Abstract
A major function of a subfamily of NLR (nucleotide-binding domain, leucine rich repeat containing or NOD-like receptor) proteins is in inflammasome activation, which has been implicated in a multitude of disease models and human diseases. This work will highlight key progress in understanding the mechanisms which activates the best studied NLRs (NLRP3, NLRC4, NAIP and NLRP1) and in uncovering new inflammasome NLRs.
Introduction
The inflammasome which leads to caspase-1 protease activation has broad biologic and clinical impact. This review will focus on recent discoveries made in the last two to three years which have uncovered new signaling pathways that leads to NLR (nucleotide-binding domain, leucine rich repeat containing or NOD-like receptor) inflammasome activation, new roles of NLRs in canonical and noncanonical inflammasome activation, and new NLR inflammasomes. The pathological effects of inflammasome activation in diseases have been reviewed extensively, and will not be discussed here (Lamkanfi and Dixit, 2012; Strowig et al., 2012).
New mechanisms of NLRP3 inflammasome activation
Despite of the rapid discovery of new activating agents of the NLRP3 inflammasome, uncovering the precise molecular mechanisms for NLRP3 inflammasome activation remains challenging. However, recent studies have identified both new mechanisms and new molecules that are involved in NLRP3 inflammasome activation in response to various stimuli.
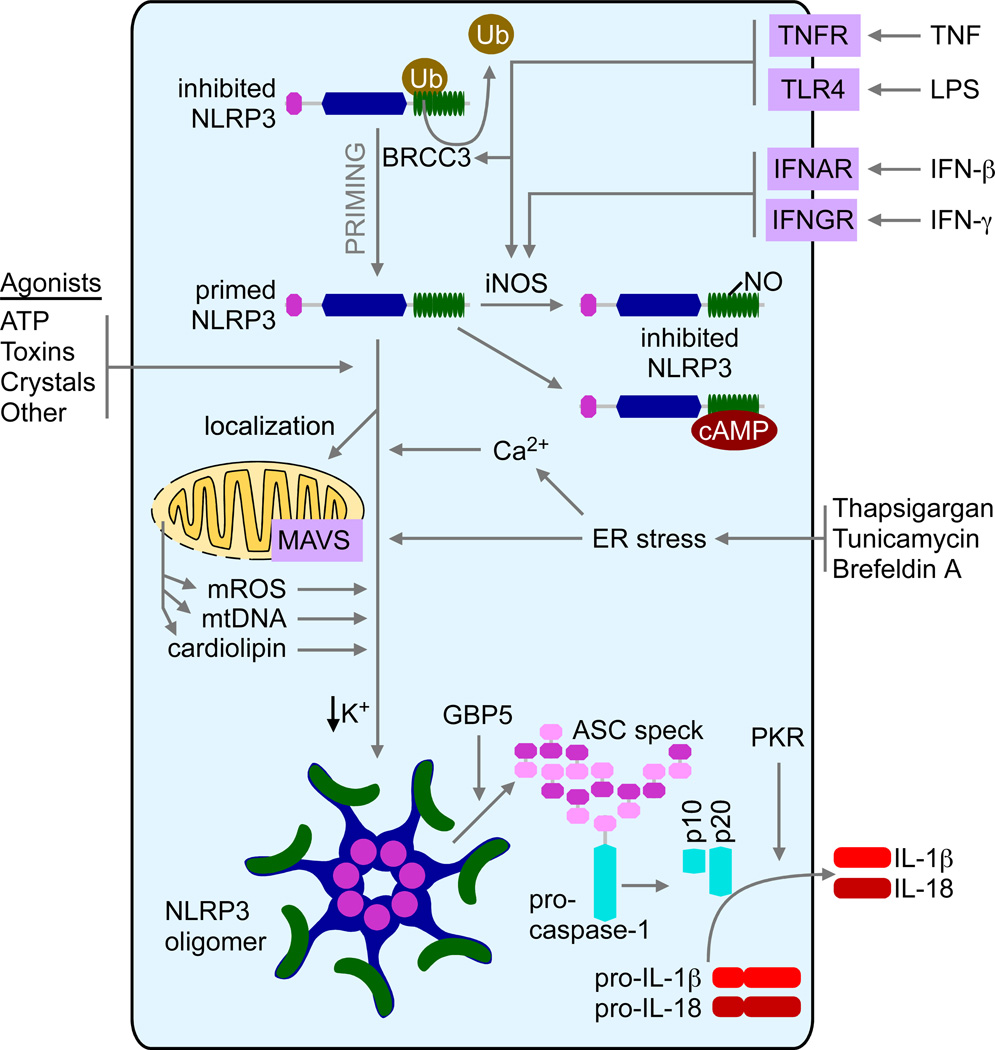
Numerous recent studies have evoked an essential role of mitochondria in NLRP3 inflammasome activation by mechanisms that could be generalized into two categories. First, the mitochondria provide an ideal platform for assembly of the NLRP3 inflammasome complex. Second, NLRP3 may be activated directly by mitochondria-derived effector molecules such as mitochondrial reactive oxygen species (mROS), mitochondrial DNA (mtDNA), and phospholipid cardiolipin (Figure 1).
Figure 1. Schematic of the NLRP3 inflammasome activation in response to mitochondria, ER and Ca2+ signals.
The activation of the NLRP3 inflammasome by its typical stimuli such as ATP, toxin and crystalline reagents involve mitochondria-derived signals such as mROS, mtDNA and cadiolipin. The relocalization of NLRP3 form the cytosol to mitochondria is MAVS-dependent and is pivotal for optimal inflammasome activation. GBP5 promotes NLRP3 signaling to ASC. ER stress has been reported to activate the NLRP3 inflammasome through a mechanism that seems not to require the typical unfolded protein response pathway. Elevated intracellular Ca2+ and/or decreased intracellular K+ are required for NLRP3 inflammasome activation.
Previous studies suggest that NLRP3 inflammasome activation induces the relocalization of NLRP3 from a cytosolic compartment to the mitochondria, based on both biochemical and imaging assays (Subramanian et al., 2013; Zhou et al., 2011). This process requires the first six amino acids at the N-terminus of the PYRIN domain of NLRP3, which are also essential for optimal NLRP3 inflammasome activation. The mitochondrial antiviral signaling (MAVS) adaptor protein is shown to mediate this process by associating with NLRP3 (Subramanian et al., 2013). Of note, macrophages form MAVS deficient (Mavs−/−) mice show a defective NLRP3 inflammasome activation in response to ATP and nigericin (a microbial toxin that acts as potassium ionophore and activates the NLRP3 inflammasome) selectively, but not to crystalline reagents such as alum and silica, suggesting that MAVS is not absolutely required for NLRP3 activation (Subramanian et al., 2013). The mitochondrial recruitment of NLRP3 and optimal NLRP3 inflammasome activation also requires the functional microtubule system and signals through a metabolic signaling axis including NAD+-sirtuin 2-tubulin-dynein (Misawa et al., 2013).
