Google Scholar]
- 23.Hellemans J, Vantrappen G, Valembois P, et al. Electrical activity of striated and smooth muscle of the esophagus. Am J Dig Dis. 1968;13:320–334. doi: 10.1007/BF02233007. [DOI] [PubMed] [Google Scholar]
- 24.Meyer GW, Castell DO. Current concepts of esophageal function. Am J Otolaryngol. 1980;1:440–446. doi: 10.1016/s0196-0709(80)80025-1. [DOI] [PubMed] [Google Scholar]
- 25.Ask P, Tibling L. Effect of time interval between swallows on esophageal peristalsis. Am J Physiol. 1980;238:G485. doi: 10.1152/ajpgi.1980.238.6.G485. [DOI] [PubMed] [Google Scholar]
- 26.Vanek AW, Diamant NE. Responses of the human esophagus to paired swallows. Gastroenterology. 1987;92:G485. doi: 10.1016/0016-5085(87)90012-6. [DOI] [PubMed] [Google Scholar]
- 27.Conklin JL, Du CA, Schulze-Delrieu K, et al. Hypertrophic smooth muscle in the partially obstructed opossum esophagus. Excitability and electrophysiological properties. Gastroenterology. 1991;101:657–663. doi: 10.1016/0016-5085(91)90522-m. [DOI] [PubMed] [Google Scholar]
- 28.Mittal RK, Ren J, McCallum RW, Shaffer HA, Jr, et al. Modulation of feline esophageal contractions by bolus volume and outflow obstruction. Am J Physiol. 1990;258:G208–G215. doi: 10.1152/ajpgi.1990.258.2.G208. [DOI] [PubMed] [Google Scholar]
- 29.Schneider JH, Peters JH, Kirkman E, et al. Are the motility abnormalities of achalasia reversible? An experimental outflow obstruction in the feline model. Surgery. 1999;125:498–503. [PubMed] [Google Scholar]
- 30.Cruiziat C, Roman S, Robert M, et al. High-resolution esophageal manometry evaluation in symptomatic patients after gastric banding for morbid obesity. Dig Liver Dis. 2011;43:116–120. doi: 10.1016/j.dld.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Khan A, Ren-Fielding C, Traube M. Potentially reversible pseudoachalasia after laparoscopic adjustable gastric banding. J Clin Gastroenterol. 2011;45:775–779. doi: 10.1097/MCG.0b013e318226ae14. [DOI] [PubMed] [Google Scholar]
- 32.Gyawali CP, Kushnir VM. High-resolution manometric characteristics help differentiate types of distal esophageal obstruction in patients with peristalsis. Neurogastroenterol Motil. 2011;23:502–e197. doi: 10.1111/j.1365-2982.2011.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roman S, Pandolfino JE, Chen J, et al. Phenotypes and clinical context of hypercontractility in high resolution esophageal pressure topography (EPT) Am J Gastroenterol. 2012;107:37–45. doi: 10.1038/ajg.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen J, Conklin JL, Freeman BW. Physiologic specialization at esophagogastric junction in three species. Am J Physiol. 1973;225:1265–1270. doi: 10.1152/ajplegacy.1973.225.6.1265. [DOI] [PubMed] [Google Scholar]
- 35.Goyal RK, Rattan S. Genesis of basal sphincter pressure: effect of tetrodotoxin on lower esophageal sphincter pressure in opossum in vivo. Gastroenterology. 1976;71:62–67. [PubMed] [Google Scholar]
- 36.Asoh R, Goyal RK. Electrical activity of the opossum lower esophageal sphincter in vivo. Gastroenterology. 1978;74:835–840. [PubMed] [Google Scholar]
- 37.Zelcer W, Weisbrodt NW. Electrical and mechanical activity of the lower esophageal sphincter in the cat. Am J Physiol. 1984;246:G243–G247. doi: 10.1152/ajpgi.1984.246.3.G243. [DOI] [PubMed] [Google Scholar]
- 38.Dektor DL, Ryan JP. Transmembrane voltage of opossum esophageal smooth muscle and its response to electrical stimulation of intrinsic nerves. Gastroenterology. 1982;82:301–308. [PubMed] [Google Scholar]
- 39.Kannan MS, Jagler LP, Daniel EE. Electrical properties of smooth muscle cell membrane of opossum esophagus. Am J Physiol. 1985;248:G342–G346. doi: 10.1152/ajpgi.1985.248.3.G342. [DOI] [PubMed] [Google Scholar]
- 40.Schulze K, Hajjar JJ, Christensen J. Regional differences in potassium content of smooth muscle from opossum esophagus. Am J Physiol. 1978;235:E709–E713. doi: 10.1152/ajpendo.1978.235.6.E709. [DOI] [PubMed] [Google Scholar]
