Abstract
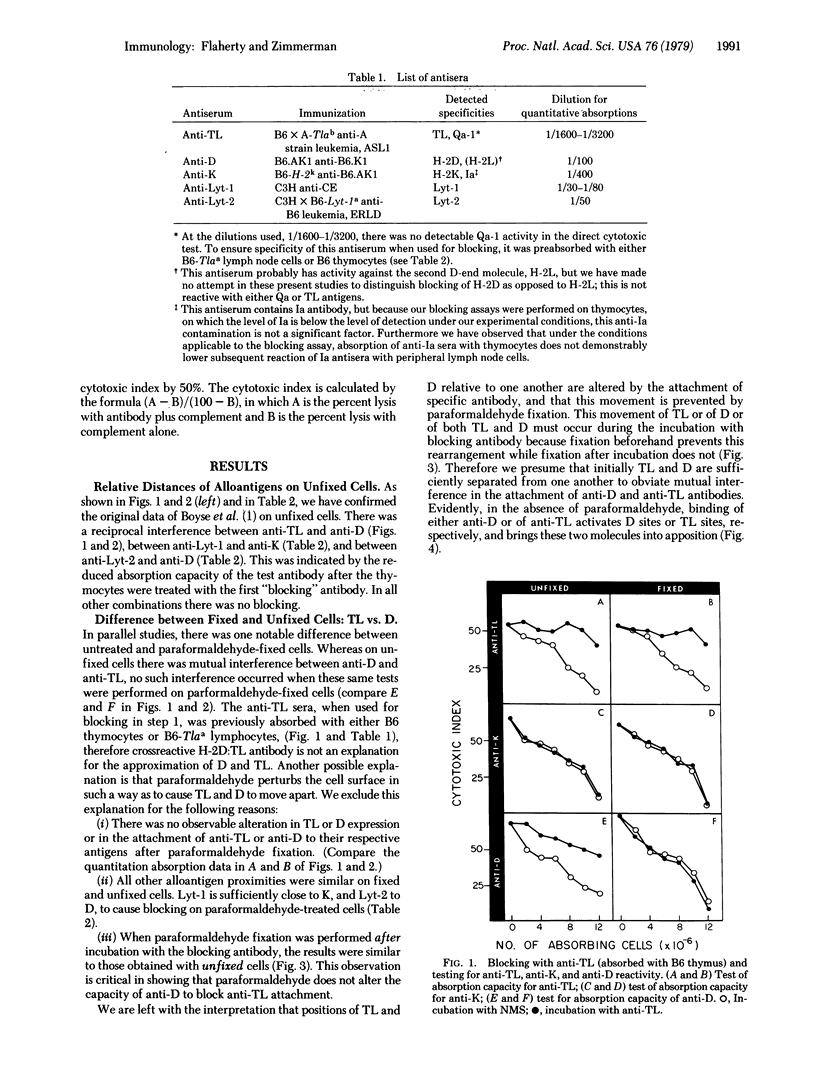
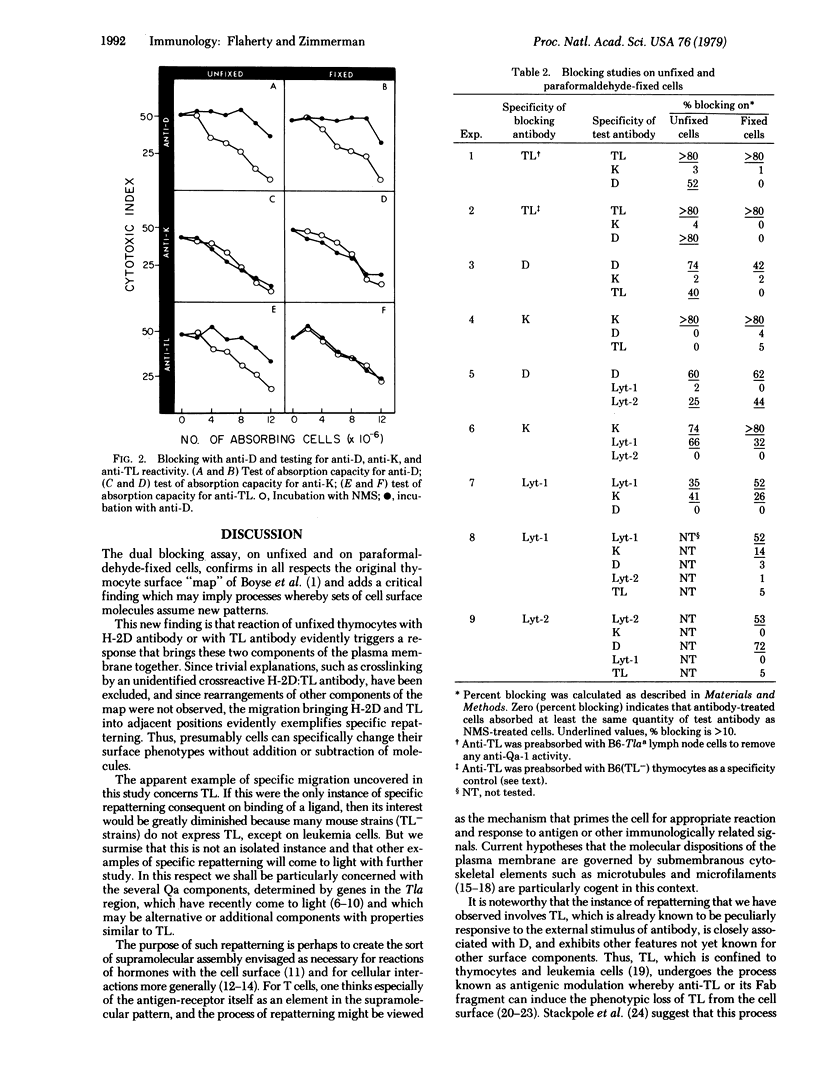
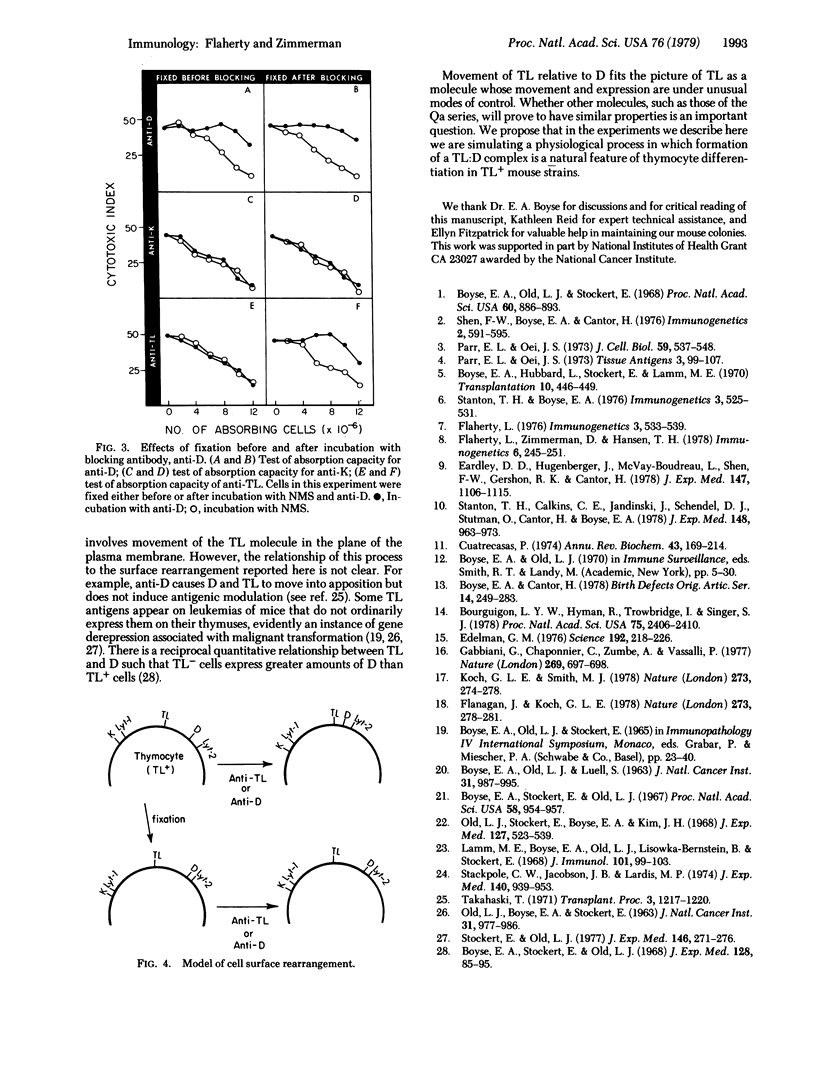
The blocking method used previously for determining the relative positions of different components of the cell surface was modified by first fixing the cells with paraformaldehyde. This technique was applied to the H-2K (K), H-2D (D), TL, Lyt-1, and Lyt-2 surface components of mouse thymocytes, and the results were compared in parallel with data obtained with the original technique with unfixed cells. Previous mapping data with unfixed cells, indicating the positions of these molecules relative to one another, were confirmed with paraformaldehyde-fixed cells, with one exception. On unfixed cells, D and TL appeared sufficiently adjacent to produce mutual interference in the attachment of anti-D and anti-TL antibodies. With paraformaldehyde-fixed cells this was not so, D and TL appearing sufficiently separated from one another to obviate interference in the attachment of anti-D and anti-TL antibodies. The previously reported close association of K with Lyt-1 and of D with Lyt-2 were demonstrable equally with unfixed and paraformaldehyde-fixed thymocytes. It is suggested that activation of D sites, and alternatively of TL sites, by antibody in the present experiments brings these two molecules into apposition and that this movement may exemplify a mechanism concerned in immunological recognition and response.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguignon L. Y., Hyman R., Trowbridge I., Singer S. J. Participation of histocompatibility antigens in capping of molecularly independent cell surface components by their specific antibodies. Proc Natl Acad Sci U S A. 1978 May;75(5):2406–2410. doi: 10.1073/pnas.75.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyse E. A., Cantor H. Immunogenetic aspects of biologic communication: a hypothesis of evolution by program duplication. Birth Defects Orig Artic Ser. 1978;14(2):249–283. [PubMed] [Google Scholar]