There has been a long-term debate about the role of mROS in NLRP3 activation. Many studies have shown that increased mROS activates the NLRP3 inflammasome (Zhou et al., 2010; Zhou et al., 2011), whereas at least one study argues that instead of activating NLRP3, increased ROS promotes NLRP3 expression at the transcriptional level (Bauernfeind et al., 2011). These discordant conclusions may be due to a wide usage of pharmacological activators or inhibitors of ROS and mitochondrial functions, which causes various outcomes depending on the dosage, duration and cell types. Of the note, another study argues that a drop in cytosolic K+, but not mitochondrial perturbation, causes NLRP3 activation (Munoz-Planillo et al., 2013). Whether this study has truly defined the convergent point between all NLRP3 agonists needs additional investigation. Mitochondria may also contribute to NLRP3 activation by releasing mtDNA (Nakahira et al., 2011; Shimada et al., 2012) or phospholipid cardiolipin (Iyer et al., 2013).
Due to the variety of molecular structures such as pattern-associated molecular pattern (PAMP) and damage-associated molecular pattern (DAMP) containing molecules that can activate the NLRP3 inflammasome, it is generally accepted that NLRP3 is not likely to recognize these motifs, but rather senses the disturbance of cellular homeostasis, i.e. cell stress, such as the changes in redox status or cellular ion concentration. One of typical cell stresses originates from the loss of endoplasmic reticulum (ER) homeostasis, namely ER stress. ER stress is induced by the accumulation of unfolded protein in the ER or the disruption of ER Ca2+ and activates the downstream unfolded protein response (UPR) (Cox et al., 1997). The role of ER stress has been implicated in a multitude of human diseases (Hotamisligil, 2010; Ozcan and Tabas, 2012; Ron and Walter, 2007; Todd et al., 2008; Zhang and Kaufman, 2008). Recent studies suggest that ER stress can activate NLRP3 (Figure 1). Multiples ER stressors induce NLRP3- and ASC (apoptosis-associated speck-like protein containing a CARD)-dependent caspase-1 cleavage followed by IL-1β maturation (Menu et al., 2012). Interestingly, none of three known UPR initiators (PERK, IRE1α and ATF6) seems to be required for ER stress-induced NLRP3 activation. Two recent studies have confirmed ER stress-activated NLRP3 and further indicated thioredoxin-interacting protein (TXNIP) as a key mediator (Lerner et al., 2012; Oslowski et al., 2012). Since TXNIP expression is increased only by ER stressors, but not by ATP, one may hypothesize that TXNIP is only required for NLRP3 activation by ER stress, but not by other stimuli. These studies may reconcile the long-term debate about whether TXNIP plays a role in NLRP3 activation (Masters et al., 2010; Zhou et al., 2010). However, significant questions remain to be answered. For example, is TXNIP-mediated NLRP3 activation cell-type specific, such as in pancreatic β cells but not in myeloid cells? Is TXNIP involved in NF-κB-dependent signal-1 (pro-IL-1β generation and NLRP3 upregulation), or signal-2 (inflammasome activation), or both, since ER stress can clearly enhance both signals? Further investigation of the mechanisms of ER stress-induced NLRP3 inflammasome activation is warranted.
The importance of intracellular Ca2+ ([Ca2+]i) in IL-1β processing (a downstream event following NLRP3 activation) was initially based on the observation that pretreatment of cells with Ca2+ chelator, BAPTA-AM, abolished ATP- and nigericin-induced IL-1β maturation (Brough et al., 2003). Although ATP or nigericin causes rapid Ca2+ influx and a sharp increase in [Ca2+]i, extracellular Ca2+ influx is not absolutely required for the cleavage of caspase-1 and IL-1β. This is due to Ca2+ release from ER, the major intracellular Ca2+ store. ATP stimulation leads to the translocation and activation of phospholipase C (PLC), which catalyzes inositol triphosphate (IP3) production. IP3 causes rapid Ca2+ release from ER by activating an IP3 receptor on ER membranes and increased [Ca2+]i subsequently triggers extracellular Ca2+ influx through store-operated Ca2+ entry (SOCE) (Feske et al., 2012). Treatment of cells with pharmacological inhibitors of PLC, IP3 receptor (IP3R) or SOCE significantly decreases NLRP3 inflammasome activation (Murakami et al., 2012) (Figure 1).
How does elevated intracellular Ca2+ promote NLRP3 inflammasome activation? One study suggests that mitochondrial damage induced by Ca2+ causes NLRP3 inflammasome activation through mROS and mtDNA release (Murakami et al., 2012). This is consistent with the concept that Ca2+ mobilization from the ER to the mitochondria occurs rapidly and mitochondrial Ca2+ overload causes mitochondrial damage (Rizzuto et al., 2012; Rizzuto et al., 1998). Therefore, inhibition of mitochondrial Ca2+ uptake by silencing mitochondrial Ca2+ uniporter (MCU) abolishes NLRP3-dependent IL-1β release (Triantafilou et al., 2013a). Two other studies have reported the effect of G protein-coupled Ca2+-sensing receptors, CASR and GPRC6A, in NLRP3 activation (Lee et al., 2012; Rossol et al., 2012). CASR activation by extracellular Ca2+ triggers positive feedback signaling cascade involving PLC-IP3-IP3R, which leads to increased [Ca2+]i and NLRP3 activation. Increased [Ca2+]i is also required for NLRP3 activation by encephalomyocarditis virus (Ito et al., 2012). CASR stimulation reduces intracellular cyclic AMP (cAMP), which has been suggested to be an inhibitor of NLRP3 (Lee et al., 2012). Other studies have reported that Ca2+-permeable channels, transient potential melastatin-like 2 (TRPM2) (Zhong et al., 2013), TRPV2 and TRPM7 (Compan et al., 2012), are required for Ca2+ influx and NLRP3 activation by crystalline reagents, liposome and extracellular hypo-osmolarity, respectively. Therefore, NLRP3 activation requires increased [Ca2+]i and Ca2+-dependent signaling.
The conceptual similarity between the inflammasome (NLR or a non-NLR sensor, ASC and caspase-1) and the apoptosome (Apaf-1, cytochrome c and caspase-9) has been increasingly appreciated: as they both form multiprotein complexes in response to specific activating signals and they both control inflammation and cell death. Recent studies reveal that the inhibitors of apoptosis protein (IAPs), which are critical inhibitors of apoptotic signaling, mediate inflammasome activation in both positive and negative manner. The cIAPs (cIAP1 and cIAP2) associate with caspase-1 and promotes its K63-linked polyubiquitination, which is essential for NLRP3 activation. The deletion of the gene encoding cIAP2 (Birc3−/−) results in impaired NLRP3 activation (Labbe et al., 2011). In contrast, the deletion of all three IAPs (XIAP, cIAP1 and cIAP2) leads to NLRP3-caspase-1-dependent and caspase-8 dependent IL-1β activation, which is mediated by RIP3 (receptor-interacting protein 3)-dependent ROS generation (Vince et al., 2012). The cause of conflicting data from the above two studies is not clear, although it may be due to usage of different gene-deletion mice strains. Therefore, targeting IAPs by Smac (second mitochondria-derived activator of caspase) mimetics could be a therapeutic regimen for IL-1β-mediated inflammatory diseases.
