Abstract
Nuclear factor erythroid-derived 2-related factor 1 (Nrf1) regulates cellular stress response genes, and has also been implicated to have a role in other cellular processes. We previously demonstrated that hepatocyte-specific deletion of Nrf1 in mice resulted in spontaneous apoptosis, inflammation, and development of liver tumors. Here, we showed that both fibroblasts derived from Nrf1-null mouse embryos, and fibroblasts bearing a conditional Nrf1 allele exhibited increased micronuclei and formation of abnormal nuclei. Lentiviral shRNA-mediated knockdown of Nrf1 in SAOS-2 cells also resulted in increased micronuclei, abnormal mitosis and multinucleated cells. Metaphase analyses showed increased aneuploidy in Nrf1-/- embryonic fibroblasts. Nuclear defects in Nrf1-deficient cells were associated with decreased expression of various genes encoding kinetochore and mitotic checkpoint proteins. Our findings suggest that Nrf1 may serve a function in maintaining genomic integrity, and Nrf1 dysregulation can induce tumorigenesis.
Introduction
Accurate chromosome segregation during mitosis is essential to maintain euploidy in eukaryotic cells. Errors in this process result in losses and gains of chromosomal material known as aneuploidy, which is associated with developmental abnormalities, syndromic conditions, and also a hallmark of cancer [1, 2]. Aneuploidy has been suggested to play a role in tumorigenesis by causing multiple genetic changes necessary for the onset and progression of cancer [3]. Faithful transmission of chromosomes relies upon the correct assembly and function of the kinetochore, which mediates attachment between chromosomes and spindle microtubules for proper chromosome segregation during mitosis [4]. The kinetochore is comprised of many different proteins including CENPs, NDC80/HEC1, NUF2, SGOL1, among others [5, 6]. To guard against aneuploidy, accurate chromosomal segregation during mitosis in eukaryotes is maintained by the spindle assembly checkpoint (SAC). The SAC prevents chromosome instability by delaying the onset of anaphase from metaphase until all of the chromosomes are properly aligned and attached to the mitotic spindle. The kinetochore serves to recruit SAC proteins such as, BUB1, BUB3, MAD2, Aurora-A and -B that monitors kinetochore microtubule attachment prior to anaphase. Altered expression or mutations of various components of the kinetochore and SAC has been linked to tumorigenesis [7-9].
Transcription of many detoxification genes and antioxidants is regulated through cis-acting sequences known as antioxidant response elements (AREs) [10]. These elements have been identified in the regulatory regions of genes involved in oxidative stress response and xenobiotic metabolism. For example, the ARE regulates expression of the catalytic and regulatory subunits of glutamylcysteine ligase involved in glutathione biosynthesis. The ARE also regulates heme oxygenase, glutathione reductases, thioredoxin reductases and ferritins, phase-2 enzyme genes including quinone reductases and glutathione-S-transferases. Phase-2 proteins catalyze the conversion of mutagenic metabolites or their precursors to less reactive and more excretable compounds.
Several members of the CNC-bZIP family have been shown to regulate ARE function [11]. The CNC family consists of four closely related members that include p45NFE2 (nuclear factor erythroid-derived 2), Nrf1 (nuclear factor erythroid-derived 2-related factor), Nrf2 and Nrf3, which are characterized by a highly conserved 43 amino acid sequence located on the N-terminal side of the basic-DNA-binding domain. Numerous studies indicate a pivotal role of Nrf2 in the protection against xenobiotics and electrophiles [ 10]. In contrast to Nrf2, less is known regarding the function of Nrf1. In vitro and in vivo studies indicate that Nrf1 modulates gene expression through the ARE, and preferentially activates a subset of oxidative stress response genes [12]. Recent studies suggest that Nrf1 is critical for both basal and inducible expression of genes encoding proteasomal subunits [13,14]. Aside from stress response, Nrf1 has been shown to regulate genes involved in development and other cellular processes. In cell development, Nrf1 has been found to activate expression of Osterix, a zinc finger transcription factor that regulates osteoblast differentiation during bone formation [15]. Nrf1 has also been reported to repress the expression of dentin sialophosphoprotein gene in terminally differentiated odontoblast [16].
Previously, we have generated hepatocyte-specific Nrf1 knockout mice (Nrf1LKO), and showed that these animals developed steatohepatitis and 100% liver tumor incidence by 12 months [17]. While it has been suggested that hepatic neoplasia is dependent on chronic liver inflammation in Nrf1LKO mice, a direct role for Nrf1 in tumorigenesis has not been ruled out. It is possible that Nrf1 may accelerate disease progression by regulating yet to be identified pathways. In this study, we used wild type and Nrf1 deficient cells to assess possible role of Nrf1 in maintaining chromosomal stability. Loss of Nrf1 was found to induce micronuclei formation and aneuploidy. In addition, we found that the expression of various genes encoding kinetochore proteins was reduced in Nrf1 deficient cells.