- 41.Schlippert W, Schulze K, Forker EL. Calcium in smooth muscle from the opossum esophagus. Proc Soc Exp Biol Med. 1979;162:354–358. doi: 10.3181/00379727-162-40681. [DOI] [PubMed] [Google Scholar]
- 42.Kwiatek MA, Nicodème F, Pandolfino JE, et al. Pressure morphology of the relaxed lower esophageal sphincter: the formation and collapse of the phrenic ampulla. Am J Physiol Gastrointest Liver Physiol. 2012;302:G389–G396. doi: 10.1152/ajpgi.00385.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dogan I, Bhargava V, Liu J, et al. Axial stretch: a novel mechanism of the lower esophageal sphincter relaxation. Am J Physiol. 2007;292:G329–G334. doi: 10.1152/ajpgi.00351.2006. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Y, Bhargava V, Mittal RK. Mechanism of stretch-activated excitatory and inhibitory responses in the lower esophageal sphincter. Am J Physiol. 2009;297:G397–G405. doi: 10.1152/ajpgi.00108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen J, Miftakhov R. Hiatus hernia: a review of evidence for its origin in esophageal longitudinal muscle dysfunction. Am J Med. 2000;108(Suppl 4a):3S–7S. doi: 10.1016/s0002-9343(99)00288-0. [DOI] [PubMed] [Google Scholar]
- 46.Yassi R, Cheng LK, Rajagopal V, et al. Modeling of the mechanical function of the human gastroesophageal junction using an anatomically realistic three-dimensional model. J Biomech. 2009;42:1604–1609. doi: 10.1016/j.jbiomech.2009.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonella J, Niel JP, Roman C. Vagal control of lower oesophageal sphincter motility in the cat. J Physiol. 1977;273:647–664. doi: 10.1113/jphysiol.1977.sp012115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodds WJ, Dent J, Hogan WJ, et al. Effect of atropine on esophageal motor function in humans. Am J Physiol. 1981;240:G290–G296. doi: 10.1152/ajpgi.1981.240.4.G290. [DOI] [PubMed] [Google Scholar]
- 49.Klein WA, Parkman HP, Dempsey DT, et al. Sphincterlike thoracoabdominal high pressure zone after esophagogastrectomy. Gastroenterology. 1993;105:1362–1369. doi: 10.1016/0016-5085(93)90140-8. [DOI] [PubMed] [Google Scholar]
- 50.Young RL, Page AJ, Cooper NJ, et al. Sensory and motor innervation of the crural diaphragm by the vagus nerves. Gastroenterology. 2010;138:1091–1101. doi: 10.1053/j.gastro.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 51.Mittal RK, Rochester DF, McCallum RW. Sphincteric action of the diaphragm during a relaxed lower esophageal sphincter in humans. Am J Physiol. 1989;256:G139–G144. doi: 10.1152/ajpgi.1989.256.1.G139. [DOI] [PubMed] [Google Scholar]
- 52.Mittal RK, Fisher M, McCallum RW, et al. Human lower esophageal sphincter pressure response to increased intra-abdominal pressure. Am J Physiol. 1990;258:G624–G630. doi: 10.1152/ajpgi.1990.258.4.G624. [DOI] [PubMed] [Google Scholar]
- 53.De Troyer A, Sampson M, Sigrist S, et al. Action of costal and crural parts of the diaphragm on the rib cage in dog. J Appl Physiol. 1982;53:30–39. doi: 10.1152/jappl.1982.53.1.30. [DOI] [PubMed] [Google Scholar]
- 54.Altschuler SM, Boyle JT, Nixon TE, et al. Simultaneous reflex inhibition of lower esophageal sphincter and crural diaphragm in cats. Am J Physiol. 1985;249:G586–G591. doi: 10.1152/ajpgi.1985.249.5.G586. [DOI] [PubMed] [Google Scholar]
- 55.Goyal RK, Rattan S. Nature of the vagal inhibitory innervation to the lower esophageal sphincter. J Clin Invest. 1975;55:1119–1126. doi: 10.1172/JCI108013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murthy KS, Zhang KM, Jin JG, et al. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am J Physiol. 1993;265:G660–G671. doi: 10.1152/ajpgi.1993.265.4.G660. [DOI] [PubMed] [Google Scholar]
- 57.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 58.Mittal RK, Holloway RH, Penagini R, et al. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601–610. doi: 10.1016/0016-5085(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 59.Holloway RH, Hongo M, Berger K, et al. Gastric distention: a mechanism for postprandial gastroesophageal reflux. Gastroenterology. 1985;89:779–784. doi: 10.1016/0016-5085(85)90572-4. [DOI] [PubMed] [Google Scholar]