Caspase-8, an important effector apoptosis caspase, can trigger IL-1β and IL-18 cleavage by the engagement of cell surface receptors including the death inducing receptor FAS (CD95) (Bossaller et al., 2012; Miwa et al., 1998), Toll-like receptors (Maelfait et al., 2008), and the C-type lectin receptor dectin-1 (Gringhuis et al., 2012). Caspase-8-mediated IL-1β activation does not require any known inflammasome-forming NLR molecules, although ASC is required. Paradoxically, Casp8 gene deletion results in a spontaneous NLRP3 inflammasome activation that is dependent on RIP3-MLKL (mixed lineage kinase domain-like protein)-mitochondrial protein phosphatase PGAM5 signaling (Kang et al., 2013). These observations indicate that casapse-8 regulates IL-1β activation by two distinct mechanisms. Active caspase-8 can directly process IL-1β in response to specific extracellular signals, whereas loss of caspase-8 causes the disruption of cell homeostasis and leads to NLRP3 activation through RIP3 signaling. Importantly, these studies are consistent with a newly identified mechanism whereby RIP3 signaling functions downstream of caspase-8 and leads to necrosis and animal lethality in the absence of caspase-8 (Kaiser et al., 2011; Oberst et al., 2011), and highlight a complex role of caspase-8-RIP3 signaling in cell death and inflammation.
Recent studies have also focused on the role of posttranslational modification in inflammasome activity. Mishra et al. has reported a regulatory mechanism limiting the activation of NLRP3, but not AIM2, inflammasome rendered by the interferon-γ-nitric oxide synthase-2-nitric oxide (IFN-γ-NOS2-NO) pathway (Mishra et al., 2013). This inhibitory effect is due to NO-mediated S-nitrosylation of NLRP3, which leads to impaired assembly of NLRP3 inflammasome. This negative regulation may be essential to dampen NLRP3 signaling, and is suggested to be a protective mechanism during endotoxin challenge (Mao et al., 2013). Furthermore, ATP-induced NLRP3 inflammasome activation is strongly inhibited when NLRP3 is ubiquitinated, or NLRP3 deubiquitination is blocked, which indicates that NLRP3 deubiquitination is an essential posttranslational protein modification for NLRP3 activation (Juliana et al., 2012; Lopez-Castejon et al., 2013; Py et al., 2013). NLRP3 deubiquitination is mediated by BRCC3, a member of JAMM domain-containing Zn2+ metalloprotease deubiquitinating enzyme family, therefore providing a novel therapeutic target for IL-1β-associated inflammatory diseases.
Novel players in NLRP3 inflammasome activation
Recent studies have reported two novel inflammasome regulators, guanylate-binding protein 5 (GBP5) (Shenoy et al., 2012) and double-stranded RNA-dependent protein kinase (PKR) (Lu et al., 2012). GBP5 belongs to the 65-kD GBP gene family and promotes NLRP3 inflammasome activation by ATP, nigericin and bacteria, but not crystalline agents. GBP5 directly binds to the PYRIN domain of NLRP3 via its GTPase domain. GBP5 forms a tetrameric structure and stimulates NLRP3-ASC oligomerization. In contrast to the selective effect of GBP5 on NLRP3, PKR has recently been shown to contribute to the activation of all known inflammasomes, including NLRP1, NLRP3, NLRP4 and AIM2 inflammasomes. Loss of PKR results in attenuated IL-1β and IL-18 cleavage in response to a broad array of stimuli. Interestingly, GBP5 and PKR have been indicated in host defense against bacterial and viral infection, respectively, therefore highlighting complex cross-regulation among multiple inflammatory signaling pathways. However, the effect of PKR on NLRP3 inflammasome has been recently disputed in one report. Using cells from two independent Pkr gene-deletion mouse strains, PKR does not show any effect in NLRP3 activation, although it does affect LPS-induced NOS2 upregulation (He et al., 2013). Since the same gene-mutant mouse stains were employed in these studies, the reason for the different conclusions remains unclear.
Other NLR inflammasomes
Seminal new findings regarding NLRC4, NLRP1 inflammasome and other inflammasome NLRs have been reported. These findings are summarized below.
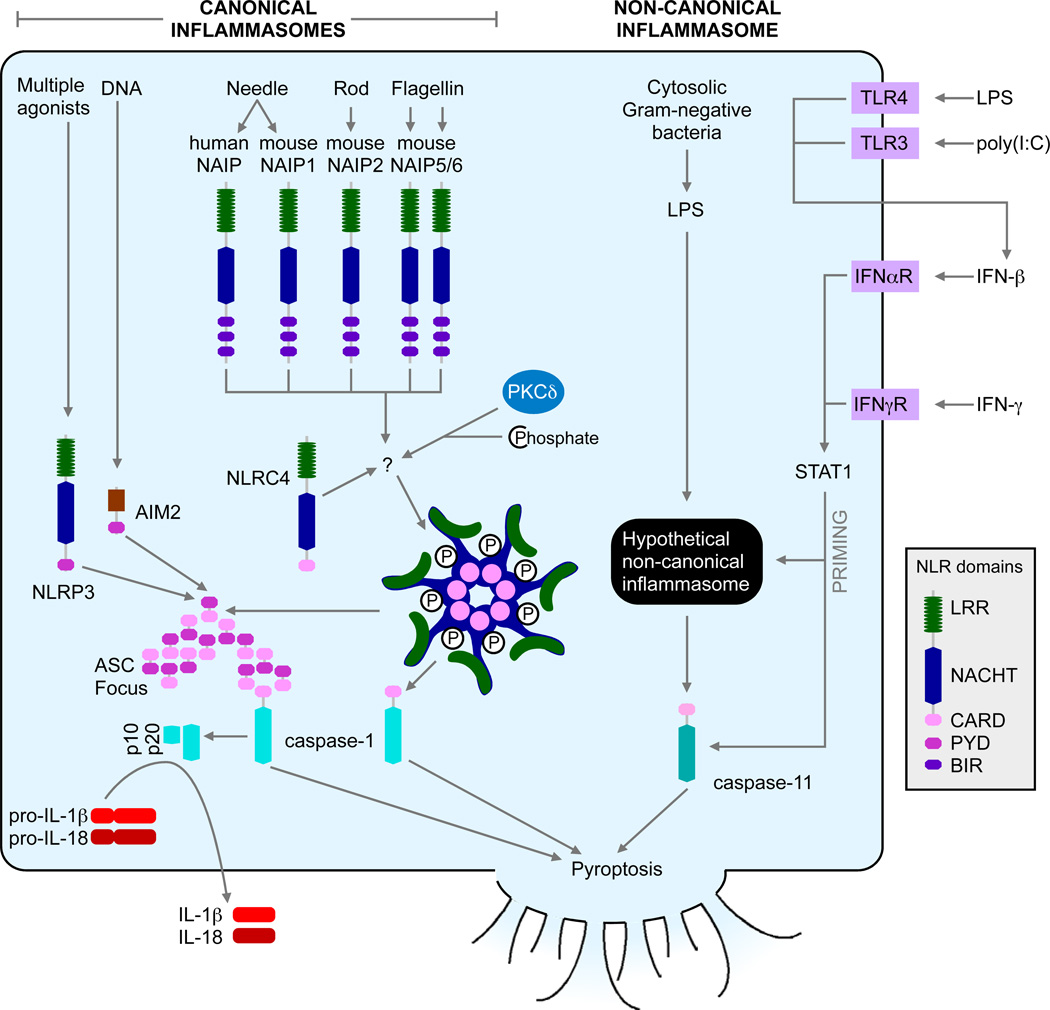
The NLRC4 inflammasome detects bacterial proteins in the cytosolic compartment as markers for the activity of bacterial type III (T3SS) and IV (T4SS) secretion systems or cytosolic invasion by flagellated bacteria. T3SS and T4SS are virulence factors that act as molecular syringes, transferring bacterial effector proteins through a hollow needle-like structure into host cell cytosol. The effector proteins reprogram host cell physiology to the benefit of the bacterial pathogen. The effector proteins are variable between bacteria, and evolve quickly, and are thus poor targets for innate immune detection. Instead, NLRC4 responds to accidental injection of three conserved proteins: flagellin, rod, and needle (Kofoed and Vance, 2011; Miao et al., 2010; Zhao et al., 2011). The reason why these proteins are accidentally injected is more thoroughly discussed elsewhere (Miao and Warren, 2010).