Results
Nrf1-/- cells exhibit increased abnormal nuclei
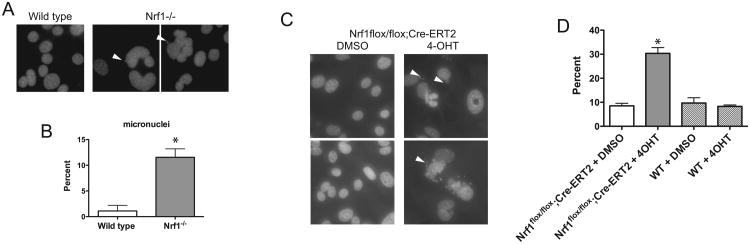
Hepatocyte-specific inactivation of Nrf1 leads to liver cancer with 100% penetrance and short tumor latency. Although inflammation has been suggested as a causative factor in neoplastic transformation of hepatocytes in Nrf1LKO mice, we speculate that loss of Nrf1 might contribute to tumorigenesis by promoting chromosome missegregation. To investigate this possibility, we examined Nrf1-/- MEF cells for micronuclei formation, which is representative of abnormal chromosome segregation during mitosis. As shown in Figure 1A, multinucleated cells and micronuclei-containing cells were readily observed in Nrf1-/- cells, while being markedly absent in wild type cells. Multinucleated and micronuclei-containing cells were detected at a 2-fold increase in Nrf1-/- cells when compared to wild type cells (Fig. 1B).
Figure 1. Nrf1 deficient cells exhibit abnormal nuclei.
(A) Micrograph of DAPI stained wild type and Nrf1-/- mouse embryonic fibroblasts. (C) Micrograph of DAPI stained DMSO- or 4OHT-treated Nrf1flox/flox;Cre-ERT2 fibroblasts. Note aberrant shaped nuclei and micronuclei (arrowheads) in Nrf1-/- cells and 4OHT-treated Nrf1flox/flox;Cre-ERT2 fibroblasts. (B, D) Histograms show mean ± standard deviation of micronuclei in control and Nrf1 knockout cells. Significant P values are indicated *(P < 0.05).
To examine the acute effects of Nrf1 ablation on genomic stability, we utilized MEF cells bearing an Nrf1 allele that can be disrupted by a tamoxifen (4OHT) inducible Cre-LoxP approach. MEF cells derived from Nrf1flox/flox;Cre-ERT2 mice have been described previously, and treatment of Nrf1flox/flox;Cre-ERT2 MEFs with 4OHT leads to the deletion of the Nrf1flox/flox conditional allele [13]. Quantitative RT-PCR analysis showed efficient inactivation of Nrf1 by 4OHT treatment of Nrf1flox/flox;Cre-ERT2 MEF cells. Nrf1 expression was reduced 80% in 4OHT-treated cells compared to vehicle control (Fig. 5B). In 4-OHT treated Nrf1flox/flox;Cre-ERT2 MEFs, increased abnormal nuclei and micronuclei were observed (Fig. 1C). The prevalence of micronuclei in 4OHT -treated Nrf1flox/flox;Cre-ERT2 MEF cells was 3-fold higher than DMSO-treated cells, whereas no difference was observed in DMSO- and 4OHT-treated control MEF cells (Fig. 1D).
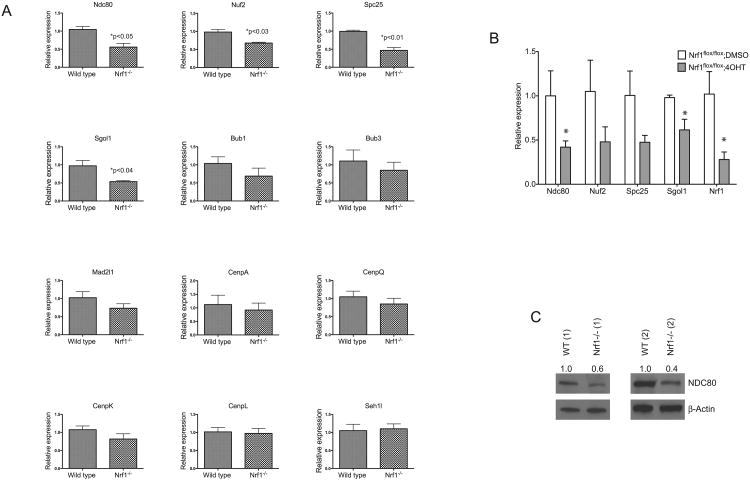
Figure 5. Nrf1-deficient cells have abnormal expression of kinetochore and spindle assembly checkpoint genes.