- 60.Pandolfino JE, Zhang QG, Ghosh SK, et al. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology. 2006;131:1725–1733. doi: 10.1053/j.gastro.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Babaei A, Bhargava V, Korsapati H, et al. A unique longitudinal muscle contraction pattern associated with transient lower esophageal sphincter relaxation. Gastroenterology. 2008;134:1322–1331. doi: 10.1053/j.gastro.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 62.Pandolfino JE, Ghosh SK, Zhang Q, et al. Upper sphincter function during transient lower oesophageal sphincter relaxation (tLOSR); it is mainly about microburps. Neurogastroenterol Motil. 2007;19:203–210. doi: 10.1111/j.1365-2982.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 63.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261:G677–G684. doi: 10.1152/ajpgi.1991.261.4.G677. [DOI] [PubMed] [Google Scholar]
- 64.Clouse RE, Staiano A, Alrakawi A, et al. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95:2720–2730. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol. 2007;293:G878–G885. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 66.Staiano A, Clouse RE. Detection of incomplete lower esophageal sphincter relaxation with conventional point-pressure sensors. Am J Gastroenterol. 2001;96:3258–3267. doi: 10.1111/j.1572-0241.2001.05323.x. [DOI] [PubMed] [Google Scholar]
- 67.Galey KM, Wilshire CL, Niebisch S, et al. Atypical variants of classic achalasia are common and currently under-recognized: a study of prevalence and clinical features. J Am Coll Surg. 2011;213:155–161. doi: 10.1016/j.jamcollsurg.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Hirano I, Tatum RP, Shi G, et al. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology. 2001;120:789–798. doi: 10.1053/gast.2001.22539. [DOI] [PubMed] [Google Scholar]
- 69.Roman S, Kahrilas PJ, Mion F, et al. Partial recovery of peristalsis after myotomy for achalasia: more the rule than the exception. JAMA Surg. 2013;148:157–164. doi: 10.1001/2013.jamasurg.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dogan I, Puckett JL, Padda BS, et al. Prevalence of increased esophageal muscle thickness in patients with esophageal symptoms. Am J Gastroenterol. 2007;102:137–145. doi: 10.1111/j.1572-0241.2006.01003.x. [DOI] [PubMed] [Google Scholar]
- 71.Friesen DL, Henderson RD, Hanna W. Ultrastructure of the esophageal muscle in achalasia and diffuse esophageal spasm. Am J Clin Pathol. 1983;79:319–325. doi: 10.1093/ajcp/79.3.319. [DOI] [PubMed] [Google Scholar]
- 72.Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology. 1996;111:648–654. doi: 10.1053/gast.1996.v111.pm8780569. [DOI] [PubMed] [Google Scholar]
- 73.Clark SB, Rice TW, Tubbs RR, et al. The nature of the myenteric infiltrate in achalasia: an immunohistochemical analysis. Am J Surg Pathol. 2000;24:1153–1158. doi: 10.1097/00000478-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 74.Villanacci V, Annese V, Cuttitta A, et al. An immunohistochemical study of the myenteric plexus in idiopathic achalasia. Clin Gastroenterol. 2010;44:407–410. doi: 10.1097/MCG.0b013e3181bc9ebf. [DOI] [PubMed] [Google Scholar]
- 75.Kilic A, Krasinskas AM, Owens SR, et al. Variations in inflammation and nerve fiber loss reflect different subsets of achalasia patients. J Surg Res. 2007;143:177–182. doi: 10.1016/j.jss.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 76.Kilic A, Owens SR, Pennathur A, et al. An increased proportion of inflammatory cells express tumor necrosis factor alpha in idiopathic achalasia of the esophagus. Dis Esoph. 2009;22:382–385. doi: 10.1111/j.1442-2050.2008.00922.x. [DOI] [PubMed] [Google Scholar]
- 77.Hoshino M, Omura N, Yano F, et al. Immunohistochemical study of the muscularis externa of the esophagus in achalasia patients. Dis Esophagus. 2013;26:14–21. doi: 10.1111/j.1442-2050.2011.01318.x. [DOI] [PubMed] [Google Scholar]