NLRC4 is unique amongst inflammasomes in that it is a downstream adaptor protein from the upstream receptor NLRs in the NAIP family (Figure 2). NAIPs appear to directly bind to one of the three agonists: flagellin, T3SS rod, or T3SS needle. For example, in C57BL/6 mice there are four NAIPs: NAIP1 detects needle (Miao, unpublished), NAIP2 detect rod, and NAIP5 and NAIP6 detect flagellins (Kofoed and Vance, 2011; Zhao et al., 2011). Interestingly, in humans there appears to be only one NAIP that responds to the needle protein (Zhao et al., 2011). How NAIPs and NLRC4 interact with each other remains unknown. They may form mixed inflammasome hubs, or NAIP may signal to activate NLRC4 (Halff et al., 2012).
Figure 2. Canonical and non-canonical inflammasome activation pathways.
Canonical inflammasomes including NLRP3, AIM2, and NLRC4 activate caspase-1. NLRP3 or AIM2 recruits caspase-1 through the adaptor protein ASC via Pyrin-Pyrin homotypic interactions. The activated caspase-1 results in its proteolytic maturation to the p10 and p20 fragments, and subsequent IL-1β and IL-18 cleavage and secretion. NLRC4 contains a CARD domain and directly binds to the CARD of caspase-1 through homotypic interaction, triggering cytokine (IL-1β and IL-18) maturation and pyroptosis. Non-canonical inflammasome activation leads to caspase-11-dependent pyroptosis. Caspase-11 activation also triggers NLRP3 activation via an unknown mechanism (not shown).
The latter hypothesis could explain the recent finding that NLRC4 phosphorylation is critical for its activity. Protein kinase C delta (PKCδ) was found to be the kinase responsible for this modification, and Prkcd−/− macrophages were mostly, but not completely, recalcitrant to NLRC4 activation (Qu et al., 2012). It is tempting to speculate that the NAIP inflammasome oligomerization somehow directs PKCδ to phosphorylate NLRC4. The NLRC4 structure was recently solved, and surprisingly apparently inactive NLRC4 is phosphorylated (Hu et al., 2013). However, the purified protein was generated in insect cells, and a construct lacking the CARD domain as well as 22 internal amino acids was used. Given the biologic evidence supporting phosphorylation as an activating step, it is possible that the phosphorylation seen in the crystal structure may not represent the true inactive form of NLRC4.
NLRP1 (DEFCAP, NAC or NALP1) is the first NLR that defined the inflammasome (Martinon et al., 2002; Moayeri et al., 2012). Human NLRP1 is genetically linked to a group of autoimmune and autoinflammatory diseases including vitiligo, autoimmune thyroid diseases and type I diabetes (Jin et al., 2007; Magitta et al., 2009). Human NLRP1 contains a N-terminal pyrin domain, a nucleotide-binding domain (NBD), a function-to-find domain (FIIND), LRR and a c-terminal CARD domain. The NLRP1 CARD is comprised of six-anti-parallel alpha helices and is similar to other death domain fold containing prominent charged patches (Jin et al., 2013), and is thought to be the key component that interacts with the CARD from procaspase-1. However, human and murine NLRP1 have very distinct structural components. Murine NLRP1 proteins do not have the pyrin domain and are encoded by three highly polymorphic paralogues, Nlrp1a, Nlrp1b and Nlrp1c. Furthermore mouse Nlrp1b mediates response to the lethal toxin (LT) of Bacillus anthracis (Boyden and Dietrich, 2006), while human NLRP1 does not. LT causes cell death due to toxin-induced lysosomal membrane permeability, cathepsin B release (Ali et al., 2011; Averette et al., 2009), and ATP leakage (Ali et al., 2011). The activator of human NLRP1 is less clear although several studies have shown that muramyl-dipeptide (MDP) can activate overexpressed NLRP1 (Faustin et al., 2007; Gregory et al., 2011). Likewise overexpression or RNA interference shows that mouse Nlrp1, when coupled with NOD2, can mediate inflammasome activation in response to MDP and titanium oxide (TiO2) (Hsu et al., 2008). However, recently Nlrp1b−/− mice have been generated, which identifies LT, but not MDP or TiO2, as the activator for murine NLRP1 inflammasome (Kovarova et al., 2012). Moreover, Nlrp1b deficiency results in an enhanced in vitro and in vivo pyroptosis, which occurs independently of IL-1β but is dependent on caspase-1, and this pyrotopsis profoundly affects pathogenesis. A separate study used N-ethyl-N-nitrosourea mutagenesis and found a single residue mutation in the FIND domain of Nlrp1a that results in a gain-of-function phenotype (Masters et al., 2012). This strain produces more caspase-1 and IL-1β but yet exhibits IL-1 receptor-independent hematopoietic cell death in response to chemotherapy or viral infection. Thus both reports underscore a strong pathologic role for Nlrp1 that is related to cell death but independent of IL-1 β and IL-1R, although the targeted cell death pathway remains to be elucidated.
NLRP1 has also been shown to be a target of viral immune evasion strategies. DNA viruses contain large genomes that encode immune evasion proteins and two viral-encoded inflammasome evasion proteins have been described. Orf63 of Kaposi’s sarcoma-associated herpesvirus (KSHV) was found to encode a NLRP1-like protein which contains the NBD and LRR domains, but lacks the PYRIN and CARD domain present in human NLRP1 (Gregory et al., 2011). Overexpression and RNA interference of Orf63 shows that it blocks NLRP1-dependent inflammasome and pyroptosis by blocking inflammasome complex assembly. Orf63 not only reduces NLRP1 but also reduces NLRP3 and NOD2-activated inflammasome, indicating a broader function in inflammasome inhibition. In a second study, a B-cell CLL/lymphoma 2 (Bcl-2) homolog of Vaccinia virus (F1L) was found to bind and inhibit NLRP1 through a small hexapeptide in F1L (Gerlic et al., 2013). Deletion of F1L in Vaccinia virus caused improved survival of the infected mice accompanied by increased caspase-1 activation and IL-1β secretion. These two studies reveal different ways by which viral-encoded proteins target the inflammasome for immune evasion.