Comparison of mRNA encoding kinetochore and spindle assembly checkpoint genes in (A) wild type and Nrf1-/- MEF cells, (B) DMSO and 4-OHT treated Nrf1flox/flox;Cre-ERT2 fibroblasts by real time RTPCR analysis. Expression levels of genes were quantitated relative to endogenous beta-tubulin-5 (Tubb5) levels as an internal reference, and calculated as 2(Ct test gene-Ct Tubb5). Fold change in expression was determined from the difference between the averaged expression levels relative to control. Mean values ± SD of each genotype are shown, P-values less than 0.05 were considered statistically significant. (C) Whole cell lysates of 2 independent cultures of wild type and Nrf1-/- MEFs were immunoblotted with anti-NDC80 antibody, and beta-Actin was used as loading control.
Knockdown of Nrf1 leads to abnormal nuclear shape and micronuclei formation
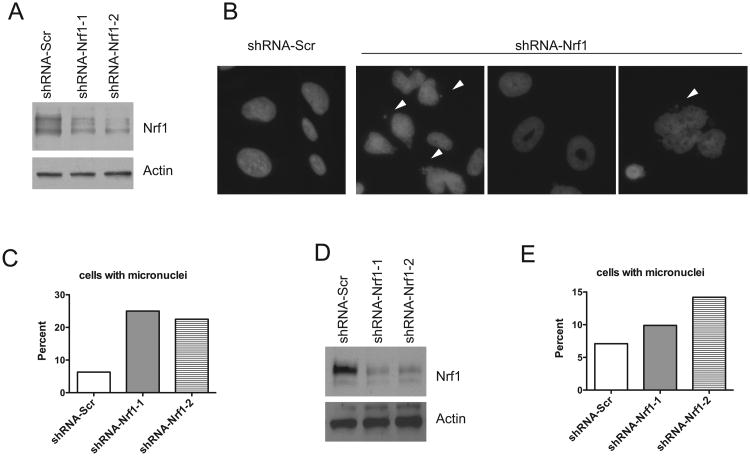
To exclude potential nonspecific effects associated with MEF cells, and to further substantiate that reducing Nrf1 function affects chromosome segregation during mitosis, we performed shRNA-mediated knockdown of endogenous Nrf1 in SAOS-2 cells. Two shRNAs against Nrf1 were used for transduction. As shown in Figure 2A, both constructs showed efficient knockdown of Nrf1 expression by Western blot analysis. SAOS-2 cells with Nrf1 knockdown exhibited a three-fold increase in the formation of micronuclei relative to the shScramble control (Fig. 2B, C). Knockdown of Nrf1 also resulted in increased number of cells with abnormally large and deformed nuclei (Fig. 2B, C). To exclude the possibility of cell type specificity, Nrf1 shRNA-mediated knockdown was performed on HCT116 cells to reaffirm the inductive effects Nrf1 depletion has on the formation of micronuclei. The depletion of Nrf1 by shRNA-mediated knockdown was verified by Western blot analysis (Fig. 2D). Similar to results obtained in SAOS-2 cells, the formation of micronuclei was increased in both Nrf1 knockdown clones of HCT116 cells (Fig. 2E).
Figure 2. Knockdown of Nrf1 leads to abnormal nuclear shape and micronuclei formation.
(A) Protein extracts of SAOS cells transduced with scramble shRNA, or Nrf1 shRNA were subjected to western blotting using the antibodies indicated. (B) Representative immunofluorescence analysis of SAOS cells transduced with scramble shRNA, or Nrf1 shRNA. Note multinuclei, donut-shaped and aberrant shaped nuclei in Nrf1 knockdown cells. Arrowhead indicates micronuclei. (C) Histograms show mean ± standard deviation of micronuclei in control and Nrf1 knockdown SAOS cells. Significant P values are indicated *(P < 0.05). (D) Protein extracts of HCT116 cells transduced with scramble shRNA, or Nrf1 shRNA were subjected to western blotting using the antibodies indicated. (E) Histograms show mean ± SD of abnormal and micronuclei in control and Nrf1 knockdown HCT116 cells. Significant P values are indicated *(P < 0.05).