- 78.De Giorgio R, Di Simone MP, Stanghellini V, et al. Esophageal and gastric nitric oxide synthesizing innervation in primary achalasia. Am J Gastroenterol. 1999;94:2357–2362. doi: 10.1111/j.1572-0241.1999.01357.x. [DOI] [PubMed] [Google Scholar]
- 79.Storch WB, Eckardt VF, Junginger T. Complement components and terminal complement complex in oesophageal smooth muscle of patients with achalasia. Cell Mol Biol. 2002;48:247–252. [PubMed] [Google Scholar]
- 80.Tøttrup A, Fredens K, Funch-Jensen P, et al. Eosinophil infiltration in primary esophageal achalasia. A possible pathogenic role. Dig Dis Sci. 1989;34:1894–1899. doi: 10.1007/BF01536708. [DOI] [PubMed] [Google Scholar]
- 81.Savarino E, Gemignani L, Zentilin P, et al. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin Gastroenterol Hepatol. 2011;9:1104–1106. doi: 10.1016/j.cgh.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Ruiz-de-Leon A, Mendoza J, Sevilla-Mantilla C, et al. Myenteric antiplexus antibodies and class II HLA in achalasia. Dig Dis Sci. 2002;47:15–19. doi: 10.1023/a:1013242831900. [DOI] [PubMed] [Google Scholar]
- 83.Moses PL, Ellis LM, Anees MR, et al. Antineuronal antibodies in idiopathic achalasia and gastro-oesophageal reflux disease. Gut. 2003;52:629–636. doi: 10.1136/gut.52.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lennon VA, Sas DF, Busk MF, et al. Enteric neuronal autoantibodies in pseudoobstruction with small-cell lung carcinoma. Gastroenterology. 1991;100:137–142. doi: 10.1016/0016-5085(91)90593-a. [DOI] [PubMed] [Google Scholar]
- 85.Lee HR, Lennon VA, Camilleri M, et al. Paraneoplastic gastrointestinal motor dysfunction: clinical and laboratory characteristics. Am J Gastroenterol. 2001;96:373–379. doi: 10.1111/j.1572-0241.2001.03454.x. [DOI] [PubMed] [Google Scholar]
- 86.Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology. 2013;144:718–725. doi: 10.1053/j.gastro.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 87.Bruley des Varannes S, Chevalier J, Pimont S, et al. Serum from achalasia patients alters neurochemical coding in the myenteric plexus and nitric oxide mediated motor response in normal human fundus. Gut. 2006;55:319–326. doi: 10.1136/gut.2005.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim HS, Park H, Lim JH, et al. Morphometric evaluation of oesophageal wall in patients with nutcracker oesophagus and ineffective oesophageal motility. Neurogastroenterol Motil. 2008;20:869–876. doi: 10.1111/j.1365-2982.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 89.Paterson WG, Beck IT, Da Costa LR. Transition from nutcracker esophagus to achalasia. A case report. J Clin Gastroenterol. 1991;13:554–558. doi: 10.1097/00004836-199110000-00016. [DOI] [PubMed] [Google Scholar]
- 90.Fontes LH, Herbella FA, Rodriguez TN, et al. Progression of diffuse esophageal spasm to achalasia: incidence and predictive factors. Dis Esophagus. 2013;26:470–474. doi: 10.1111/j.1442-2050.2012.01377.x. [DOI] [PubMed] [Google Scholar]
- 91.Khatami SS, Khandwala F, Shay SS, et al. Does diffuse esophageal spasm progress to achalasia? A prospective cohort study. Dig Dis Sci. 2005;50:1605–1610. doi: 10.1007/s10620-005-2903-3. [DOI] [PubMed] [Google Scholar]
- 92.Storch WB, Eckardt VF, Wienbeck M, et al. Autoantibodies to Auerbach’s plexus in achalasia. Cell Mol Biol. 1995;41:1033–1038. [PubMed] [Google Scholar]
- 93.Facco M, Brun P, Baesso I, et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol. 2008;103:1598–1609. doi: 10.1111/j.1572-0241.2008.01956.x. [DOI] [PubMed] [Google Scholar]
- 94.Birgisson S, Galinski MS, Goldblum JR, et al. Achalasia is not associated with measles or known herpes and human papilloma viruses. Dig Dis Sci. 1997;42:300–306. doi: 10.1023/a:1018805600276. [DOI] [PubMed] [Google Scholar]
- 95.Niwamoto H, Okamoto E, Fujimoto J, et al. Are human herpes viruses or measles virus associated with esophageal achalasia? Dig Dis Sci. 1995;40:859–864. doi: 10.1007/BF02064992. [DOI] [PubMed] [Google Scholar]