NLRP1 is also the first NLR shown to undergo proteolytic processing and two cleavage sites have been characterized. The first is located in the FIIND domain that resides between the LRR and CARD domain. FIIND shares similarity with the ZU5-UPA domain of an autoproteolytic protein, leading the authors to investigate and confirm that NLRP1 FIIND domain undergoes autoproteolysis (D'Osualdo et al., 2011). Another group confirmed this finding and found that auto-cleavage of NLRP1 enhances inflammasome activation suggesting that FIIND protein processing is an important activation step (Finger et al., 2012). Another report showed that this C-terminal cleavage site in mouse FIIND is important for spontaneous inflammasome activation in a reconstitution system and introduction of an artificial protease site into an Nlrp1b protein from a LT resistance strain (NOD) restores spontaneous inflammasome activation (Frew et al., 2012). A second cleavage sequence was initially found in the N-terminus of rat Nlrp1a protein. Anthrax LT resistant vs. sensitive rat strains are polymorphic at this cleavage site, which affects the ability of LT to cleave this site. Cleavable Nlrp1b is associated with LT sensitivity (Levinsohn et al., 2012). In contrast to the clear association of LT resistance or sensitivity to cleavage of rat Nlrp1a, mouse Nlrp1b variants are cleaved by LT regardless of LT sensitivity or resistance. This suggests that LT resistance or sensitivity in mice cannot be solely explained by polymorphisms at this cleavage site (Hellmich et al., 2012). However cleavage of this site in mouse Nlrp1b does have a functional consequence as reported in a recent paper (Chavarria-Smith and Vance, 2013). These authors noted that mouse recombinant Nlrp1b can be cleaved by LT at a site that shares similarity with the rat Nlrp1b cleavage site. Mutation of this site results in the loss of cleavage by LT and a correlative loss of inflammasome activation. Furthermore, replacement of this cleavage site with an artificial TEV (Tobacco Etch Virus) protease cleavage-site results in inflammasome activation in the presence of TEV, supporting a functional role for the proteolytic processing of Nlrp1b at this N-terminal site.
Several NLR family members are found to exhibit inflammasome function but interestingly these proteins also exhibit other functions, prominent among them is the negative regulation of NF-κB activation. How inflamamsome activation and NF-κB inhibition intersect is unclear, although prior work has shown a negative relationship between NF-κB and caspase-1 activation in that IKKβ (inhibitor of κB kinase β) deletion causes increased caspase-1 activation and pro-IL-1β processing (Greten et al., 2007). Since many of these NLRs are studied in gene-deletion mice, this approach lends credence regarding these functional studies. The following summarizes the findings of these new inflamamsome NLRs.
NLRP6 (PYPAF5) was originally found to synergize with ASC to cause NF-κB and caspase-1 activation in overexpressed systems (Grenier et al., 2002). Analysis of Nlrp6−/− mice shows that they are uniformly more susceptible to chemical-induced colitis and colitis-associated tumorigenesis (Chen et al., 2011; Elinav et al., 2011; Normand et al., 2011). Meanwhile, one group has shown that Nlrp6−/− mice have reduced IL-18 and expanded pathobiont bacteria, Bacteroidetes (Prevotellaceae) and TM7 microbiota, accompanied by spontaneous and induced colitis (Elinav et al., 2011). Because Asc−/− and Casp1−/−Casp11−/− mice mice mice show strikingly similar phenotypes, these authors conclude that NLRP6 is an inflammasome NLR (Elinav et al., 2011). These studies have been extended to a model for non-alcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) and found that NLRP6, NLRP3 and IL-18 negatively regulate NAFLD and NASH progression (Henao-Mejia et al., 2012). Two other reports have also studied Nlrp6−/− mice in DSS-induced colitis and colitis-associated tumorigenesis. One report has shown that Nlrp6 deletion results in enhanced inflammation and inflammatory cytokines including IL-1β mRNA and epithelial cell proliferation that likely contributes to tumorigenesis (Chen et al., 2011). This report also showed that Nlrp6−/− mice have reduced IL-18 protein without an associated decrease in pro-IL-18 transcript. Another report found that Nlrp6 is expressed primarily by colonic myofibroblasts, and its deletion similarly exacerbated chemically induced colitis and tumorigenesis. The investigators found a role for NLRP6 in promoting tissue repair, and its absence caused colonic proliferation and increased chemokines; IL-1β was not significantly altered, and treatment with an IL-1R antagonist did not affect the disease but the disease is similar to that exhibited by Asc−/− and Casp1−/− mice (Normand et al., 2011). In addition to a role in assembling the inflammasome, another report has shown that the deletion of Nlrp6 results in resistance to bacterial infections, accompanied by enhanced NF-κB and mitogen activated protein kinase (MAPK)-dependent cytokines and chemokine secretion (Anand et al., 2012). The parallel findings in Asc−/−, Casp1−/− and Nlrp6−/− mice with reduced IL-18 in these mice suggest that NLRP6 is most likely an inflammasome, however key features that define the inflammasome such as NLRP6-dependent pro-caspase-1, IL-1β and IL-18 processing remain to be demonstrated. Additionally, the results also show that NLRP6 can attenuate inflammatory mediators.
NLRP7 (PYPAF3) is found in humans and not mice. It was initially shown to be a negative feed-back regulator of IL-1β secretion in reconstituted inflammasome (Kinoshita et al., 2005). A genetic study of two families with familial recurrent hydatidiform moles (HM), a rare abnormal pregnancy comprised of hyperproliferating trophoblasts, identified NLRP7 as a candidate gene(Murdoch et al., 2006), which was confirmed in multiple families and ethnic groups (Kou et al., 2008; Wang et al., 2009). In a follow-up study, peripheral blood mononuclear cells (PBMCs) were isolated from six HM patients with different NLRP7 mutations, and all but one secreted reduced IL-1β and TNF-α upon LPS treatment compared to healthy controls (Messaed et al., 2011). The report implicates a secretory function for NLRP7 rather than pro-IL-1β processing. The same study showed that WT NLRP7 inhibits IL-1β maturation while a nonsense mutation does not exhibit this function. In contrast, a recent report has applied RNAi screening and shows that NLRP7 mediates inflammasome activation by mycoplasma acylated lipopeptide (acLP) and other acLPs in a human macrophage cell line (Khare et al., 2012). Inflammasome reconstitution assays reveals that NLRP7 but not NLRP3 or NOD1 causes lipopeptide-induced inflammasome, and HM-linked mutations are more potent activators of the inflammasome. It is possible that the use of different cell sources, test systems and activators resulted in these opposing findings.