Knockdown of Nrf1 in SAOS-2 cells leads to increased chromosome missegragation
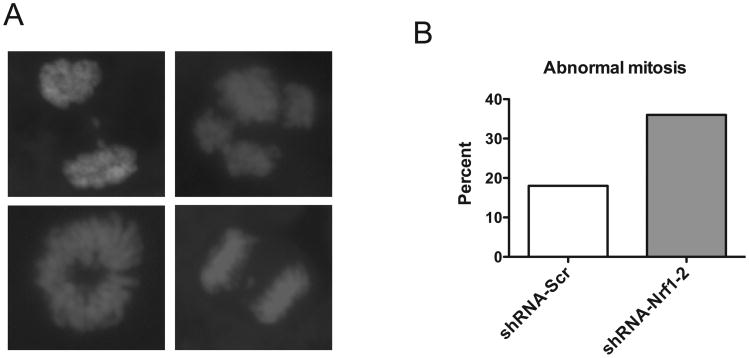
Next, we determined whether reduction in Nrf1 function leads to defective chromosome segregation during mitosis. Control and knockdown mitotic cells were stained for DNA and the frequency of aberrant mitotic phenotype was found to be 2-fold higher in Nrf1 knockdown cells compared to shScramble control cells (Fig. 3). These abnormalities included cells with lagging chromosomes, anaphase bridges, and misaligned chromosomes (Fig. 3). A similar spectrum of mitotic aberrations was also observed in knockout MEF cells (data not shown). These results suggest that loss of Nrf1 disrupted normal mitosis.
Figure 3. Knockdown of Nrf1 in SAOS cells leads to abnormal chromosome segregation.
Micrographs of DAPI stained SAOS cells transduced with Nrf1 shRNA showing atypical anaphase figures (multipolar centrosome, abnormal chromosome segregation and anaphase bridge). Histogram shows percentage of abnormal mitotic figures in control and Nrf1 knockdown cells.
Nrf1-/- MEF cells exhibit increased chromosomal instability
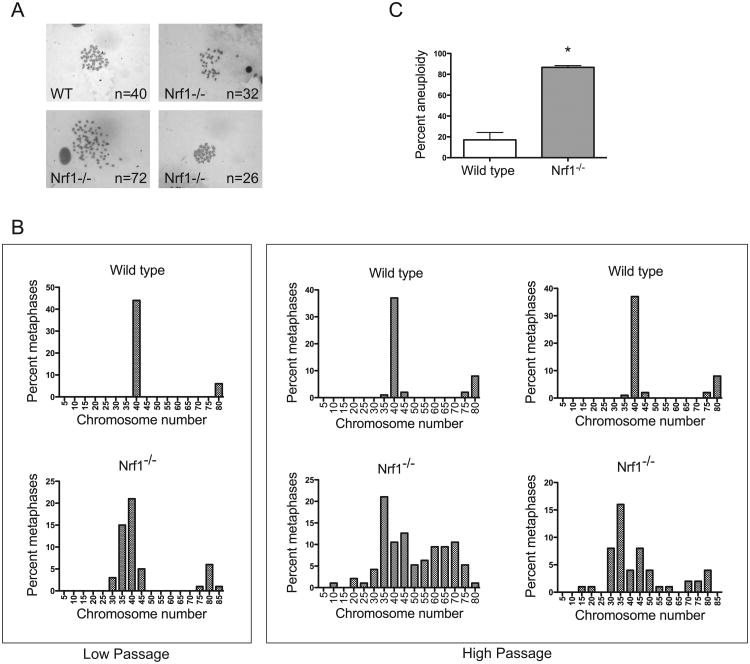
Micronuclei formation often leads to genomic instability, and is considered a hallmark of aneuploidy. We next examined whether Nrf1-deficient cells are prone to aneuploidy, by comparing metaphase spreads of wild type and Nrf1-/- MEFs. At early passage (P3), wild type MEF cells exhibited cells with a diploid number of chromosomes (Fig. 4B). However, contrary to wild type MEF cells, low passage Nrf1-/- cells exhibited abnormal chromosome number (Fig. 4B). Metaphase spreads of two independent high passage wild type and Nrf1-/- MEF cell cultures were also analyzed. At higher passages, the frequency of wild type cells bearing abnormal number of chromosomes was slightly elevated (Fig. 4B and C). In contrast, Nrf1-/- MEF cells possessed a significant increase in the frequency of aneuploidy (Fig. 4B and C). The aneuploidy observed in Nrf1-/- MEF cells is consistent with genetic instability.
Figure 4. Nrf1-/- cells exhibit increased chromosomal instability.
(A) Representative Giemsa-stained metaphase spread of wild type MEF showing normal (40) number of chromosomes. (B) Representative Giemsa-stained metaphase spread of Nrf1-/- MEF showing aneuploidy. (C) Analysis of the percentage of aneuploid cells in MEF cultures. Values are from three independent wild type and Nrf1-/- MEF cultures. 95 metaphases were counted for each MEF line. (D) Distribution of chromosome numbers of wild type and Nrf1-/- MEF lines. Data shown are combined values from two independent MEF lines per genotype.