- Boyse E. A., Hubbard L., Stockert E., Lamm M. E. Improved complementation in the cytotoxic test. Transplantation. 1970 Nov;10(5):446–449. doi: 10.1097/00007890-197011000-00019. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Old L. J., Stockert E. An approach to the mapping of antigens on the cell surface. Proc Natl Acad Sci U S A. 1968 Jul;60(3):886–893. doi: 10.1073/pnas.60.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyse E. A., Stockert E., Old L. J. Isoantigens of the H-2 and Tla loci of the mouse: interactions affecting their representation on thymocytes. J Exp Med. 1968 Jul 1;128(1):85–95. doi: 10.1084/jem.128.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyse E. A., Stockert E., Old L. J. Modification of the antigenic structure of the cell membrane by thymus-leukemia (TL) antibody. Proc Natl Acad Sci U S A. 1967 Sep;58(3):954–957. doi: 10.1073/pnas.58.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Hugenberger J., McVay-Boudreau L., Shen F. W., Gershon R. K., Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978 Apr 1;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Flanagan J., Koch G. L. Cross-linked surface Ig attaches to actin. Nature. 1978 May 25;273(5660):278–281. doi: 10.1038/273278a0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Zumbe A., Vassalli P. Actin and tubulin co-cap with surface immunoglobulins in mouse B lymphocytes. Nature. 1977 Oct 20;269(5630):697–698. doi: 10.1038/269697a0. [DOI] [PubMed] [Google Scholar]
- Koch G. L., Smith M. J. An association between actin and the major histocompatibility antigen H-2. Nature. 1978 May 25;273(5660):274–278. doi: 10.1038/273274a0. [DOI] [PubMed] [Google Scholar]
- Lamm M. E., Boyse E. A., Old L. J., Lisowska-Bernstein B., Stockert E. Modulation of TL (thymus-leukemia) antigens by Fab-fragments of TL antibody. J Immunol. 1968 Jul;101(1):99–103. [PubMed] [Google Scholar]