- 96.Robertson CS, Martin BA, Atkinson M. Varicella-zoster virus DNA in the oesophageal myenteric plexus in achalasia. Gut. 1993;34:299–302. doi: 10.1136/gut.34.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Castagliuolo I, Brun P, Costantini M, et al. Esophageal achalasia: is the herpes simplex virus really innocent? J Gastrointest Surg. 2004;8:24–30. doi: 10.1016/j.gassur.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Wattchow DA, Costa M. Distribution of peptide-containing nerve fibres in achalasia of the oesophagus. J Gastroenterol Hepatol. 1996;11:478–485. doi: 10.1111/j.1440-1746.1996.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 99.Gaumnitz EA, Bass P, Osinski MA, et al. Electrophysiological and pharmacological responses of chronically denervated lower esophageal sphincter of the opossum. Gastroenterology. 1995;109:789–799. doi: 10.1016/0016-5085(95)90386-0. [DOI] [PubMed] [Google Scholar]
- 100.Kushnir V, Sayuk GS, Gyawali CP. Multiple rapid swallow responses segregate achalasia subtypes on high-resolution manometry. Neurogastroenterol Motil. 2012;24:1069–e561. doi: 10.1111/j.1365-2982.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Savojardo D, Mangano M, Cantù P, et al. Multiple rapid swallowing in idiopathic achalasia; evidence for patients’ heterogeneity. Neurogastroenterol Motil. 2008;19:263–269. doi: 10.1111/j.1365-2982.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 102.Goldblum JR, Whyte RI, Orringer MB, et al. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18:327–337. [PubMed] [Google Scholar]
- 103.Csendes A, Smok G, Braghetto I, et al. Gastroesophageal sphincter pressure and histological changes in distal esophagus in patients with achalasia of the esophagus. Dig Dis Sci. 1985;30:941–945. doi: 10.1007/BF01308293. [DOI] [PubMed] [Google Scholar]
- 104.Hong SJ, Bhargava V, Jiang Y, et al. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology. 2010;139:102–111. doi: 10.1053/j.gastro.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mittal RK, Hong SJ, Bhargava V. Longitudinal muscle dysfunction in achalasia and its relevance. J Neurogastroenterol Motil. 2013;19:126–136. doi: 10.5056/jnm.2013.19.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kwiatek KA, Post J, Pandolfino JE, et al. Transient lower esophageal sphincter relaxation in achalasia: everything but LES relaxation. Neurogastroenterol Mot. 2009;21:1250–1255. doi: 10.1111/j.1365-2982.2009.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parrilla P, Martinez de Haro LF, et al. Factors involved in the return of peristalsis in patients with achalasia of the cardia after Heller’s myotomy. Am J Gastroenterol. 1995;90:713–717. [PubMed] [Google Scholar]
- 108.Zaninotto G, Costantini M, Anselmino M, et al. Onset of oesophageal peristalsis after surgery for idiopathic achalasia. Br J Surg. 1995;82:1532–1534. doi: 10.1002/bjs.1800821125. [DOI] [PubMed] [Google Scholar]
- 109.Tatum RP, Wong JA, Figueredo EJ, et al. Return of esophageal function after treatment for achalasia as determined by imped-ance-manometry. J Gastrointest Surg. 2007;11:1403–1409. doi: 10.1007/s11605-007-0293-x. [DOI] [PubMed] [Google Scholar]
- 110.Nicodème F, Ruigh A, Xiao Y, et al. A comparison of symptom severity and bolus retention to Chicago Classification esophageal topography metrics in achalasia. Clin Gastroenterol Hepatol. 2013;11:131–137. doi: 10.1016/j.cgh.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Metzger M, Caldwell C, Barlow AJ, et al. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009;136:2214–2225. doi: 10.1053/j.gastro.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 112.Kulkarni S, Becker L, Pasricha JP. Stem cell transplantation in neurodegenerative disorders of the gastrointestinal tract: future or fiction? Gut. 2012;61:613–621. doi: 10.1136/gut.2010.235614. [DOI] [PMC free article] [PubMed] [Google Scholar]