Nrf1-deficient cells have abnormal expression of kinetochore and spindle assembly checkpoint genes
Given that Nrf1 is a transcription factor, we investigated whether loss of Nrf1 function affects the expression of genes involved in the maintenance of genomic stability. RNA was extracted from wild type and Nrf1-/- MEF cells, and the expression levels of various genes encoding components of the kinetochore and SAC were analyzed by quantitative RT-PCR. In Nrf1-/- MEF cells, kinetochore genes Ndc80, Nuf2, Spc25 and SAC gene Sgol1 were reduced approximately two-fold when compared to wild type cells (Fig. 5A). However, expression of other kinetochore and SAC genes such as Bub1, Bub3, and CENPs did not exhibit altered levels (Fig. 5A and data not shown). In accord with RT-PCR results, Western blotting revealed lowered NDC80 levels in Nrf1-/- MEF cells (Fig. 5C). Protein levels for Spc25 and Nuf2 were not analyzed due to lack of suitable antibodies.
To determine if the cause of variance in gene expression observed in Nrf1-/- MEF cells is a direct result of Nrf1 deficiency, quantitative RT-PCR analysis was performed on Nrf1flox/flox;Cre-ERT2 MEF cells. Inducible inactivation of Nrf1 by 4-OHT was verified by qRT-PCR (Fig. 5B). Transcripts of Ndc80, Nuf2, Spc25, and Sgol1 were similarly reduced in Nrf1flox/flox;Cre-ERT2 MEF cells after 4-OHT treatment (Fig. 5B). These data suggest that down-regulation of Ndc80, Nuf2, Spc25, and Sgol1 is specific to loss of Nrf1 function.
Discussion
The function of Nrf1 has been defined in terms of its role in regulating the transcription of genes involved in cellular stress response. However, there is evidence to suggest a role for Nrf1 in other cellular functions such as development and inflammation. In the present study, we examined the effects of Nrf1 deficiency on genomic stability in cultured cells. We demonstrate here that loss of Nrf1 function using either primary Nrf1-/- MEF cells or MEF cells rendered Nrf1 deficient acutely by tamoxifen-inducible conditional system lead to increased formation of micronuclei, abnormal nuclei and multinucleated cells. Moreover, shRNA mediated knockdown of Nrf1 produced a similar result in SAOS and HCT116 cells, indicating that the defects are specific to Nrf1 deficiency. The influence Nrf1 has on genomic integrity was further highlighted when all three Nrf1-deficient fibroblast cell lines exhibited an increased incidence of aneuploidy with each passage of cells. It is known that altered expression of genes encoding components of the kinetochore and SAC could trigger aneuploidy [7-9]. Our results demonstrate that expression of kinetochore genes Ndc80, Nuf2, Spc25 and spindle assembly gene Sgo1 are down regulated in Nrf1-/- MEF cells.
The Nrf1 associated mechanisms that underlie the maintenance of genome integrity are largely unknown, but they could play an integral role in the prevention of abnormal kinetochore formation or the reduction of SAC function. In eukaryotic cells, faithful chromosome segregation during mitosis is crucial for maintaining genomic integrity. This function is mediated by the kinetochore that resides at the centromere and binds to spindle microtubules for chromosome segregation. It has been shown that altered expression of genes encoding components of the kinetochore apparatus can lead to chromosome segregration defects and consequent aneuploidy [7-9]. The Ndc80 complex is an important component of the kinetochore that mediates proper metaphase chromosome alignment and anaphase segregation. [19-21]. The Ndc80 complex consists of 4 different proteins including, Ndc80/Hec1, Nuf2, Spc24 and Spc25. Dysregulated expression of Ndc80 has been observed in a variety of human cancers [22, 23]. This conserved protein complex is suited to mediate the interface between essential kinetochore function of binding microtubules and regulating cell cycle in response to aberrant kinetochore function. In addition, the Ndc80 complex is required for SAC function through the localization of other kinetochore proteins Cenp-A, Mad1, Mad2, Bub1, Zw10 and Bub3 to the kinetochore. Our results demonstrate that expression of Ndc80, Nuf2, and Spc25 are down regulated in Nrf1-/- MEF cells.
Segregation is also monitored by the spindle assembly checkpoint, a surveillance mechanism that is part of the kinetochore apparatus that blocks the progression of mitosis unless all chromosomes are properly assembled to the spindle [6]. A key protein in SAC function is Sgol1, which protects sister chromatids from precocious separation. Failure of the spindle assembly checkpoint machinery to detect and signal for repair results in aneuploidy that is frequently observed in many types of cancer cells [9]. Our results show a reduction in the transcript level encoding Sgol1 in Nrf1-/- MEF cells, and decreased levels of Sgol1 lead to chromosomal instability in colon cancer cells [24]. Therefore, our observation of increased micronuclei formation and aneuploidy in Nrf1 deficient cells could be the result of segregation defects caused by abnormal kinetochore formation or SAC function. Thus, it is likely that reduction in Ndc80, Nuf2, Spc24 and Sgol1 exerts a synergistic effect on genomic stability in Nrf1 knockout cells.