NLRP12 (Monarch-1 or PYPAF7) exhibits at least two functions in human macrophage cell lines: as an inhibitor of the noncanonical and the canonical NF-κB pathway (Lich et al., 2007; Williams et al., 2005), along with as an inducer of ASC speck formation, a surrogate marker for inflammasome activation (Wang et al., 2002). Gene-deficient mice have revealed similar outcomes. Two reports have shown that Nlrp12−/− mice exhibited increased noncanonical and canonical NF-κB activation as well as strong extracellular regulated kinase (ERK) induction in chemical-induced colitis and colitis-associated tumorigenesis models (Allen et al., 2012; Zaki et al., 2011). Both studies found that the Nlrp12 deficiency results in prolonged and/or increased cytokine secretion including IL-1β in the affected tissues. However a different group has found that a recombinant Yersinia strain but not several other bacteria can cause Nlrp12-dependent inflammasome activation (Vladimer et al., 2012). Again, divergent models differentially affecting cell or tissue-specific Nlrp12 might explain these different functions.
NLRC5 is now widely accepted as a inducer of class I major histocompatibility complex (MHC) glycoprotein expression (reviewed in (Kobayashi and van den Elsen, 2012)). However NLRC5 also exhibits diverse effects on innate responses in vitro, but this review will only focus on its potential role in inflammasome activation. Three groups have used overexpression and siRNA targeting of Nlrc5 in a human macrophage cell line, primary PBMC or bronchial cells to link it to inflammasome and caspase-1 activation (Davis et al., 2011; Kumar et al., 2011; Triantafilou et al., 2013b). One showed the interaction of NLRC5 with NLRP3 and their shared function in response to NLRP3-inflammasome activators in human macrophage cell lines and primary cells (Davis et al., 2011). A recent report corroborated the functional and physical interaction of NLRC5 and NLRP3 in bronchial cells infected with Rhinovirus during inflammasome activation (Triantafilou et al., 2013b). Finally a study of Nlrc5−/− mouse cells reveals deficiencies in response to NLRP3 activators such as monosodium urate (MSU), alum and ATP (Yao et al., 2012). However, other Nlrc5−/− strains have not exhibited defects in inflammasome activation, an issue that could arise due to different gene targeting strategies used by these different studies. In addition, human and mouse NLRC5 may play different roles in inflammasome activation, thus resulting in different findings in these two species.
Non-canonical inflammasome pathway activating caspase-11
Caspase-1 deficient mice have long been known to be resistant to LPS induced septic shock (Li et al., 1995). However, all existing Casp1−/− mice were recently shown to also lack Casp11 due to backcrossing a passenger mutation from the Casp11 deficient 129 mouse strain into C57BL/6 (Kayagaki et al., 2011). Caspase-1 promotes both pyroptosis and processing and secretion of IL-1β and IL-18. In contrast, caspase-11 primarily triggers pyroptosis, although it can induce IL-1β secretion by a pathway in collaboration with NLRP3, ASC, and caspase-1 (Kayagaki et al., 2011) (Figure 2). To differentiate these two inflammasome pathways, the caspase-1 activating platforms are now referred to as the “canonical inflammasomes”, while the hypothetical platform(s) that activates caspase-11 is the “non-canonical inflammasome”. Examination of Casp11−/− mice on the C57BL/6 background reveals that most of the known inflammasome agonists activate the canonical inflammasome pathways independently of caspase-11. Thus, ATP, cytosolic flagellin, and cytosolic DNA activate caspase-1 via the NLRP3, NLRC4, and AIM2 canonical inflammasomes, respectively, in agreement with prior thinking (Kayagaki et al., 2011). In contrast, caspase-11, and not caspase-1, is detrimental during endotoxic shock (Kayagaki et al., 2011; Wang et al., 1998). It has been found that caspase-11 specifically differentiates Gram-negative bacteria that invade the cytosol from those that remain extracellular or confined to the vacuole, thus protecting mice against lethal infection by Burkholderia thailandensis and B. pseudomallei. In contrast, vacuolar bacteria such as S. typhimurium are poorly detected by caspase-11, while as ∆sifA mutant that destabilize the vacuole and enters the cytosol is detected (Aachoui et al., 2013).
In addition to cytosolic bacteria, caspase-11 will respond to vacuolar bacteria, but under delayed kinetics (Broz et al., 2012; Gurung et al., 2012; Kayagaki et al., 2011; Rathinam et al., 2012). It is hypothesized that this results from aberrant translocation of the caspase-11 agonist from the vacuole into the cytosol. The notable exception is L. pneumophila where the ∆sdhA mutant of L. pneumophila, which aberrantly ruptures the vacuole, is detected by caspase-11 while vacuolar L. pneumophila are not (Aachoui et al., 2013). Conversely, similar infections in LPS primed macrophages at higher multiplicities of infection (MOI) results in rapid activation of caspase-11 by wild type bacteria, but not by T4SS mutants (Case et al., 2013; Casson et al., 2013). The explanation for these divergent results undoubtedly lies in the different priming stimuli and MOIs used. We hypothesize that at higher MOI in primed macrophages a T4SS effector imbalance occurs resulting in vacuolar leakage or destabilization.
Several groups have examined the priming requirements of the caspase-11 pathway (Figure 2). Caspase-11 is normally non-responsive, unless macrophages are primed with a signal that activates the transcription factor STAT1. This can be achieved by any stimuli that induces IFN-β secretion in an autocrine or paracrine manner, such as LPS or poly(I:C), or by direct application of IFN-β or IFN-γ, which both signal through STAT1 (Aachoui et al., 2013; Broz et al., 2012; Case et al., 2013; Gurung et al., 2012; Rathinam et al., 2012). The molecular mechanisms underlying this priming effect are not yet clear.
Cytosolic LPS activates caspase-11, triggering shock independently of TLR4
We (Hagar and Miao, In press) and others (Kayagaki et al., 2013) have identified LPS as the cytosolic agonist detected through caspase-11 (Figure 2). Caspase-11 responds to cytosolic lipid A species with five or six acyl groups, but not to species with four acyl groups, indicating structural specificity. This expands the role of LPS as a key microbial pattern detected by the innate immune system: extracellular and vacuolar LPS is detected through TLR4, while cytosolic LPS is detected through caspase-11. Interestingly, both Tlr4−/− and Casp11−/− mice are resistant to classical LPS challenge (Kayagaki et al., 2011; Takeuchi et al., 1999; Wang et al., 1998). We hypothesize that endotoxic shock involves a two-step process: 1) TLR4 detects extracellular LPS and primes the caspase-11 pathway; 2) LPS aberrantly enters the cytosol and activates caspase-11, whose downstream mediators cause shock. Indeed, mice primed with sublethal LPS become hypersusceptible to subsequent low dose LPS challenge (LPS → LPS). Priming could also be accomplished by poly(I:C), as poly(I:C) → LPS mice also succumb. While Tlr4−/− mice are resistant to LPS → LPS challenge, they still succumbed to poly(I:C) → LPS challenge. Therefore, during endotoxic shock TLR4 primes the caspase-11 pathway, but can be replaced by alternate priming stimuli including via TLR3. Indeed, Casp11−/− mice are more resistant to this challenge, although some mice still succumb. These results corroborate the in vitro data showing that caspase-11 responds to LPS independently of TLR4 (Kayagaki et al., 2013) (Hagar and Miao, In press). These studies demonstrate that TLR4 is dispensable during experimental endotoxic shock, and proves that a second LPS detection pathway causes mortality.