- Meyer D., McKee P. A., Hoyer L. W., Zimmerman T. S., Gralnick H. R. Molecular biology of factor VIII/von Willebrand Factor. Thromb Haemost. 1978 Oct 31;40(2):245–251. [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A. ANTIGENIC PROPERTIES OF EXPERIMENTAL LEUKEMIAS. I. SEROLOGICAL STUDIES IN VITRO WITH SPONTANEOUS AND RADIATION-INDUCED LEUKEMIAS. J Natl Cancer Inst. 1963 Oct;31:977–995. [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A. ANTIGENIC PROPERTIES OF EXPERIMENTAL LEUKEMIAS. I. SEROLOGICAL STUDIES IN VITRO WITH SPONTANEOUS AND RADIATION-INDUCED LEUKEMIAS. J Natl Cancer Inst. 1963 Oct;31:977–995. [PubMed] [Google Scholar]
- Old L. J., Stockert E., Boyse E. A., Kim J. H. Antigenic modulation. Loss of TL antigen from cells exposed to TL antibody. Study of the phenomenon in vitro. J Exp Med. 1968 Mar 1;127(3):523–539. doi: 10.1084/jem.127.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr E. L., Oei J. S. Immobilization of membrane H-2 antigens by paraformaldehyde fixation. J Cell Biol. 1973 Nov;59(2 Pt 1):537–542. doi: 10.1083/jcb.59.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr E. L., Oei J. S. Paraformaldehyde fixation of mouse cells with preservation of antibody-binding by the H-2 locus. Tissue Antigens. 1973;3(2):99–107. doi: 10.1111/j.1399-0039.1973.tb00984.x. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W., Jacobson J. B., Lardis M. P. Antigenic modulation in vitro. I. Fate of thymus-leukemia (TL) antigen-antibody complexes following modulation of TL antigenicity from the surfaces of mouse leukemia cells and thymocytes. J Exp Med. 1974 Oct 1;140(4):939–953. doi: 10.1084/jem.140.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. H., Calkins C. E., Jandinski J., Schendel D. J., Stutman O., Cantor H., Boyse E. A. The Qa-1 antigenic system. Relation of Qa-1 phenotypes to lymphocyte sets, mitogen responses, and immune functions. J Exp Med. 1978 Oct 1;148(4):963–973. doi: 10.1084/jem.148.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., Old L. J. Preleukemic expression of TL antigens in x-irradiated C57BL/6 mice. J Exp Med. 1977 Jul 1;146(1):271–276. doi: 10.1084/jem.146.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]