Currently, it is not known whether any of the genes identified by RT-PCR are direct targets of Nrf1. Sequence analyses revealed potential ARE sites located upstream from the transcription start sites of Ndc80, Nuf2 and Spc25 genes that could explain the decrease in expression of these transcripts in Nrf1 knockout cells. Moreover, it is interesting to note that transcription of human Ndc80/Hec1 gene promoter is regulated by activating transcription factor 4 (ATF4) [25], and ATF4 has been implicated as a binding partner for Nrf1 [26]. The possibility that expression of these genes is Nrf1 dependent requires further experimentation.
Our results here also provide a potential molecular link between Nrf1 and cancer. Previously, it was shown that Nrf1LKO mice spontaneously developed steatohepatitis and liver tumors [17]. However, the precise mechanism of tumorigenesis in Nrf1LKO livers remains uncertain. It is a that tumorigenesis is a multistep process involving the accumulation of mutations at various genetic loci, as well as the acquisition of genomic instability [27]. The prevalence of cancer in Nrf1LKO mice may stem from sustained proliferation of hepatocytes in an inflammatory environment combined with DNA damage caused by increased oxidative stress. Our current findings suggest that dysregulation of mitotic checkpoint genes may also contribute by abating genomic integrity in Nrf1-deficient cells. The existence of aneuploidy in cells could result in the loss of tumor suppressors or the gain of oncogenes aiding initiation and progression of tumorigenesis in Nrf1LKO livers. Nonetheless, our observations have provided insight requiring further experimentation in Nrf1KO animals.
Finally, it is widely known that soy products have beneficial properties that help battle many diseases. Genistein, the most abundant isoflavone compound found in soy products was shown to have therapeutic effects on a multitude of diseases including cancer [28]. Moreover, pretreatment with genistein has been shown to protect against dimenthybenz[a]anthracene-induced micronuclei formation and chromosomal structural abnormalities in the bone marrow [29], while a recent study indicates genistein's ability to activate Nrf1 [30]. Overall, the protective effects of genistein have been attributed to the activation of oxidative stress response and expression of antioxidant genes. However, these studies together with our results suggest the possibility that genistein's beneficial health effects in the prevention of cancer development arise from the induction of Nrf1, which leads to the improvement of genetic stability. Further studies exploring the mechanism underlying Nrf1 activation by genistein would be of great interest.
In summary, this study shows that loss of Nrf1 function can cause genomic instability, and suggests that the role of Nrf1 in cellular homeostasis extends beyond oxidative and proteolytic stress response to include maintenance of genomic integrity. Thus Nrf1 may have important implications for the understanding of how genome stability is promoted.
Materials and Methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), McCoy's 5a medium, fetal bovine serum (FBS) and calf serum were purchased from Invitrogen (Carlsbad, CA). Antibody for Nrf1 was previously described [18]. Biotinylated anti–rabbit IgG and 3, 3′ diaminobenzadine (DAB) substrate kit are from Vector labs (Burlingame, CA). Chemiluminescent detection system was purchased from Piercenet (Rockford, IL).
Cell lines
HCT116 colon cancer cell lines were a generous gift from Dr. Bert Vogelstein (Johns Hopkins University Medical Institutions, Baltimore, MD), and SAOS-2 cells were from ATCC (Manassas, VA). HCT116 cells were cultured in McCoy's 5a containing 10% FBS, 1% non-essential amino acids, 1% HEPES, and 1% Pen-Strep. SAOS-2 cells were cultured in 15% FBS, 1% L-glutamine, and 1% Pen-Strep. Nrf1-/- MEF cells were cultured in DMEM as described previously [13].
Lentivirus production and infection
MISSION shRNA lentiviral constructs for Nrf1 were purchased from Sigma. Virus production was done according to manufacturer's protocol. Briefly, HEK293 cells were cotransfected with LKO.1 lentiviral, Delta VPR 8.9 and VSV-G plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Virus-containing supernatant was collected 48 h after transfection and filtered through a 0.45 um cellulose acetate filter (Millipore). Infections of cells were carried out in the presence of 10 μg/mL polybrene and 10 mM HEPES. After transduction, puromycin was used to select for stable clones.
Western Analysis
Protein lysates from cells were prepared using NP-40 lysis buffer and electrophoresed on SDS-polyacrylamide gels. Proteins were subsequently transferred onto nitrocellulose membranes and blocked in 5% milk at room temperature for one hour. Primary antibody incubation was done overnight at 4°C followed by incubation with a horseradish peroxidase-conjugated secondary antibody. Proteins were detected by chemiluminescence.