Eicosanoid mediators triggered by inflammasome activation in vivo
Poly(I:C) → LPS challenge results in mortality within two hours, which is kinetically similar to shock triggered after rapid activation of the canonical NLRC4 inflammasome activation in vivo. To activate the NLRC4 inflammasome in vivo, the anthrax lethal toxin was modified to remove the enzymatic subunit and replaced with flagellin (von Moltke et al., 2012). This FlaTox injects flagellin into the cytosol of host cells, where it is detected by the canonical NLRC4 inflammasome. Mice injected with FlaTox die within 30 minutes in the absence of priming, as the NLRC4 pathway is largely priming independent. This mortality was found to be independent of IL-1β and IL-18, and rather was due to massive eicosanoid production, resulting in fluid extravasation, hemoconcentration, and resulting shock from loss of blood volume. Mice can be rescued by cyclooxygenase-1 (COX-1) deficiency, which prevents eicosanoid production (von Moltke et al., 2012). Similarly, during poly(I:C) → LPS challenge, COX-1 inhibitors rescue the mice (Hagar and Miao, unpublished). Thus, two in vivo models indicate that both canonical inflammasome pathways activating caspase-1 and non-canonical inflammasome pathways activating caspase-11 can cause shock via eicosanoid production. The mechanism by which caspase-1 or caspase-11 trigger eicosanoid production remains to be determined, but appears to involve calcium influx activating cyclic phospholipase A2 (cPLA2), and also is enhanced by MYD88 and TRIF signaling (von Moltke et al., 2012).
Conclusion
This review provides an updated synopsis of the rapidly evolving field of the biology of the inflammasome with a focus on the NLR proteins. Novel pathways and molecules including mitochondrial changes, calcium regulators and ER stress inducers are found to lie upstream of the NLRP3 inflammasome, while the NLRC4-NAIP inflammasome is found to interact with its ligand through NAIP with NLRC4 undergoing post- translational modification and activation. Key advances have also been made in NLRP1 which is found to cause profound changes in pyroptosis, and undergoes post-translational proteolysis that are necessary for its activation. In addition, several new NLRs are found to exhibit inflammasome function. In addition to the canonical caspase-1 activating inflammasome, the non-canonical caspase-11 inflammasome is found to be differentially triggered by cytosolic and vacuolar bacteria, and most importantly by cytosolic LPS. It is apparent that recent progress has generated an arsenal of information regarding inflammasomes. Challenges are to understand how the different activation pathways of inflammasome converge or diverge, and how the different NLR inflammasomes are activated in molecular terms.
ACKNOWLEDGMENTS
This work was supported by NIH grants R37-AI029564, U54-AI057157 (SERCEB), U19AI077437, U19AI067798 and CA156330 awarded to J.P.-Y.T., NIH grants AI097518 and AI057141 awarded to E.A.M, NIH grant K01DK098307 awarded to H.W. H.W. is a recipient of Postdoctoral Fellowship of the American Heart Association (Mid-Atlantic Affiliate) and Postdoctoral Fellowship of Cancer Research Institute. We thank Dr. Moayeri for helpful discussions. The authors have declared that no conflict of interest exists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011;35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averette KM, Pratt MR, Yang Y, Bassilian S, Whitelegge JP, Loo JA, Muir TW, Bradley KA. Anthrax lethal toxin induced lysosomal membrane permeabilization and cytosolic cathepsin release is Nlrp1b/Nalp1b–dependent. PLoS One. 2009;4:e7913. doi: 10.1371/journal.pone.0007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, Mocarski ES, Subramanian D, Green DR, Silverman N, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. Journal of immunology. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nature genetics. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, Verkhratsky A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. Journal of immunology. 2003;170:3029–3036. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, Roy CR. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson CN, Copenhaver AM, Zwack EE, Nguyen HT, Strowig T, Javdan B, Bradley WP, Fung TC, Flavell RA, Brodsky IE, Shin S. Caspase-11 Activation in Response to Bacterial Secretion Systems that Access the Host Cytosol. PLoS Pathog. 2013;9:e1003400. doi: 10.1371/journal.ppat.1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Smith J, Vance RE. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog. 2013;9:e1003452. doi: 10.1371/journal.ppat.1003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Liu M, Wang F, Bertin J, Nunez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. Journal of immunology. 2011;186:7187–7194. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan V, Baroja-Mazo A, Lopez-Castejon G, Gomez AI, Martinez CM, Angosto D, Montero MT, Herranz AS, Bazan E, Reimers D, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Cox JS, Chapman RE, Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell. 1997;8:1805–1814. doi: 10.1091/mbc.8.9.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Osualdo A, Weichenberger CX, Wagner RN, Godzik A, Wooley J, Reed JC. CARD8 and NLRP1 undergo autoproteolytic processing through a ZU5-like domain. PLoS One. 2011;6:e27396. doi: 10.1371/journal.pone.0027396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BK, Roberts RA, Huang MT, Willingham SB, Conti BJ, Brickey WJ, Barker BR, Kwan M, Taxman DJ, Accavitti-Loper MA, et al. Cutting edge: NLRC5-dependent activation of the inflammasome. Journal of immunology. 2011;186:1333–1337. doi: 10.4049/jimmunol.1003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Molecular cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Feske S, Skolnik EY, Prakriya M. Ion channels and transporters in lymphocyte function and immunity. Nature reviews. Immunology. 2012;12:532–547. doi: 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, Bertin J, Gough PJ. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. The Journal of biological chemistry. 2012;287:25030–25037. doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew BC, Joag VR, Mogridge J. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 2012;8:e1002659. doi: 10.1371/journal.ppat.1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlic M, Faustin B, Postigo A, Yu EC, Proell M, Gombosuren N, Krajewska M, Flynn R, Croft M, Way M, et al. Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7808–7813. doi: 10.1073/pnas.1215995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S, Reed JC, Ting JP, Damania B. Discovery of a viral NLR homolog that inhibits the inflammasome. Science. 2011;331:330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier JM, Wang L, Manji GA, Huang WJ, Al-Garawi A, Kelly R, Carlson A, Merriam S, Lora JM, Briskin M, et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS letters. 2002;530:73–78. doi: 10.1016/s0014-5793(02)03416-6. [DOI] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nature immunology. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- Gurung P, Malireddi RK, Anand PK, Demon D, Walle LV, Liu Z, Vogel P, Lamkanfi M, Kanneganti TD. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. The Journal of biological chemistry. 2012;287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halff EF, Diebolder CA, Versteeg M, Schouten A, Brondijk TH, Huizinga EG. Formation and structure of a NAIP5-NLRC4 inflammasome induced by direct interactions with conserved N- and C-terminal regions of flagellin. The Journal of biological chemistry. 2012;287:38460–38472. doi: 10.1074/jbc.M112.393512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Franchi L, Nunez G. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. European journal of immunology. 2013;43:1147–1152. doi: 10.1002/eji.201243187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmich KA, Levinsohn JL, Fattah R, Newman ZL, Maier N, Sastalla I, Liu S, Leppla SH, Moayeri M. Anthrax lethal factor cleaves mouse nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS One. 2012;7:e49741. doi: 10.1371/journal.pone.0049741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Yan C, Liu P, Huang Z, Ma R, Zhang C, Wang R, Zhang Y, Martinon F, Miao D, et al. Crystal Structure of NLRC4 Reveals Its Autoinhibition Mechanism. Science. 2013 doi: 10.1126/science.1236381. [DOI] [PubMed] [Google Scholar]
- Ito M, Yanagi Y, Ichinohe T. Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome. PLoS Pathog. 2012;8:e1002857. doi: 10.1371/journal.ppat.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, et al. Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation. Immunity. 2013 doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Curry J, Smith P, Jiang J, Xiao TS. Structure of the NLRP1 caspase recruitment domain suggests potential mechanisms for its association with procaspase-1. Proteins. 2013;81:1266–1270. doi: 10.1002/prot.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. The New England journal of medicine. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. The Journal of biological chemistry. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3- mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, et al. Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4. Science. 2013 doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity. 2012;36:464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Wang Y, Hasegawa M, Imamura R, Suda T. PYPAF3, a PYRIN-containing APAF-1-like protein, is a feedback regulator of caspase-1-dependent interleukin-1beta secretion. The Journal of biological chemistry. 2005;280:21720–21725. doi: 10.1074/jbc.M410057200. [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, van den Elsen PJ. NLRC5: a key regulator of MHC class I-dependent immune responses. Nature reviews. Immunology. 2012;12:813–820. doi: 10.1038/nri3339. [DOI] [PubMed] [Google Scholar]
- Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou YC, Shao L, Peng HH, Rosetta R, del Gaudio D, Wagner AF, Al-Hussaini TK, Van den Veyver IB. A recurrent intragenic genomic duplication, other novel mutations in NLRP7 and imprinting defects in recurrent biparental hydatidiform moles. Mol Hum Reprod. 2008;14:33–40. doi: 10.1093/molehr/gam079. [DOI] [PubMed] [Google Scholar]
- Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, Xiang Z, Lommatzsch SE, Huang MT, Ting JP, Koller BH. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. Journal of immunology. 2012;189:2006–2016. doi: 10.4049/jimmunol.1201065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Pandey S, Zou J, Kumagai Y, Takahashi K, Akira S, Kawai T. NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. Journal of immunology. 2011;186:994–1000. doi: 10.4049/jimmunol.1002094. [DOI] [PubMed] [Google Scholar]
- Labbe K, McIntire CR, Doiron K, Leblanc PM, Saleh M. Cellular Inhibitors of Apoptosis Proteins cIAP1 and cIAP2 Are Required for Efficient Caspase-1 Activation by the Inflammasome. Immunity. 2011;35:897–907. doi: 10.1016/j.immuni.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annual review of cell and developmental biology. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, et al. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, Liu S, Sastalla I, Leppla SH, Moayeri M. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012;8:e1002638. doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178:1256–1260. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
- Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E, Brough D. Deubiquitinases regulate the activity of caspase-1 and interleukin-1beta secretion via assembly of the inflammasome. The Journal of biological chemistry. 2013;288:2721–2733. doi: 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. The Journal of experimental medicine. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magitta NF, Boe Wolff AS, Johansson S, Skinningsrud B, Lie BA, Myhr KM, Undlien DE, Joner G, Njolstad PR, Kvien TK, et al. A coding polymorphism in NALP1 confers risk for autoimmune Addison's disease and type 1 diabetes. Genes Immun. 2009;10:120–124. doi: 10.1038/gene.2008.85. [DOI] [PubMed] [Google Scholar]
- Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang B, He Z, Zeng Y, Hu Y, Sun S, et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013;23:201–212. doi: 10.1038/cr.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ, et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K, Tschopp J. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaed C, Akoury E, Djuric U, Zeng J, Saleh M, Gilbert L, Seoud M, Qureshi S, Slim R. NLRP7, a nucleotide oligomerization domain-like receptor protein, is required for normal cytokine secretion and co-localizes with Golgi and the microtubule-organizing center. The Journal of biological chemistry. 2011;286:43313–43323. doi: 10.1074/jbc.M111.306191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Warren SE. Innate immune detection of bacterial virulence factors via the NLRC4 inflammasome. Journal of clinical immunology. 2010;30:502–506. doi: 10.1007/s10875-010-9386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nature immunology. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- Mishra BB, Rathinam VA, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, Sassetti CM. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nature immunology. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nature medicine. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Sastalla I, Leppla SH. Anthrax and the inflammasome. Microbes and infection / Institut Pasteur. 2012;14:392–400. doi: 10.1016/j.micinf.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch S, Djuric U, Mazhar B, Seoud M, Khan R, Kuick R, Bagga R, Kircheisen R, Ao A, Ratti B, et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nature genetics. 2006;38:300–302. doi: 10.1038/ng1740. [DOI] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9601–9606. doi: 10.1073/pnas.1100981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–328. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Molecular cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Komuves L, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490:539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nature reviews. Molecular cell biology. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, Schubert K, Schoneberg T, Schaefer M, Krugel U, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The Adaptor MAVS Promotes NLRP3 Mitochondrial Localization and Inflammasome Activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Hughes TR, Triantafilou M, Morgan BP. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013a doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Kar S, van Kuppeveld FJ, Triantafilou M. Rhinovirus-Induced Calcium Flux, Triggers NLRP3 and NLRC5 Activation in Bronchial Cells. American journal of respiratory cell and molecular, biology. 2013b doi: 10.1165/rcmb.2013-0032OC. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37:96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, Vance RE. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CM, Dixon PH, Decordova S, Hodges MD, Sebire NJ, Ozalp S, Fallahian M, Sensi A, Ashrafi F, Repiska V, et al. Identification of 13 novel NLRP7 mutations in 20 families with recurrent hydatidiform mole; missense mutations cluster in the leucine-rich region. J Med Genet. 2009;46:569–575. doi: 10.1136/jmg.2008.064196. [DOI] [PubMed] [Google Scholar]
- Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS, Bertin J. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. The Journal of biological chemistry. 2002;277:29874–29880. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, et al. The CATERPILLER protein monarch-1 is an antagonist of toll-like, receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. The Journal of biological chemistry. 2005;280:39914–39924. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Wang Y, Chen F, Huang Y, Zhu S, Leng Q, Wang H, Shi Y, Qian Y. NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res. 2012;22:836–847. doi: 10.1038/cr.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, Qiao L. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun. 2013;4:1611. doi: 10.1038/ncomms2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]