Preparation and examination of metaphase spreads
Cells were harvested after treatment with 100 ng/ml nocodazole and swollen in a hypotonic buffer (10mM Tris-HCl pH 7.5, 10mM NaCl, 5mM MgCl2) for 15 min at 37°C. Cells were fixed with freshly made Carnoy's solution (75% methanol, 25% acetic acid), and the fixative was changed several times. For spreading, cells were dropped on to slides. Slides were air dried at room temperature and stained with 5% Giemsa at pH 6.8 for 7 min, washed briefly in deionized water, air dried, and mounted with Permount.
RNA Isolation and Quantitative RT-PCR
RNA was extracted using UltraSpec RNA (BiotecX, Houston, TX). cDNAs were synthesized from 10μg total RNA in 20μL reactions containing 1× RT buffer, 1mM dNTPs, 0.3μg random hexamer, 40 units of RNase inhibitor, and 250 units of Moloney murine leukemia virus reverse transcriptase. Reverse transcription reactions were incubated at 72°C for 5 min and then 25°C for 10 min, followed by 42°C for 60 min. Aliquots of cDNA were amplified in Step One Plus PCR machine (Applied Biosystem) using FastStart SyBr Green reagent (Roche, Indianapolis, IN). Each cDNA amplification was conducted in duplicates comprising 20μL reaction volumes. PCR cycling conditions consist of 95°C for 15 min and 45 cycles of 95°C for 30 s, 60°C for 30 s, and 68°C for 45 s. Expression levels were calculated relative to the endogenous control levels of beta-tubullin-5 (Tubb5). Relative expression was quantitated using the following formula 2(Ct test gene – Ct Tubb5).
Statistical Analysis
Data was expressed as means ± standard deviation. For statistical comparison, Student's t-test was used. P-values less than 0.05 were considered statistically significant.
Primer Sequences
| Gene | Forward | Reverse |
|---|---|---|
| mTubb5 | GATCGGTGCTAAGTTCTGGGA | AGGGACATACTTGCCACCTGT |
| mSeh1L | CACAAGGACCTCATCCACGAT | TCTCGCTCTTATCCCACACCT |
| mNdc80 | CTGAGTACACACAAACCGACAT | TGTCCGCTAGTCCTTTTCCCA |
| mNuf2 | TGCCGATGAACATAGAAGTCAC | ACTGGTAAGAAGCCCTCCATTA |
| mSpc25 | GAGCTGACTGCTAAAATCCAGG | CTCTTCCTAGCGTACTCTTCCTT |
| mSgol1 | CCTCCAGGGCCAACATCAAGG | AAGGCTGTGATGGGCGAGAGG |
| mMad2l1 | GTGGCCGAGTTTTTCTCATTTG | AGGTGAGTCCATATTTCTGCACT |
| mChek1 | GTTAAGCCACGAGAATGTAGTGA | GATACTGGATATGGCCTTCCCT |
| mCenpL | AACTCACGGATATTGAAGTGCAT | CACAGAGCCAGGATACTGCTT |
| mCenpQ | AGAGCCAATAAGGAAGCGGAT | AACATACGTTGCTTGTCCTGTTA |
| mCenpK | TGAACTGACAACTAAGATCCGTG | AAAAAGCACCCAAGGTAAGCA |
| mBub1 | GCTGCCAACCTGCCAGCTCTT | TGATGGCTGCACTTTGCATGG |
| mBub3 | CACTGAAGCCCTCCGGACGAT | AGGGACAGCCAAACGGAACCA |
| mCenpA | AGGGCGGACTCCCCTAAGCTG | GCGAGTGGGCAGACCTCTGTG |
Acknowledgments
We thank Jocelyn Lee for technical assistance. This research was supported by the NIH grants CA091907 and NS065223.
Abbreviations
- Nrf1
Nuclear factor erythroid-derived 2-related factor 1
- SAC
spindle assembly checkpoint
- MEF
mouse embryonic fibroblast
Footnotes
The content is solely the responsibility of the authors, and they have no conflicts of interest to disclose.
References
- 1.Callier P, Faivre L, Cusin V, Marle N, Thauvin-Robinet C, Sandre D, Rousseau T, Sagot P, Lacombe E, Faber V, et al. Microcephaly is not mandatory for the diagnosis of mosaic variegated aneuploidy syndrome. Am J Med Genet A. 2005;137:204–207. doi: 10.1002/ajmg.a.30783. [DOI] [PubMed] [Google Scholar]
- 2.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 3.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musacchio A. Spindle assembly checkpoint: the third decade. Philos Trans R Soc Lond B Biol Sci. 2011;366:3595–3604. doi: 10.1098/rstb.2011.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleveland DW, Mao Y, Sullivan KF. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 6.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 7.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 8.Hernando E, Nahle Z, Juan G, Diaz-Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 9.Weaver BA, Cleveland DW. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 2007;67:10103–10105. doi: 10.1158/0008-5472.CAN-07-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. The Journal of biological chemistry. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motohashi H, O'Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 12.Biswas M, Chan JY. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol Appl Pharmacol. 2010;244:16–20. doi: 10.1016/j.taap.2009.07.034. S0041-008X(09)00324-X [pii]10.1016/j.taap.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CS, Lee C, Hu T, Nguyen JM, Zhang J, Martin MV, Vawter MP, Huang EJ, Chan JY. Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc Natl Acad Sci U S A. 2011;108:8408–8413. doi: 10.1073/pnas.1019209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. S1097-2765(10)00240-6 [pii]10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing W, Singgih A, Kapoor A, Alarcon CM, Baylink DJ, Mohan S. Nuclear factor-E2-related factor-1 mediates ascorbic acid induction of osterix expression via interaction with antioxidant-responsive element in bone cells. The Journal of biological chemistry. 2007;282:22052–22061. doi: 10.1074/jbc.M702614200. [DOI] [PubMed] [Google Scholar]
- 16.Narayanan K, Ramachandran A, Peterson MC, Hao J, Kolsto AB, Friedman AD, George A. The CCAAT enhancer-binding protein (C/EBP)beta and Nrf1 interact to regulate dentin sialophosphoprotein (DSPP) gene expression during odontoblast differentiation. The Journal of biological chemistry. 2004;279:45423–45432. doi: 10.1074/jbc.M405031200. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z, Chen L, Leung L, Yen TS, Lee C, Chan JY. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci U S A. 2005;102:4120–4125. doi: 10.1073/pnas.0500660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Kwok AM, Chan JY. The p65 isoform of Nrf1 is a dominant negative inhibitor of ARE-mediated transcription. The Journal of biological chemistry. 2007;282:24670–24678. doi: 10.1074/jbc.M700159200. [DOI] [PubMed] [Google Scholar]
- 19.Wigge PA, Kilmartin JV. The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J Cell Biol. 2001;152:349–360. doi: 10.1083/jcb.152.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharadwaj R, Qi W, Yu H. Identification of two novel components of the human NDC80 kinetochore complex. The Journal of biological chemistry. 2004;279:13076–13085. doi: 10.1074/jbc.M310224200. [DOI] [PubMed] [Google Scholar]
- 21.DeLuca JG, Dong Y, Hergert P, Strauss J, Hickey JM, Salmon ED, McEwen BF. Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell. 2005;16:519–531. doi: 10.1091/mbc.E04-09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bieche I, Vacher S, Lallemand F, Tozlu-Kara S, Bennani H, Beuzelin M, Driouch K, Rouleau E, Lerebours F, Ripoche H, et al. Expression analysis of mitotic spindle checkpoint genes in breast carcinoma: role of NDC80/HEC1 in early breast tumorigenicity, and a two-gene signature for aneuploidy. Mol Cancer. 2011;10:23. doi: 10.1186/1476-4598-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–1521. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwaizumi M, Shinmura K, Mori H, Yamada H, Suzuki M, Kitayama Y, Igarashi H, Nakamura T, Suzuki H, Watanabe Y, et al. Human Sgo1 downregulation leads to chromosomal instability in colorectal cancer. Gut. 2009;58:249–260. doi: 10.1136/gut.2008.149468. [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, Li L, Qiao X, Liu J, Yao X. Functional characterization of the promoter of human kinetochore protein HEC1: novel link between regulation of the cell cycle protein and CREB family transcription factors. Biochim Biophys Acta. 2007;1769:593–602. doi: 10.1016/j.bbaexp.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Masuoka HC, Townes TM. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood. 2002;99:736–745. doi: 10.1182/blood.v99.3.736. [DOI] [PubMed] [Google Scholar]
- 27.Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet. 2012;13:189–203. doi: 10.1038/nrg3123. [DOI] [PubMed] [Google Scholar]
- 28.Wiegand H, Wagner AE, Boesch-Saadatmandi C, Kruse HP, Kulling S, Rimbach G. Effect of dietary genistein on Phase II and antioxidant enzymes in rat liver. Cancer Genomics Proteomics. 2009;6:85–92. [PubMed] [Google Scholar]
- 29.Pugalendhi P, Manoharan S, Panjamurthy K, Balakrishnan S, Nirmal MR. Antigenotoxic effect of genistein against 7,12-dimethylbenz[a]anthracene induced genotoxicity in bone marrow cells of female Wistar rats. Pharmacol Rep. 2009;61:296–303. doi: 10.1016/s1734-1140(09)70035-0. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Montes E, Pollard SE, Vauzour D, Jofre-Montseny L, Rota C, Rimbach G, Weinberg PD, Spencer JP. Activation of glutathione peroxidase via Nrf1 mediates genistein's protection against oxidative endothelial cell injury. Biochem Biophys Res Commun. 2006;346:851–859. doi: 10.1016/j.bbrc.2006.05.197. [DOI] [PubMed] [Google Scholar]